Method Article
Three-Dimensional Culture of Murine Colonic Crypts to Study Intestinal Stem Cell Function Ex Vivo
W tym Artykule
Podsumowanie
The present protocol describes establishing a murine colonic organoid system to study the activity and functioning of colonic stem cells in a claudin-7 knockout model.
Streszczenie
The intestinal epithelium regenerates every 5-7 days, and is controlled by the intestinal epithelial stem cell (IESC) population located at the bottom of the crypt region. IESCs include active stem cells, which self-renew and differentiate into various epithelial cell types, and quiescent stem cells, which serve as the reserve stem cells in the case of injury. Regeneration of the intestinal epithelium is controlled by the self-renewing and differentiating capabilities of these active IESCs. In addition, the balance of the crypt stem cell population and maintenance of the stem cell niche are essential for intestinal regeneration. Organoid culture is an important and attractive approach to studying proteins, signaling molecules, and environmental cues that regulate stem cell survival and functions. This model is less expensive, less time-consuming, and more manipulatable than animal models. Organoids also mimic the tissue microenvironment, providing in vivo relevance. The present protocol describes the isolation of colonic crypts, embedding these isolated crypt cells into a three-dimensional gel matrix system and culturing crypt cells to form colonic organoids capable of self-organization, proliferation, self-renewal, and differentiation. This model allows one to manipulate the environment-knocking out specific proteins such as claudin-7, activating/deactivating signaling pathways, etc.-to study how these effects influence the functioning of colonic stem cells. Specifically, the role of tight junction protein claudin-7 in colonic stem cell function was examined. Claudin-7 is vital for maintaining intestinal homeostasis and barrier function and integrity. Knockout of claudin-7 in mice induces an inflammatory bowel disease-like phenotype exhibiting intestinal inflammation, epithelial hyperplasia, weight loss, mucosal ulcerations, epithelial cell sloughing, and adenomas. Previously, it was reported that claudin-7 is required for intestinal epithelial stem cell functions in the small intestine. In this protocol, a colonic organoid culture system is established to study the role of claudin-7 in the large intestine.
Wprowadzenie
Intestinal organoid culture is a three-dimensional (3D) ex vivo system in which stem cells are isolated from the intestinal crypts of primary tissue and plated into a gel matrix1,2. These stem cells are capable of self-renewal, self-organization, and organ functionality2. Organoids mimic the tissue microenvironment and are more similar to in vivo models than two-dimensional (2D) in vitro cell culture models, although less manipulatable than cells3,4. This model eliminates obstacles encountered in 2D models, such as a lack of proper cell-cell adhesions, cell-matrix interactions, and homogenous populations, and also reduces the limitations of animal models, including high costs and lengthy periods of time5. Intestinal organoids-also referred to as colonoids for those grown from colonic crypt-derived stem cells-are essentially mini-organs that contain an epithelium including all cell types that would be present in vivo, as well as a lumen. This model allows manipulation of the system to study many aspects of the intestine, such as the stem cell niche, intestinal physiology, pathophysiology, and gut morphogenesis3,5,6. It also provides a great model for drug discovery, studying human intestinal disorders such as inflammatory bowel disease (IBD) and colorectal cancer, patient-specific personalized treatment development, and studying tissue regeneration4,7,8,9. In addition, the organoid system can also be used to study cellular communication, drug metabolism, viability, proliferation, and response to stimuli7,8. While animal models may be used to test potential therapeutics for intestinal pathologic conditions, they are quite limited, as studying multiple drugs at once poses a challenge. There are more confounding variables in vivo, and associated cost and time are high and long, respectively. On the other hand, the organoid culture system allows for the screening of many therapeutics at once in a shorter time period and also allows for personalized treatment through the use of patient-derived organoid culture4,8. The ability of colonic organoids to mimic tissue organization, microenvironment, and functionality also makes them an excellent model for studying regeneration and tissue repair9. Our lab has established a small intestine organoid culture system to study the effect of claudin-7 on small intestine stem cell functions10. In this study, a large intestinal organoid culture system is established to study stem cells' ability, or lack of ability, to self-renew, differentiate, and proliferate in a conditional claudin-7 knockout (cKO) model.
Claudin-7 is a very important tight junction (TJ) protein that is highly expressed in the intestine and is essential for maintaining TJ function and integrity11. cKO mice suffer from an IBD-like phenotype, exhibiting severe inflammation, ulcerations, epithelial cell sloughing, adenomas, and increased cytokine levels11,12. While it is widely accepted that claudins are vital for epithelial barrier function, new roles for claudins are emerging; they are involved in proliferation, migration, cancer progression, and stem cell function10,12,13,14,15,16,17. It is currently unknown how claudin-7 impacts the stem cell niche and function of colonic stem cells. As the intestine rapidly self-renews approximately every 5-7 days, maintenance of the stem cell niche and proper functioning of the active stem cells is vital18. Here, a system is established to examine the potential regulatory effects of claudin-7 on the colonic stem cell niche.
Protokół
All animal experiments and procedures were approved by the East Carolina University (ECU) Animal Care and Use Committee (IACUC) and conducted in compliance with guidelines from the National Institutes of Health and ECU on laboratory animal care and use. Inducible, intestinal-specific claudin-7 knockout mice were generated by crossing C57BL6 claudin-7-flox transgenic mice with Villin-CreERT2 mice19. Male and female mice aged 3 months were used in this study.
1. Reagent/equipment preparation
- Cool the following reagents/equipment before starting their associated experiments: phosphate buffered saline (PBS) for washing colon tissue during crypt isolation; a rocker/rotator (place in a 4 °C refrigerator) for incubation with epithelial dissociation media.
- Remove the gel matrix (see Table of Materials) from -20 °C and thaw on ice before plating.
- Pre-cool the centrifuge to 4 °C prior to spinning down crypts for plating.
- Cool 0.1% sodium citrate buffer (see Table of Materials) and keep on ice prior to in situ cell death detection.
- Warm the following reagents before starting their associated experiments: a 96-well culture plate for 24 h before plating; L-WRN media (see Table of Materials) before adding to plated crypts and before each media change.
- Heat the water bath to 94 °C before staining.
2. Murine colonic crypt isolation
- Prepare the necessary media following the steps below.
NOTE: The media volumes are calculated here for the colonic tissue of two mice.- Prepare epithelial dissociation media: 30 mL of 1x PBS + 400 µL of 0.5 M EDTA + 50 µL of 10 mM Y-27632 dihydrochloride (see Table of Materials). Keep on ice until use.
- Prepare crypt dissociation media: 10 mL of 1x PBS + 10 µL of 10 mM Y-27632 dihydrochloride. Keep on ice until use.
- Supplement L-WRN media: 50 mL of L-WRN media + 47.5 mL of DMEM high glucose with L-Glutamine + 500 µL of L-glutamine + 500 µL of pennicilin/streptomycin + 1,000 µL of B-27 supplement (50x) + 500 µL of N2 supplement (100x) + 50 µL of HEPES (1 M) buffer solution (see Table of Materials).
- Filter the complete (supplemented) L-WRN media and aliquot for storage at -20 °C for up to 3 months.
NOTE: Thawed media is stable at 4 °C for up to 2 weeks.
- Perform the crypt isolation.
- For general anesthesia, add 1 mL of isoflurane to cotton and place it inside a plastic cassette within a 0.09 cubic feet anesthesia chamber (see Table of Materials). Place the mouse in the anesthesia chamber until breathing halts (approximately 3-5 min) and then perform cervical dislocation20.
- Make an approximately 2 in incision down the midline of the mouse, pinning the back skin to expose the abdomen. Isolate the colon by cutting just below the cecum from the proximal side and above the rectum from the distal side21.
- Using forceps, remove the adipose tissue attached to the colon. Gently push feces out utilizing the flat end of the forceps and cut the tissue open longitudinally.
- Wash the tissue 10-15 times with cold 1x PBS using forceps to "swirl" the tissue around in PBS between washes.
- Using clean, sharp scissors, cut the tissue into small pieces, approximately 3-5 mm in size.
- Repeat the process for the second mouse and combine the tissue pieces in a 50 mL tube containing cold epithelial dissociation media (step 2.1.1).
- Incubate the colon tissue pieces in epithelial dissociation media for 90 min at 4 °C with gentle rocking.
- Allow the tissue fragments to sink to the bottom of the tube, then carefully discard the epithelial dissociation media without disrupting the tissue. Repeat this process when washing the tissue 10-15 times with cold 1x PBS. Discard as much PBS as possible during the final wash.
- Add crypt dissociation media (step 2.1.2) to the 50 mL tube containing colon tissue pieces and continuously shake for 5-10 min by hand.
NOTE: The media must become cloudy from the dissociated crypts. - Under the cell culture hood, filter the tissue and media with a 70 µm nylon cell strainer (see Table of Materials) into a fresh 50 mL tube.
- Centrifuge at 200 x g for 10 min at room temperature and discard the supernatant without disturbing the crypt-containing pellet.
NOTE: Depending on the equipment, one may transfer the strained media to a 15 mL tube for centrifuging. - Resuspend the pellet in ~3-4 mL of cold 1x PBS.
- Pipette 10 µL of the isolated crypts in a line on a microscope slide. Under the microscope, count the number of full, long crypts to estimate crypt concentration per 10 µL.
- Calculate the appropriate volume of crypts to spin down in order to plate 10 crypts/µL in a 96-well plate.
3. Crypt plating
- Centrifuge an appropriate volume of isolated crypts in a 1.5 mL microcentrifuge tube at 200 x g for 5 min at 4 °C.
- Carefully remove the supernatant using a 1,000 µL pipette without disrupting the pelleted crypts.
- Add 100 µL of gel matrix (enough for nine wells) to the pelleted crypts and pipette carefully to avoid introducing air bubbles.
NOTE: See the Discussion section for a detailed description of how to mix the gel matrix and crypts. Typically, 100 µL is sufficient for nine wells. - Allow the gel matrix to partially solidify (~1-2 min).
- Plate 10 µL of the gel matrix mixed with the crypts in each well of a pre-warmed 96-well culture plate to form a dome shape.
NOTE: Place the dome in the center of the well. Be careful not to allow the gel matrix to spread to the sides of the well. See the Discussion section for a detailed description on solidification and plating. - Allow the gel matrix to be fully set for 10-20 min in an incubator at 37 °C with 5% CO2.
- Prepare the final working solution of L-WRN media.
- Add 100 µL pennicilin/streptomycin to a 10 mL aliquot of L-WRN media (see step 2.1.3) (stable for 2 weeks at 4 °C).
- Add supplements to 900 µL of L-WRN media with pennicilin/streptomycin: 0.9 µL of 1 mg/mL EGF + 0.9 µL of 10 mM Y-27632 dihydrochloride.
NOTE: The volumes are based upon nine wells. Y-27632 dihydrochloride is only added on day 0 (at the time of plating).
- Add 100 µL of media to each well. Be careful not to disrupt the dome.
- Incubate at 37 °C with 5% CO2 for 24 h.
4. Creating claudin-7 knockout in culture
- Allow the crypts to grow normally in culture for 24 h after plating.
- In a 1.5 mL microcentrifuge tube, add 2.7 µL of 1 mmol/L 4-hydroxytamoxifen (4OH-Tamoxifen) to 900 µL of L-WRN media (final concentration of 3 µmol/L) + 0.9 µL of 1 mg/mL EGF.
NOTE: The stock solution of 4OH-Tamoxifen is prepared by mixing powdered 4OH-Tamoxifen (see Table of Materials) with 1x PBS to a concentration of 1 mmol/L. - Remove old media from the wells via vacuum suction.
- Add 100 µL of 4OH-Tamoxifen-containing media to each well and label them as claudin-7 cKO/4OH-TAM.
NOTE: DMSO needs to be added to the control wells. Fresh 4OH-Tamoxifen-containing media must be added every 2 days. - Incubate at 37 °C until the next media change (~2-3 days).
5. Colonic organoid maintenance
NOTE: Media must be changed every 2-3 days. Culture up to 12 days.
- Prepare a fresh final working solution of L-WRN media: 900 µL of L-WRN media + 0.9 µL of 1 mg/mL EGF.
- Remove old media from the wells via vacuum suction. Add fresh media to the wells.
NOTE: Be careful not to disrupt the dome. - Incubate at 37 °C until the next media change.
NOTE: Cultures can be maintained and passaged for the duration desired for the experiment. The cultures for the present study were typically maintained for between 9-12 days, but one may wish to continue further, for 15 or 20 days.
6. Harvesting and embedding colonic organoids
- Remove old media from the wells via vacuum suction.
- Fix the organoids with 4% paraformaldehyde (PFA) for 1 h at room temperature.
NOTE: PFA is a toxic material. Take precautions when using this reagent. - Remove 4% PFA from the wells via vacuum suction and add 30% sucrose for 24 h at 4 °C.
- Label a plastic mold and fill it to 90% with optimum cutting temperature (OCT) compound (see Table of Materials).
- Remove 30% sucrose from the wells via vacuum suction. Add 10 µL of 1x PBS to each well.
- With a pipette tip, gently scratch the bottom of the well to dissociate the dome containing organoids.
- With a pipette, remove the PBS containing the dissociated organoids and load the liquid into the mold containing OCT.
NOTE: Avoid introducing bubbles into the OCT compound. - Continue this process until all organoids have been removed from all wells.
- In a stainless-steel Dewar flask, add dry ice pellets and 2-methylbutane (enough to cover the dry ice pellets) (see Table of Materials).
- Steadily hold the organoid-containing OCT block above 2-methylbutane to flash freeze.
- Store the organoid-containing OCT block at -80 °C until ready to section (can be stored for up to 1 year).
7. Immunofluorescence
- Section the organoid-containing OCT block at a 5 µm thickness using a cryostat (see Table of Materials) at -20 °C.
- For each section, check under the microscope to ensure an organoid was captured. When sectioning is complete, store the slides at -80 °C until ready to stain (can be stored for up to 6 months).
- Heat the slides in 10 mM sodium citrate buffer for 10 min at 94 °C.
NOTE: The buffer is made by dissolving sodium citrate in deionized water. Adjust the pH to 6 with hydrochloric acid. - Allow the slides to cool on the benchtop for 20 min. Rinse with distilled water for 5 min.
- Assemble the slides in a staining rack (see Table of Materials) with 0.2% Triton X-100 and incubate with 100 mM glycine for 15 min.
- Rinse three times with 1x PBS for 5 min each. Block with 5% bovine serum albumin (BSA) for 45 min at room temperature.
- Incubate with claudin-7 anti-murine rabbit primary antibody22 (diluted in 1% BSA) overnight at 4 °C. Rinse three times with 1x PBS for 10 min each.
- Incubate with Cy3 anti-rabbit secondary antibody23 (diluted in 1% BSA) for 1 h at room temperature. Rinse three times with 1x PBS for 10 min each.
- Mount the slides with an appropriate mounting medium (see Table of Materials) with DAPI and add a coverslip.
8. Cell death detection
- Fix the organoids inside the wells with 4% paraformaldehyde for 1 h at room temperature. Rinse with 1x PBS for 5 min.
- Incubate the culture plate on ice with 0.1% Triton X-100 in cold 0.1% sodium citrate buffer for 2 min. Rinse twice with 1x PBS for 5 min each.
- Prepare TUNEL reaction24,25 using TMR Red, an in situcell death detection kit (see Table of Materials). Add 50 µL of TUNEL reaction reagents to each well.
- Incubate in a humidified atmosphere for 1 h at 37 °C in the dark.
NOTE: All the remaining steps must be completed in the dark. - Rinse three times with 1x PBS for 5 min each. Incubate with 1:2,500 Hoechst (diluted in 1x PBS, see Table of Materials) for 3 min.
- Rinse three times with 1x PBS for 5 min each. Utilize the TRITC filter to visualize images on a fluorescent microscope (see Table of Materials).
Wyniki
In order to examine the regulatory effects of claudin-7 on colon stem cells, colonic crypts were isolated from murine colon tissue as described above and shown in Figure 1A. Once the crypts were isolated from the primary tissue, they were plated in a 3D matrix in a 96-well plate to grow for 11 days (Figure 1). Normal healthy crypts will close the lumen and become spheroids by day 2 and eventually begin budding and forming the various epithelial cell types at approximately day 5 (Figure 1B). Colonoids were allowed to grow until day 11, where they were then harvested for further experiments (Figure 1B). To knockout claudin-7 in culture, the crypts were allowed to grow normally for 24 h. After 24 h, the crypts were treated with 3 µmol/L 4OH-Tamoxifen (TAM) and cultured for 10 additional days. The culture medium containing fresh 4OH-TAM was changed every 2 days. DMSO was used as a vehicle in control wells. Claudin-7 deficient crypts (claudin-7 KO) failed to form proper spheroids and began rapidly dying after 1 day of 4OH-TAM treatment (Figure 1B).
Figure 2A highlights the successful growth of colonoids from normal crypts containing claudin-7 (control) as they progress throughout the 9 days of culture. These crypts began to form spheroids by day 2, started budding on day 5, and continued growing and budding until they were harvested on day 9 (Figure 2A). In contrast, crypts lacking claudin-7 (claudin-7 KO) deteriorated very quickly (Figure 2B). Approximately 2-3 days after treatment with 4OH-TAM, claudin-7 KO crypts had not formed healthy-looking spheroids and appeared only as circular clumps of cells (Figure 2B). Crypts isolated from wild-type mice were treated with 4OH-TAM to confirm there is no toxic effect due to Tamoxifen treatment; these crypts were able to survive and grow normally. To examine claudin-7 deletion and the survival condition of claudin-7 KO colonoids, an immunostaining method and an in situ cell death detection kit were utilized (Figure 3). Immunofluorescent staining for claudin-7 in harvested control and cKO organoids confirmed successful knockout of claudin-7 in culture (Figure 3A). Day 9 control colonoids exhibited very little apoptotic signal (Figure 3B); however, claudin-7 KO colonoids displayed high apoptosis (Figure 3B). Without claudin-7, the stem cells could not survive, self-renew, or differentiate to form colonoids.

Figure 1: Schematic representation showing crypt isolation and colonoid growth. (A) Graphical depiction of the crypt isolation process, plating in the 3D matrix, and growth until harvest. (B) Timeline of experiments and colonoid growth in control and claudin-7 KO-derived crypts. Please click here to view a larger version of this figure.
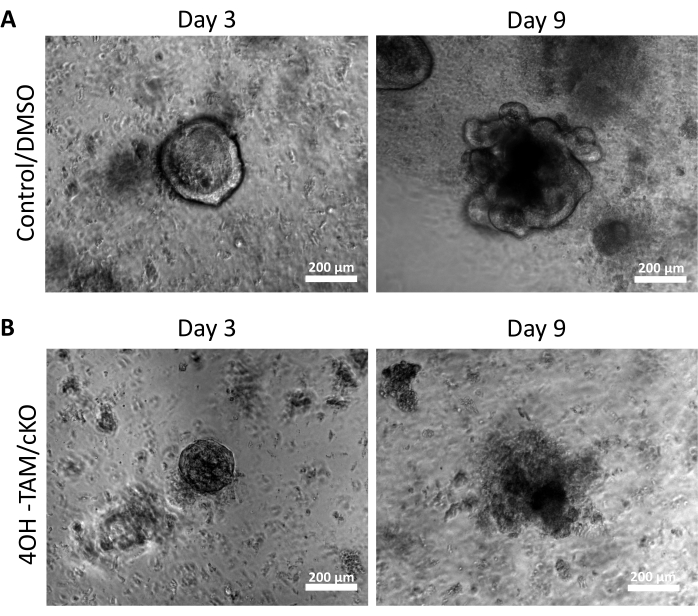
Figure 2: Claudin-7 deficient colonoids are unable to survive and grow. (A) Representative images of control/DMSO organoids on day 3 and day 9. (B) Representative images of 4OH-TAM/cKO organoids on day 3 and day 9, n = 10. Scale bars = 200 µm. Please click here to view a larger version of this figure.
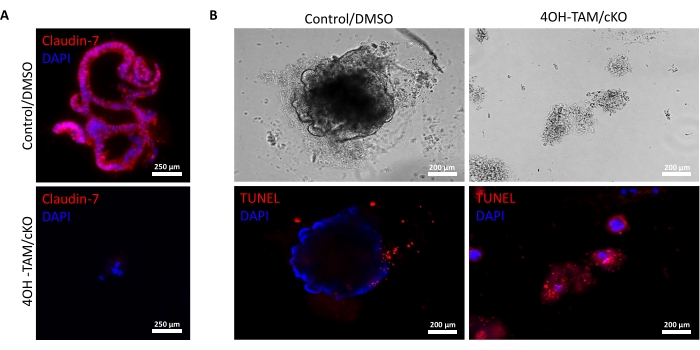
Figure 3: Claudin-7 deficient colonoids rapidly undergo apoptosis. (A) Claudin-7 staining in day 9 control/DMSO and claudin-7 cKO/4OH-TAM organoids, n = 3. Scale bars = 250 µm. (B) Apoptotic staining in day 9 control and claudin-7 cKO/4OH-TAM colonoids, n = 3. Scale bars = 200 µm. Please click here to view a larger version of this figure.
Dyskusje
Organoid culture is an excellent model for studying stem cell function, intestinal physiology, drug discovery, human intestinal diseases, and tissue regeneration and repair7,8,9,10,11,26. While it has many advantages, it can be challenging to establish. Care must be taken in all steps throughout the protocol, but most importantly during the plating stage. When mixing the isolated crypts with a gel matrix, ensure to thoroughly pipette up and down to break up the crypt pellet formed following centrifugation and distribute the crypts evenly throughout the matrix. Concurrently, avoid introducing air bubbles into the matrix while pipetting. In order to do this, pipetting must be done slowly, with the pipette tip toward the bottom of the 1.5 mL tube.
Additionally, the gel matrix must not be fully solidified throughout this process. To prevent premature solidification, mix by careful pipetting, then place the tube on ice, and repeat this process. Once the isolated crypts and gel matrix are sufficiently mixed, allow the gel matrix to solidify partially. This process may take 1-5 min, depending on the type/brand of gel matrix used. It must resemble a gel that will move slightly if the tube is tipped, but should not be too runny that it would spill out if inverted. At this point, one may begin plating 10 µL into the center of each well. The gel matrix must form a 3D dome and should not touch the sides of the well. If the gel matrix spreads and hits the wall of the well, it is not solidified enough; wait until it is sufficiently solidified to form a dome, as the crypts will not survive and grow if the dome is not formed. Once plating is completed properly, and crypts are sufficiently supplemented, as explained above, the organoids are expected to grow without issue.
This protocol establishes a colon organoid system with or without claudin-7 to observe its effects on colonic stem cell survival. While colon organoid culture is an innovative and advantageous system, the model still has limitations. Depending on the type of study, the lack of immune cells and the microbiota in intestinal organoids may be an advantage or disadvantage26. For the present study, it is advantageous to investigate claudin-7's regulatory role on stem cell functions without the immune component. It was concluded that a certain effect is specifically due to claudin-7, rather than other potential variables such as the immune response that would be present in in vivo animal models. Conversely, this factor may be a limitation for other types of studies. Establishing colon organoid culture may also be more costly and time intensive than traditional 2D cell lines. However, they can mimic the cellular microenvironment of tissues providing in vivo relevance, are much more representative of tissue than 2D cell culture, and are still less costly than animal models4,7. Given intestinal organoid culture's vast application and enormous potential, this system is likely to become the ideal model in laboratory research worldwide.
Ujawnienia
The authors declare no conflicts of interest.
Podziękowania
This study was funded by NIH DK103166.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.09 cubic feet space-saver vacuum desiccator | United States Plastic Corp | 78564 | anesthesia chamber |
| 0.5 M EDTA pH 8.0 | Invitrogen | AM9261 | |
| 1.5 mL microcentrifuge tubes | ThermoFisher | 69715 | |
| 15 mL conical centrifuge tubes | Fisher Scientific | 14-959-53A | |
| 1x Dulbecco’s Phosphate buffered saline | Gibco | 14190-144 | |
| 2-methylbutane | Sigma | 277258 | |
| 4% paraformaldehyde | ThermoFisher | J61899.AK | |
| 4-hydroxytamoxifen (4OH-TAM) | Sigma | 579002 | |
| 50 mL conical centrifuge tubes | Fisher Scientific | 14-432-22 | |
| 70 µm nylon cell strainer | Corning | 352350 | |
| 96 well culture plate | Greiner Bio-One | 655180 | |
| B-27 Supplement (50x) | Gibco | 12587-010 | |
| Bovine serum albumin | Fisher Scientific | BP1605-100 | |
| Claudin-7 anti-murine rabbit antibody | Immuno-Biological Laboratories | 18875 | |
| Cover glass (24 x 50-1.5) | Fisher Scientific | 12544E | |
| Cryomolds | vwr | 25608-916 | |
| Cultrex RCF BME, Type 2 | R&D Systems | 3533-005-02 | gel matrix |
| Cy3 anti-rabbit antibody | Jackson Immunoresearch | 111-165-003 | |
| Dewar Flask | Thomas Scientific | 1173F61 | |
| DMEM High Glucose with L-Glutamine | ATCC | 30-2002 | |
| EVOS FLoid Imaging System | ThermoFisher | 4477136 | |
| Fluoro-Gel II with DAPI | Electron Microscopy Sciences | 17985-50 | |
| GlutaMAX (100x) | Gibco | 35050-061 | |
| Glycine | JT Baker | 4059-02 | |
| HEPES (1 M) Buffer Solution | Gibco | 15630-080 | |
| Hoechst | ThermoFisher | 62249 | |
| In situ cell death detection kit, TMR Red | Roche | 12156792910 | |
| Isoflurane | Pivetal | 07-893-8440 | |
| L-WRN Media | Harvard Medical School Gastrointestinal Organoid Derivation and Culture Core | N/A | |
| Mouse surgical kit | Kent Scientific Corporation | INSMOUSEKIT | |
| Murine EGF | PeproTech | 315-09-500UG | |
| N2 Supplement (100x) | Gibco | 17502-048 | |
| Optimum cutting temperature (OCT) compound | Agar Scientific | AGR1180 | |
| Penicillin-Streptomycin | Gibco | 15140-122 | |
| Sequenza Rack | vwr | 10129-584 | |
| Sodium Citrate | Fisher Scientific | S-279 | |
| Sucrose | Sigma | S9378 | |
| Triton X-100 | Sigma | X100 | |
| Vacuum filter (0.22 µm; cellulose acetate) | Corning | 430769 | |
| Y-27632 dihydrochloride | Tocris Bioscience | 1254 |
Odniesienia
- Hughes, C. S., Postovit, L. M., Lajoie, G. A. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 10 (9), 1886-1890 (2010).
- Sato, T., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 141 (5), 1762-1772 (2011).
- Wallach, T. E., Bayrer, J. R. Intestinal organoids: new frontiers in the study of intestinal disease and physiology. Journal of Pediatric Gastroenterology and Nutrition. 64 (2), 180-185 (2017).
- Shankaran, A., Prasad, K., Chaudhari, S., Brand, A., Satyamoorthy, K. Advances in development and application of human organoids. 3 Biotech. 11 (6), 257 (2021).
- Angus, H., Butt, A., Schultz, M., Kemp, R. Intestinal organoids as a tool for inflammatory bowel disease research. Frontiers in Medicine. 6, 334 (2020).
- Fan, Y., Davidson, L. A., Chapkin, R. S. Murine colonic organoid culture system and down stream assay applications. Methods in Molecular Biology. 1576, 171-181 (2019).
- Gupta, N., et al. Microfluidics-based 3D cell culture models: Utility in novel drug discovery and delivery research. Bioengineering and Translational Medicine. 1 (1), 63-81 (2016).
- Yoo, J., Donowitz, M. Intesitnal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases. World Journal of Gastroenterology. 25 (30), 4125-4147 (2019).
- Qu, M., et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Research. 31 (3), 259-271 (2021).
- Xing, T., et al. Tight junction protein claudin-7 is essential for intestinal epithelial stem cell self-renewal and differentiation. Cellular and Molecular Gastroenterology and Hepatology. 9 (4), 641-659 (2020).
- Ding, L., et al. Inflammation and disruption of the mucosal architecture in claudin-7-deficient mice. Gastroenterology. 142 (2), 305-315 (2012).
- Lu, Z., Ding, L., Lu, Q., Chen, Y. H. Claudins in intestines: distribution and functional significance in health and diseases. Tissue Barriers. 1 (3), 24978 (2013).
- Ding, L., Lu, Z., Lu, Q., Chen, Y. H. The claudin family of proteins in human malignancy: a clinical perspective. Cancer Management and Research. 5, 367-375 (2013).
- Bhat, A. A., et al. Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis. Oncogene. 34 (35), 4570-4580 (2015).
- Lu, Z., et al. A non-tight junction function of claudin-7-interaction with integrin signaling in suppressing lung cancer cell proliferation and detachement. Molecular Cancer. 14, 120 (2015).
- Wang, K., Xu, C., Li, W., Ding, L. Emerging clinical significance of claudin-7 in colorectal cancer: a review. Cancer Management and Research. 10, 3741-3752 (2018).
- Wang, K., et al. Claudin-7 downregulation induces metastasis and invasion in colorectal cancer via the promotion of epithelial-mesenchymal transition. Biochemical and Biophysical Research Communications. 508 (3), 797-804 (2019).
- Wang, F., et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology. 145 (2), 383 (2013).
- Li, W., et al. Severe intestinal inflammation in the small intestine of mice induced by controllable deletion of claudin-7. Digestive Diseases and Sciences. 63 (5), 1200-1209 (2018).
- Donovan, J., Brown, P. Euthanasia. Current Protocols in Immunology. 73 (1), (2006).
- Khalil, H., Nie, W., Edwards, R. A., Yoo, J. Isolation of primary myofibroblasts from mouse and human colon tissue. Journal of Visual Experiments. (80), e50611 (2013).
- Sugimoto, K., et al. Cell adhesion signals regulate the nuclear receptor activity. Proceedings of the National Academy of Sciences. 116 (49), 24600-24609 (2019).
- Mansour, H., et al. Connexin 30 expression and frewuency of connexin heterogeneity in astrocyte gap junction plaques increase with age in the rat retina. PLoS One. 8 (3), 57038 (2013).
- Miranda, M., et al. Antioxidants rescue photoreceptors in rd1 mice: relationship with thiol metabolism. Free Radical Biology and Medicine. 48 (2), 216-222 (2010).
- Wang, L., et al. Mesenchymal stromal cells ameliorate oxidative stress-induced islet endothelium apoptosis and functional impairment via Wnt4-β-catenin signaling. Stem Cell Research and Therapy. 8 (1), 188 (2017).
- Almeqdadi, M., Mana, M., Roper, J., Yilmaz, O. Gut organoids: mini-tissues in culture to study intestinal physiology and disease. American Journal of Physiology-Cell Physiology. 317 (3), 405-419 (2019).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone