Method Article
Preparation of Washed Human Platelets for Quantitative Metabolic Flux Studies
In This Article
Summary
Platelet metabolism is of interest, particularly as it relates to the role of platelet hyper- and hypoactivity in bleeding and thrombotic disorders. Isolating platelets from plasma is necessary for some metabolic assays; presented here is a method for isolating of intracellular metabolites from washed platelets.
Abstract
Platelets are blood cells that play an integral role in hemostasis and the innate immune response. Platelet hyper- and hypoactivity have been implicated in metabolic disorders, increasing risk for both thrombosis and bleeding. Platelet activation and metabolism are tightly linked, with the numerous methods to measure the former but relatively few for the latter. To study platelet metabolism without the interference of other blood cells and plasma components, platelets must be isolated, a process that is not trivial because of platelets shear sensitivity and ability to irreversibly activate. Presented here is a protocol for platelet isolation (washing) that produces quiescent platelets that are sensitive to stimulation by platelet agonists. Successive centrifugation steps are used with the addition of platelet inhibitors to isolate platelets from whole blood and resuspend them in a controlled, isosmotic buffer. This method reproducibly produces 30%–40% recovery of platelets from whole blood with low activation as measured by markers of granule secretion and integrin activity. Platelet count and fuel concentration can be precisely controlled to allow the user to probe a variety of metabolic situations.
Introduction
Platelets are small (2–4 µm diameter), anucleate cells that play an important role in hemostasis, the tightly regulated process of clot formation1. While vital for vascular integrity, platelets are also implicated in adverse health events. Platelets are involved in deep vein thrombosis (DVT) and arterial thrombosis (AT), which are clots that occlude blood vessels, leading to diminished blood supply locally, or, if pieces of the clot break off (embolize), they can block blood supply to the lungs, heart, or brain2,3,4,5,6,7. Platelet hyperreactivity is a comorbidity of hypertension, diabetes, and cancer, leading to increased incidence of DVT and AT8,9,10. Platelet activation and metabolism are tightly linked11,12, leading to increased interest in targeting platelet metabolism as a therapeutic strategy13,14. There is debate about the exact metabolic rewiring that occurs upon activation, and this is an active field of study15. This increased interest in platelet dysfunction in disease and its ties to metabolism underscores the need for a repeatable method to isolate platelets and study their metabolism.
Human platelets are typically obtained by venipuncture and then isolated from whole blood. Washed platelets are separated from whole blood via successive washing and centrifugation steps16. This was originally done by Mustard’s group17, and modified slightly by Cazenave’s group18. Another alternative is gel filtered platelets, which can be obtained from platelet rich plasma (PRP) by size exclusion chromatography using a packed column of agarose gel beads19. Many washing protocols exist for both human and rodent blood, and are optimized for various assays20,21,22,23, but not for measuring platelet metabolism.
Techniques to study platelet metabolism include bioenergetic measurements via Seahorse XF analyzer11,24,25,26,27, extracellular flux measurements11,13,24, metabolomics14,28, and isotope assisted metabolic flux analysis (13C-MFA)29. In metabolomic studies, the goal is typically to determine altered pathways between two different conditions (for example, resting vs activated platelets14). Metabolomic studies involve the use of liquid chromatography-mass spectrometry (LC-MS). These studies can be done for intra- or extracellular metabolites and are frequently coupled with pathway analysis or principal component analysis (PCA)14,28. Isotope assisted metabolic flux analysis (13C-MFA) involves feeding cells a labeled substrate known as a tracer, and measuring how this tracer propagates through a reaction network with LC-MS. This technique allows for the calculation of fluxes through metabolic pathways with reaction level resolution29,30. In whole blood and platelet rich plasma (PRP), fuel concentration (glucose, glutamine, acetate, etc) is subject to donor-to-donor variability, and albumin and sex hormone binding globulin present in plasma can alter the active concentration of hormones, drugs, and other biologically relevant molecules31. Washed platelets offer a method to suspend platelets in a user-defined medium, including known fuel concentrations, that is compatible with 13C-MFA32.
Described here is a method for platelet washing to produce platelets that can be used in metabolic assays. The protocol produces quiescent platelets with low red blood cell and white blood cell contamination. Platelet activation status was monitored via flow cytometry of platelet activation markers. This protocol reproducibly achieves at least 30%–40% platelet recovery relative to the platelet count in whole blood. The washed platelets obtained with this technique are suitable for the metabolic analysis techniques, and the intracellular metabolite extraction method can be tailored to analysis of the user’s choosing (LC-MS, GC-MS, photometric assay, etc).
Protocol
The study received Institutional Review Board approval from the University of Colorado Anschutz Medical Campus. Written consent was obtained from all study participants. Participants reported they did not consume alcohol for the previous 48 h or non-steroidal anti-inflammatory drugs (NSAIDs) for the previous ten days. This project is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R61HL141794.
1. Blood collection
- Set up for blood draw. It is recommended blood collection is carried out by a trained phlebotomist.
- Perform venipuncture on the inner arm using a 19 G needle.
- Collect the first ~2 mL into an additive-free vacutainer and dispose. This is to remove chemical signaling molecules from damaged endothelial cells that may activate platelets. After the initial 2 mL is collected, remove tourniquet to reduce shear stress on platelets.
- Collect the rest of blood into antiocoagulent citrate dextrose (ACD-A) vacutainers in a ratio of 14:3 (blood:ACD-A). Gently invert each vacutainer after blood collection to mix blood and anticoagulant.
2. Platelet Washing
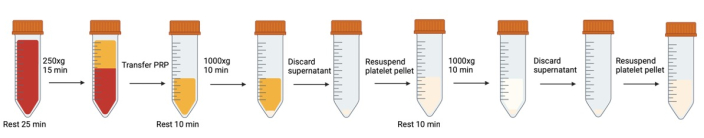
Figure 1: Successive centrifugation and resuspension steps involved in platelet washing. Please click here to view a larger version of this figure.
NOTE: Avoid air bubble generation. Use transfer pipettes to remove bubbles when they are formed, especially prior to centrifuging. Each time the tubes of blood/platelets are open/closed, its recommended to breath into the tube prior to closing the cap to increase CO2 level.
- Pre-heat centrifuge to 37 °C to reduce temperature effects. Between each centrifugation step in the protocol, maintain the centrifuge at 37 °C.
- Preheat water bath to 37 °C. Place modified Tyrode’s buffer with glucose in the water bath.
NOTE: modified Tyrode’s buffer recipe can be found in Supplementary File 1. - Combine collected whole blood from all vacutainers into 50 mL polypropylene conical tubes. Using a bevel cut pipette tip cut at a 45° angle, take appropriate sample volume to conduct a cell count (see Cell Counting).
- Remove any bubbles generated by carefully aspirating with a narrow bore transfer pipette.
- Let blood rest in water bath for 25 minutes at 37 °C This allows platelets in a reversibly activated state (from being removed from the body, transferred from tube to tube, etc.) to return to a resting state.
- To prevent activation during centrifugation, add 500 nM prostaglandin I2 (PGI2) and 0.02 U/mL of apyrase to whole blood and mix gently by inverting once. Remove any bubbles generated using a narrow bore transfer pipette.
NOTE: Instructions for proper PGI2 and apyrase recipe can be found in Supplementary File 1. - Centrifuge whole blood to obtain platelet rich plasma (PRP) (15 minutes at 250 x g for 42 mL of whole blood) with no brake (37 °C).
- Gently collect the PRP (top yellow layer above the buffy coat and red blood layer) into a new 50 mL conical tube using a wide bore transfer pipette.
- When transferring, tilt a clean conical tube and gently run the PRP down the side of the tube. Avoid air bubbles.
- Leave approximately 3 mm of PRP behind to avoid disturbing the buffy coat (the layer of white blood cells between the PRP and the red blood). If disturbed, this will look like a sudden swirl of white in the transfer pipette.
- Take cell count (See Counting Platelets) to determine resuspension volume for next wash step.
- Allow to rest for 10 minutes at 37 °C.
- Using a bevel cut pipette tip, take sample for flow cytometry (see Flow Cytometry).
- Add 500 nM PGI2 and 0.02 U/mL apyrase to PRP and mix by gently inverting once.
- Centrifuge for 10 minutes at 1000 x g at 37 °C (acceleration:0 brake: 0). Do not use brake. The pellet is not compact, and sudden brake can cause remixing.
- While centrifuging, determine resuspension volume. Assume 75% recovery. Make cell density approximately 3x105 cells/μL.
- Aspirate supernatant using a plastic wide bore transfer pipette for the bulk volume and a 1 mL pipette (uncut tip) for the remainder of the liquid near the pellet. Avoid touching pellet at bottom of tube.
- Add 500 nM PGI2 and 0.02 U/mL of apyrase to modified Tyrode’s buffer. Slowly add calculated amount of buffer from step 16 by trickling down the side of the conical tube.
- Using a bevel cut tip and a 1 mL pipette tip, gently resuspend pellet by gently pipetting up and down several times.
- Set volume to 300 μL, depress plunger all the way while above the liquid line, then place pipette below liquid and come up to first stop. This will allow the scientist to resuspend the pellet without accidentally creating air bubbles that may activate the platelets.
- Occasionally, there is a ring of visible red blood cells at the bottom of the pellet. Avoid resuspending this.
- Once the pellet is resuspended, use a wide bore transfer pipette to transfer resuspended pellet to a new conical tube, leaving any red cells or visibly clumped cells behind.
- Repeat steps 11-20. Ensure to add 500 nM PGI2 and 0.02 U/mL from a fresh aliquot to modified Tyrode’s buffer (do not re-use modified Tyrode’s buffer from step 18, PGI2 is too unstable).
- Take a sample for a cell count. Adjust platelet concentration as necessary with modified Tyrode’s buffer.
- Use a bevel cut pipette tip to take a sample for flow cytometry.
- Allow the platelets to rest for 1 h at 37 °C to allow time for the inhibitors to wear off and allow any reversibly activated platelet to return to a resting state.
- Gently mix with a wide bore pipette. Take samples for flow cytometry.
- The washed, resting platelets are now ready to be used for metabolic analysis.
3. Counting platelets
- Platelets can be counted using either an automated blood cell counter (follow manufacturer’s instructions) or a hemocytometer33.
4. Flow Cytometry
- Preparation
- Detailed protocols and reviews of best practices for setting up antibody mixes and preparing the flow cytometer to measure platelet activation can be found elsewhere34,35.
- Sampling
- When taking a sample for flow cytometry, collect platelet suspension using a bevel cut pipette tip. Slowly add this to microcentrifuge tube with antibodies, then flick gently to mix. Allow to incubate for 30 s.
- Using bevel cut pipette tip, transfer platelet suspension/antibody mix to appropriate well on 96 well plate.
- Immediately add fixant to well to fix cells.
- Run on flow cytometer within 8 h of fixation.
- Agonist sensitivity tests
- After washing, set aside 2 15 mL conical tubes, one for a resting control and one as a thrombin activated control.
- Gently pipette 100 μL of platelet suspension into each conical tube using a bevel cut pipette tip. Allow to rest at 37 °C for 1 h.
- After the hour rest, add 0.1 U/mL of thrombin to one tube (instructions for thrombin preparation can be found in Supplementary File 1), and vehicle to the other. Incubate at 37 °C for 15 min.
- Take a flow cytometry sample of each tube to determine platelet sensitivity to agonist.
5. Sampling for Quantitative Metabolic Flux Analysis
- Quenching
NOTE: Quenching metabolism is a necessary step for measuring accurate metabolic fluxes. Rapidly chilling cells and maintaining their temperature at or below 4 °C slows metabolism enough that it can be assumed to be essentially halted so that the sampled cells accurately reflect the metabolism of cells from the bulk. There are a variety of methods that can be used, but to balance the need for rapid chilling and minimizing leakage, use cold ( -4 °C) normal saline36. If salt will interfere with subsequent analysis, another fluid can be used (methanol/water, ethanol, etc.)37.- Prepare and freeze aliquots of normal saline. Make each saline aliquot at 6x the volume of the desired sample.
NOTE: normal saline recipe can be found in Supplementary File 1. - Pre-chill microcentrifuge to 0 °C.
- Collect platelet suspension in partially frozen normal saline (< -4 °C) in a 1:6 ratio (eg, collect 150 μL of platelet suspension into 750 μL of saline in microcentrifuge tubes).
NOTE:Do not leave the platelet/saline solution on ice for more than 15 min. - Centrifuge at 16,000 x g, 0 °C for 10 min.
- Save the supernatant for external metabolite analysis, and the pellet for intracellular metabolite extraction and measurement. Store both at -20 °C until ready for analysis.
- Repeat this process over desired time interval and number of sample time points.
- Prepare and freeze aliquots of normal saline. Make each saline aliquot at 6x the volume of the desired sample.
- Intracellular Metabolite Extraction
- Add 0.5 mL of pre-chilled 7:3 methanol-water at -20 °C to the quenched pellet. Vortex vigorously for 1 min.
- Freeze in liquid nitrogen, thaw at 0 °C, then repeat for 2 more cycles.
- Centrifuge the suspensions at 16,000 x g for 10 minutes at -4 °C.
- Collect the supernatant in a new microcentrifuge tube.
- Use the pellet from step 3 and repeat the extraction protocol (steps 1-3) with 50:50 methanol:water. Add the second collected extract to the first. Dry overnight.
- Resuspend the dried extract in 150 µL of LC-MS grade water (or other dissolvent appropriate for intended analysis). Mix for 15 minutes at 4 °C. Briefly vortex and transfer to 0.22 μm microcentrifuge tubes to remove cell debris. Centrifuge for 5 minutes at 16,000 x g and 4 °C.
- Remove from centrifuge, pipette an additional 50 μL optima water onto the filter, and centrifuge again (5 min, 16,000 x g and 4 °C) to rinse the filter. Collect for analysis.
Results
Representative results in Figure 2 represent 6 different blood donors, including 3 males and 3 females. The platelet yield relative to whole blood is shown in Figure 2A. Final platelet recovery was an average of 52% ± 3% (standard deviation, n=6). Final platelet count compared to white blood cell contamination was measured using an automated hematology analyzer. White blood cell counts were less than 0.1% of total cells (Figure 2B). Through the wash process and the hour rest, platelets maintained low P-selectin exposure, but responded strongly to thrombin treatment (Figure 2C). Bound fibrinogen spikes after the first 1000 x g spin, but returns to below 5% after the second 1000 x g spin. Like P-selectin exposure, bound fibrinogen drastically increases following thrombin treatment after the hour rest (Figure 2D). Representative gating for size, singlets and CD42a positivity are shown in Figure 3A-C. Events that pass these successive gates are used to look for P-selectin exposure and bound fibrinogen. Representative gating for P-selectin positive and fibrinogen positive platelets are shown in Figure 3D-E. Figure 3D shows a platelet sample treated with vehicle control and Figure 3E shows a platelet sample 15 minutes after 0.1 U/mL of thrombin was added.
Washed platelets were used to conduct a quantitative uptake and excretion experiment, shown in Figure 4. Platelets were washed, and 5 mmol/L of glucose and 20 mmol/L of acetate were added to the final washed suspensions. 0.1 U/mL of thrombin or vehicle was added to washed platelet suspensions after an hour rest. Samples were collected and quenched every 15 minutes after thrombin addition for 30 minutes. The supernatants were used to measure changes in concentration of extracellular lactate, glucose, and acetate using automated photometric assays. Flux was calculated by taking the regression slope of the concentration of the metabolite over time. Figure 4 shows the calculated metabolite fluxes for three representative donors. Lactate fluxes for all donors was positive, indicating lactate was being produced, and glucose and acetate fluxes were negative, indicating they were being consumed. While there is variation in the fold change, thrombin treatment led to an increase in the magnitude of fluxes compared to resting for each donor.

Figure 2: (A) Percent of platelets recovered relative to whole blood at each step of the wash process. n=6, error bars represent standard deviation. (B) Platelet and leukocyte cell count at the end of the wash process. (C) Percent of platelets positive for P-Selectin expression, measured by flow cytometry. n=6, error bars represent standard deviation. (D) Percent of platelets positive for fibrinogen, measured by flow cytometry. n=6, error bars represent standard deviation. . Please click here to view a larger version of this figure.

Figure 3: Representative gating for platelet flow cytometry. Platelets are (A) gated for size with forward scatter (FSC) and side scatter (SSC), (B) then gated for single cells with FSC-width and FSC-height, and (C) gated for CD42a positivity. Events that pass these successive gates are used to look for activation markers. (D) P-selectin and fibrinogen expression for a resting control platelet sample. Activation markers measured here are P-Selectin PECy5 and FITC-Fibrinogen. (E) P-selectin and fibrinogen expression for a 0.1 U/mL thrombin treated platelet sample. Activation markers measured here are P-Selectin PECy5 and FITC-Fibrinogen.. Please click here to view a larger version of this figure.
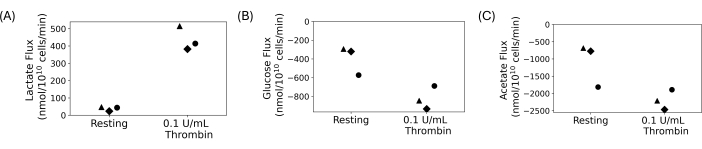
Figure 4: (A) Lactate excretion rates for three different donors for a resting and thrombin activated condition. (B) Glucose uptake rates for three different donors for a resting and thrombin activated condition. (C) Acetate uptake rates for three different donors for a resting and thrombin activated condition. Please click here to view a larger version of this figure.
Supplementary File 1: Buffer and reagent preparation recipes and instructions. Please click here to download this File.
Discussion
Platelets are very sensitive to their environment, including shear stress and presence of agonists38,39. This makes platelets challenging to handle and isolate, making the use of inhibitors and wide bore pipettes crucial40. Proper storage and preparation of PGI2 is vital, as failing to prepare PGI2 in basic PBS will result in rapid degradation of PGI241. To minimize the risk of shear induced activation, use wide bore pipettes, bevel cut pipette tips, and a 19-gauge phlebotomy needle.
Inevitably, a portion of the platelets will be lost at each step of the wash process (Figure 2A). Not all platelets will end up in the plasma layer, and collecting plasma too close to the buffy coat layer risks white blood cell contamination. In addition, a population of platelets clump and sink to the bottom upon resuspension, emphasizing the importance of transferring the resuspended pellet to a clean tube after each resuspension. Notice in Figure 2D, there is an increase in fibrinogen binding as after the first 1000 x g spin which then goes down after the second 1000 x g spin. The samples for flow cytometry were collected after a 10-minute rest period in each case. It is possible that part of this population recovers its quiescent nature, but because this population is present after the first 10-minute rest, part of this activated population may clump and fall to the bottom of the tube upon resuspension.
While the use of flow cytometry is not strictly necessary, it is beneficial to monitor if the platelets are quiescent and able to respond to agonists at the end of the wash process. In addition, particularly if a large portion of the platelets appear visually clumpy during the wash, performing flow cytometry at each step can help identify where the problem is arising. Though uncommon, approximately 1 of 20 donor experiments must be excluded because of spontaneous platelet activation during the rest period. Flow cytometry analysis of platelet activation markers is a valuable tool to validate the quiescence and sensitivity to agonist prior to experiments involving platelets.
To measure platelet response to agonist using flow cytometry, some studies allow the platelets to incubate in the agonist and antibody cocktail for 10-30 minutes at room temperature before fixing42,43,44. After the hour rest, a 20-minute incubation at room temperature prior to fixing the resting control platelets results in an overestimate of activation. This activation does not occur when platelets are incubated for 20 minutes at 37 °C, suggesting this apparent activation occurs because the inhibitors have worn off and the sudden temperature change activates the platelets. Waiting 20 minutes to fix during the washing process also does not induce this artificial activation, which could be due to attenuation of the action of the inhibitors during the hour rest. To capture the true activation state of the platelets, it is recommended to perform the incubation at 37 °C or to fix them after only a short (30 second) incubation with antibodies.
Washed platelets offer a way to study platelets without the interference of other blood cells and plasma components. They are ideal for studies in which the extracellular environment must be manipulated, including metabolic studies. The density of the final platelet suspension is tunable and able to be controlled to minimize variability in platelet counts between people. For metabolic studies, custom concentrations of platelet carbon substrates can be controlled. Representative results shown in Figure 4 are from an uptake and excretion experiment in which glucose and acetate were added as carbon substrates, and lactate was produced. While there is biologic variability between donors in Figure 4, lactate production increases upon thrombin treatment, while glucose and acetate consumption increases. This agrees with literature; platelets are known to increase their rate of aerobic glycolysis and oxidative phosphorylation upon activation13,14,15,45. This technique can be applied to study platelets under different nutrient conditions or with different ions in the final platelet suspension.
While washed platelet studies are valuable, it is important to keep in mind that the artificial environment does not recapitulate the environment in vivo. Therefore, washed platelets should be used only in situations where investigators are interested in platelet function alone. As with all in vitro studies, the results must be interpreted cautiously. It is recommended that the platelets be resuspended in modified Tyrode’s buffer with a calcium concentration of 2 mM after the final wash. The P-selectin and fibrinogen binding expression have only been measured up to 1.5 hours after the wash process (2.5 hours including the hour rest), so it is recommended the scientist use the washed platelet suspension within that time window, or otherwise validate the fidelity of the platelets. This study did not measure other classic metrics of platelet functionality, such as aggregation.
Washed platelets prepared with our protocol are quiescent, validated by flow cytometric measurements of P-selectin and fibrinogen. The metabolic substrates, hormones, and platelet agonists in the extracellular environment can be precisely controlled, allowing the scientist to study platelet metabolism in a system of their choosing.
Disclosures
The authors have no conflict of interest to report.
Acknowledgements
The authors would like to acknowledge Dr. Emily Janus-Bell and Clarisse Mouriaux from the lab of Dr. Pierre Mangin and Katrina Bark from Dr. Jorge DiPaola’s lab for their guidance and advice.
Materials
| Name | Company | Catalog Number | Comments |
| 0.22 µM filter Spin-X tubes | Millapore-Sigma | CLS8160 | Reagent prep |
| 19 G x 3/4" needle | McKesson Corporation | 448406 | Phlebotomy |
| 21 G 1.5 inch needle with luer lock | Amazon | B0C39PJD23 | Reagent prep |
| 96 well plate, half area | Greiner Bio-One | 675101 | Flow cytometry |
| ACD-A vaccutainers | Fisher Scientific | 364606 | Phlebotomy |
| Adapter | McKesson Corporation | 609 | Phlebotomy |
| Alcohol swab | VWR | 15648-916 | Phlebotomy |
| Apyrase from potatoes | Sigma | A6410-100UN | Reagent prep |
| CD42a Monoclonal Antibody | Thermo Fisher Scientific | 48-0428-42 | Flow cytometry |
| Chilled microcentrifuge | ThermoFisher Scientific | 75002441 | Quenching |
| D-Glucose | Sigma | G7021 | Reagent prep |
| FITC Anti-Fibrinogen antibody | Abcam | 4217 | Flow cytometry |
| Flow cytometer | Beckman Coulter | 82922828 | Flow cytometry |
| Gauze | VWR | 76049-110 | Phlebotomy |
| Glycerol | Sigma Aldrich | G5516 | Reagent prep |
| HEPES | Sigma Aldrich | H4034 | Reagent prep |
| Human alpha-thrombin | Prolytix | HCT-0020 | Flow cytometry |
| KCl | Sigma Aldrich | P9333 | Reagent prep |
| KH2PO4 | Sigma Aldrich | P5655 | Reagent prep |
| MgCl2 | Sigma | M8266 | Reagent prep |
| Microcentrifuge tubes | VWR | 87003-292 | General |
| Na2HPO4 | Sigma | S3264 | Reagent prep |
| NaCl | Sigma Aldrich | S7653 | Reagent prep |
| NaHCO3 | Sigma Aldrich | S5761 | Reagent prep |
| Narrow bore transfer pipette | VWR | 16001-176 | Platelet washing |
| Paraformaldehyde solution, 4% in PBS | Santa Cruz Biotechnology | sc-281692 | Flow cytometry |
| PECy5 Mouse Anti-Human CD62P | BD Pharmingen | 551142 | Flow cytometry |
| Plate cover | Thermo Fisher Scientific | AB0626 | Flow cytometry |
| Polypropylene 15 mL conical tubes | VWR | 89039-658 | Reagent prep |
| Polypropylene 50 mL conical tubes | VWR | 352070 | Platelet washing |
| Prostaglandin I2 (sodium salt) | Cayman Chemical | 18220 | Reagent prep |
| SKC Inc. C-Chip Disposable Hemocytometers | Fisher Scientific | 22-600-100 | Cell counting |
| Syringe | BD Pharmingen | 14-823-41 | Reagent prep |
| Tourniquet | VWR | 76235-371 | Phlebotomy |
| Vacutainer needle holder | BD | 364815 | Phlebotomy |
| Vortexer | VWR | 102091-234 | Reagent prep |
| Water bath | Thermo Fisher Scientific | TSGP02 | Platelet washing |
| Wide bore transfer pipette | VWR | 76285-362 | Platelet washing |
References
- Versteeg, H. H., Heemskerk, J. W., Levi, M., Reitsma, P. H. New fundamentals in hemostasis. Physiol Rev. 93 (1), 327-358 (2013).
- Webera, M., et al. Enhanced platelet aggregation with trap-6 and collagen in platelet aggregometry in patients with venous thromboembolism. Thromb Res. 107, 325-328 (2002).
- Herbert, J. M., Bernat, A., Maffrand, J. P. Importance of platelets in experimental venous thrombosis in the rat. Blood. 80 (9), 2281-2286 (1992).
- Puurunen, M. K., Hwang, S. J., O'donnell, C. J., Tofler, G., Johnson, A. D. Platelet function as a risk factor for venous thromboembolism in the framingham heart study. Thromb Res. 151, 57-62 (2017).
- Montoro-Garcia, S., Schindewolf, M., Stanford, S., Larsen, O. H., Thiele, T. The role of platelets in venous thromboembolism. Semin Thromb Hemost. 42 (3), 242-251 (2016).
- Koupenova, M., Kehrel, B. E., Corkrey, H. A., Freedman, J. E. Thrombosis and platelets: An update. Eur Heart J. 38 (11), 785-791 (2017).
- Yeung, J., Li, W., Holinstat, M. Platelet signaling and disease: Targeted therapy for thrombosis and other related diseases. Pharmacol Rev. 70 (3), 526-548 (2018).
- Ferreiro, J. L., Gomez-Hospital, J. A., Angiolillo, D. J. Platelet abnormalities in diabetes mellitus. Diab Vasc Dis Res. 7 (4), 251-259 (2010).
- Buergy, D., Wenz, F., Groden, C., Brockmann, M. A. Tumor-platelet interaction in solid tumors. Int J Cancer. 130 (12), 2747-2760 (2012).
- Gkaliagkousi, E., Passacquale, G., Douma, S., Zamboulis, C., Ferro, A. Platelet activation in essential hypertension: Implications for antiplatelet treatment. Am J Hypertens. 23 (3), 229-236 (2010).
- Fidler, T. P., et al. Deletion of GLUT1 and GLUT3 reveals multiple roles for glucose metabolism in platelet and megakaryocyte function. Cell Rep. 20 (4), 881-894 (2017).
- Fidler, T. P., et al. Glucose metabolism is required for platelet hyperactivation in a murine model of type 1 diabetes. Diabetes. 68 (5), 932-938 (2019).
- Kulkarni, P. P., et al. Aerobic glycolysis fuels platelet activation: Small-molecule modulators of platelet metabolism as anti-thrombotic agents. Haematologica. 104 (4), 806-818 (2019).
- Ghatge, M., Flora, G. D., Nayak, M. K., Chauhan, A. K. Platelet metabolic profiling reveals glycolytic and 1-carbon metabolites are essential for gp vi-stimulated human platelets-brief report. Arterioscler Thromb Vasc Biol. 44 (2), 409-416 (2024).
- Ravera, S., Signorello, M. G., Panfoli, I. Platelet metabolic flexibility: A matter of substrate and location. Cells. 12 (13), (2023).
- Hechler, B., Dupuis, A., Mangin, P. H., Gachet, C. Platelet preparation for function testing in the laboratory and clinic: Historical and practical aspects. Res Pract Thromb Haemost. 3 (4), 615-625 (2019).
- Mustard, J. F., Perry, D. W., Ardille, N. G., Packham, M. A. Preparation of suspensions of washed platelets from humans. British Journal of Haematology. 22, 193-204 (1972).
- Cazenave, J. P., et al. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol Biol. 272, 13-28 (2004).
- Fine, K., Ashbrook, P., Brigden, L., Maldonado, J., Didishelm, P. Gel-filtered human platelets. Ultrastructure, function, and role of proteins in inhibition of aggregation by aspirin. Am J Pathol. 84 (1), (1976).
- Weiss, L., et al. An optimized protocol to isolate quiescent washed platelets from human whole blood and generate platelet releasate under clinical conditions. STAR Protoc. 4 (2), 102150 (2023).
- Burzynski, L. C., Pugh, N., Clarke, M. C. H. Platelet isolation and activation assays. Bio Protoc. 9 (20), e3405 (2019).
- Aurbach, K., Spindler, M., Haining, E. J., Bender, M., Pleines, I. Blood collection, platelet isolation and measurement of platelet count and size in mice-a practical guide. Platelets. 30 (6), 698-707 (2018).
- Narciso, M. G., Nasimuzzaman, M. Purification of platelets from mouse blood. J Vis Exp. (147), (2019).
- Aibibula, M., Naseem, K. M., Sturmey, R. G. Glucose metabolism and metabolic flexibility in blood platelets. J Thromb Haemost. 16 (11), 2300-2314 (2018).
- Ravi, S., et al. Metabolic plasticity in resting and thrombin-activated platelets. PLoS One. 10 (4), e0123597 (2015).
- Nayak, M. K., et al. an inhibitor of pyruvate dehydrogenase kinases, inhibits platelet aggregation and arterial thrombosis. Blood Advances. 2, (2018).
- Hadley, J. B., et al. Hormones, age, and sex affect platelet responsiveness in vitro. Transfusion. 62 (9), 1882-1893 (2022).
- Paglia, G., et al. Comprehensive metabolomic study of platelets reveals the expression of discrete metabolic phenotypes during storage. Transfusion. 54 (11), 2911-2923 (2014).
- Sake, C. L., et al. Isotopically nonstationary (13)c metabolic flux analysis in resting and activated human platelets. Metab Eng. 69, 313-322 (2022).
- Antoniewicz, M. R. A guide to metabolic flux analysis in metabolic engineering: Methods, tools and applications. Metab Eng. 63, 2-12 (2021).
- Larsen, M. T., Kuhlmann, M., Hvam, M. L., Howard, K. A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol Cell Ther. 4 (3), (2016).
- Balduini, C. L., Noris, P. P. latelet count and aging. Haematologica. 9 (6), 953-955 (2014).
- JoVE Science Education Database. Basic methods in cellular and molecular biology. Using a hemacytometer to count cells. , (2023).
- Spurgeon, B. E. J., Naseem, K. M. Platelet flow cytometry: Instrument setup, controls, and panel performance. Cytometry B Clin Cytom. 98 (1), 19-27 (2020).
- Van Velzen, J. F., Laros-Van Gorkom, B. A., Pop, G. A., Van Heerde, W. L. Multicolor flow cytometry for evaluation of platelet surface antigens and activation markers. Thromb Res. 130 (1), 92-98 (2012).
- Sake, C. L., Newman, D. M., Boyle, N. R. Evaluation of quenching methods for metabolite recovery in photoautotrophic synechococcus sp. Pcc 7002. Biotechnol Prog. 36 (5), e3015 (2020).
- Sapcariu, S. C., et al. Simultaneous extraction of proteins and metabolites from cells in culture. MethodsX. 1, 74-80 (2014).
- Rana, A., Westein, E., Niego, B., Hagemeyer, C. E. Shear-dependent platelet aggregation: Mechanisms and therapeutic opportunities. Front Cardiovasc Med. 6, 141 (2019).
- Ding, J., et al. Quantification of shear-induced platelet activation: High shear stresses for short exposure time. Artif Organs. 39 (7), 576-583 (2015).
- Ardlie, N., Perry, D., Packham, M., Mustard, J. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 136 (4), (1971).
- Rao, G. H. R., Reddy, K. R., Hagert, K., White, J. G. Influence of ph on the prostacyclin (pg12) mediated inhibition of platelet function. Prostaglandins Medicine. 4, 263-273 (1980).
- Navred, K., et al. A simplified flow cytometric method for detection of inherited platelet disorders-a comparison to the gold standard light transmission aggregometry. PLoS One. 14 (1), e0211130 (2019).
- Aldrighi, J. M., et al. Platelet activation status decreases after menopause. Gynecol Endocrinol. 20 (5), 249-257 (2005).
- Bausset, O., et al. Formulation and storage of platelet-rich plasma homemade product. Biores Open Access. 1 (3), 115-123 (2012).
- Nayak, M. K., et al. The metabolic enzyme pyruvate kinase m2 regulates platelet function and arterial thrombosis. Blood. 137 (12), 1658-1668 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved