Method Article
Rapid in vivo Drug Response Prediction Using Leukemia Cell Grafts in Zebrafish Embryos
In This Article
Summary
This protocol provides step-by-step instructions for generating and troubleshooting human acute lymphoblastic leukemia (ALL) xenografts from cell lines and fresh patient material in transiently immunosuppressed zebrafish embryos, along with guidelines for drug response assessment using flow cytometry. The experimental pipeline can also be adapted for solid tumors.
Abstract
Zebrafish xenotransplantation is a pivotal technique for investigating human cancer pathogenesis and predicting individual drug responses. This document introduces a streamlined protocol (ZefiX) for expanding primary B-cell precursor acute lymphoblastic leukemia (BCP-ALL) patient samples or immortalized cell lines in transiently immunosuppressed zebrafish embryos, utilizing flow cytometry for high-resolution single-cell analysis of treatment responses. Compared to solid tumor engraftments, leukemia cells profit significantly from a morpholino antisense oligonucleotide-based suppression of macrophage and neutrophil differentiating factors during the assay. Flow cytometry analysis of dissociated graft cells enables precise evaluation of cell count, proliferation rate, and vitality after treatment on a per-cell basis. This approach has been validated using targeted therapeutics such as venetoclax and dasatinib, with treatment outcomes compared to clinical records of related patient samples and traditional 2D culture controls. Notably, the protocol is completed within 7 days, aligning with clinical decision-making timelines. The methodology is adaptable for testing selected drugs in various cancer types, including solid tumors, thereby supporting personalized therapeutic strategies. However, limitations on the number of drugs that can be assessed, likely due to pharmacokinetic constraints in zebrafish embryos, should be considered.
Introduction
Zebrafish xenotransplantation has become a crucial in vivo model for understanding cancer pathogenesis and predicting drug responses1,2,3,4,5. Animal models remain critical for preclinical drug testing, and the zebrafish model offers significant advantages over other in vivo systems, including high throughput and cost-efficiency6,7,8. This model could also aid personalized treatment response predictions, including molecular targeted therapies and CAR-T cell therapy9,10,11,12.
BCP-ALL can particularly benefit from zebrafish xenografting, as expanding primary patient cells in culture remains challenging13. There is an undeniable need for novel treatment approaches in ALL. Despite a high remission rate of 80%-85% in children with BCP-ALL, the long-term survival rates for patients with relapsed or refractory disease range only between approximately 30%-60 %14,15,16. In such cases, drug testing using the proposed pipeline could be integrated into the clinical setting to identify the optimal patient-specific therapy14,15. This personalized approach can be crucial when dealing with multiple drug resistances, significantly reducing the treatment burden for patients by avoiding ineffective or suboptimal drugs with severe side effects.
Several features make zebrafish embryo xenografting a suitable model. The genetic similarities between humans and zebrafish - 70% genetic homology and 84% shared disease-linked genes - support gene-drug interaction studies17. Using a transgenic host embryo can thus reveal genetic predispositions affecting drug susceptibility18. Alternatively, cells with specific genetic modifications can be transplanted to evaluate whether the drug sensitivity or resistance aligns with in vitro findings. Zebrafish embryo xenografts also provide insights into the potential systemic effects of drugs. Although organ development in 2-3 days old embryos is not fully mature, the organs are correctly localized and partly share the cellular composition with their adult counterparts19.
Further advantages of this model include that only a few cancer cells are needed for engraftment, maintaining host embryos is simple, as no feeding is required within the first 5 days of life, and injection success can be rapidly assessed due to the transparency and size of the embryos. A unique feature is that only innate immunity is active at this developmental stage, facilitating efficient engraftment20. In the ZefiX protocol described here (see summary in Figure 1), immunodeficiency is further enhanced by suppressing the innate immune system during the first 4 days of life using stable Morpholino antisense oligonucleotides targeting spi1 and csf3r, which block macrophage and neutrophil differentiation21,22,23.
This protocol also differs from previous zebrafish xenotransplantation protocols, which were primarily developed for solid tumor grafts and typically use whole-mount imaging-based drug response assessment methods. ZefiX is optimized for liquid cancer cells, such as BCP-ALL cells, and has been successfully used to expand fresh or fresh-frozen patient material21. ZefiX can also be adapted for adherent cancer cells by selecting appropriate enzymes for tissue dissociation.
Another major advantage is the downstream analysis using flow cytometry, which offers several benefits: (i) a large number of graft cells can be processed quickly, allowing for robust statistical analysis at the single-cell level, (ii) proliferation rate and viability can be assessed simultaneously in individual cells, and (iii) flow cytometers are commonly available in clinical research settings, enabling drug response evaluation of graft cells on a single cell level within a few hours. To ensure reproducibility, this protocol provides a standardized pipeline from preparation through transplantation to flow cytometry analysis, allowing for drug response prediction in ALL cells within a week.
Protocol
All zebrafish experiments comply with the Charité-Universitätsmedizin Berlin Research Institutes for Experimental Medicine guidelines and official authorities. All studies involved zebrafish embryos < 6 days post fertilization (dpf), exempting them from the Protection of Animals Act. Zebrafish (Danio rerio) were raised and maintained at the animal facility of Charité-Universitätsmedizin Berlin, Berlin, Germany, according to standard protocols. They were housed at 28 °C with a 14 h light and 10 h dark cycle. Wild-type fish of AB or TüLF strains were used for all experiments.
NOTE: Establishing optimal treatment conditions for each desired drug prior to its ZefiX application includes several necessary steps. First, determine the half-maximal inhibitory concentration (IC50) of each drug using a suitable cell line within a conventional 2D culture system. Based on prior experience, effective drug concentrations for ZefiX treatment may be 5x - 50x greater than those used under typical cell culture conditions21,24. Prior to treating engrafted embryos, it is essential to assess toxicity within the non-transplanted host embryos using the established concentration range. After evaluating toxicity, expose cell-line-engrafted embryos to a variety of drug concentrations around 50x the IC50 value previously determined in 2D culture. If the grafted cells show no response to dosages up to 100x the IC50, the drug may be considered ineffective for ZefiX. To potentially enhance efficacy, one option is to precondition graft cells with the drug shortly before their transplantation into embryos25. See Table 1 for all the solutions used here.
1. Day 1: Preparation for the experiment
- E3 medium preparation: Prepare 2 L of autoclaved E3 medium to be used for embryo maintenance.
- Morpholino antisense oligonucleotides (MO) preparation: Prepare a 50 µM stock solution containing both MOs in a 1.5 mL microcentrifuge tube using nuclease-free water. Store the stock solution at room temperature (RT). Prepare the MOs for injection by incubating the solution on a heating block at 65 °C for 10 min.
NOTE: The MOs are directed against spi1 and csf3r to inhibit macrophage and neutrophil cell differentiation, as described in references22,23,26. - Preparation of injection plates
- To prepare 4-5 plates, dissolve 1% agarose in E3 medium to create a 100 mL solution. For transplantation plates, pour ~20 mL of the solution into each 10 cm Petri dish, ensuring they are half-filled. Swirl to evenly distribute the liquid.
- For Morpholino injection plates, place the injection mold onto the liquid agarose in two Petri dishes, ensuring no bubbles form. Cover the dishes by tilting the lids and leave them at RT until the agarose has solidified. Once solidified, remove the mold and store the plates upside down at 4 °C in a sealed plastic bag.
- Preparation of injection needles
- For Morpholino injections: Generate needles from 10 cm capillaries using a needle-puller and break off tips to achieve an estimated diameter of 10 µm (as described in27).
- For cell transplantation: Use commercially available pre-pulled blunt-end injection needles with a 20 µm outer diameter.
- Graft cell culture: Split cells (e.g., Nalm6) aiming at a target density of 70%-80% on day 4 in a T175 cell culture flask with RPMI medium supplemented with 10% FCS and 1% P/S (RPMI-complete). Split the cells 3-4 times before use to ensure a proper proliferation rate.
NOTE: For the preparation of fresh or fresh/frozen patient material for transplantation, instructions can be found in step 4.3. Cells are then prepared on the day of the transplantation. - Zebrafish breeding: Set up wild-type zebrafish in breeding tanks in the afternoon, keeping males and females separated.
NOTE: Zebrafish were raised and staged as described in the reference28. Time references (hpf or dpf) denote hours or days post-fertilization.
2. Day 2: Morpholino injection
- Supplement 500 mL of autoclaved E3 medium with 1% penicillin/streptomycin (E3/P/S) and fill two 10 cm Petri dishes. Take the injection plate out of the fridge to prewarm at RT.
- Stagger gate opening of the breeding tanks so that fertilization can only take place in a fraction of tanks at a time (depending on injection speed) to ensure timely microinjections of Morpholino solution into zebrafish embryos at the one-cell stage28,29. Start by opening one or two gates depending on the speed of the injector, when the one batch of eggs is readily injected open another gate or two gates and so forth.
- Transfer fertilized eggs in batches of 100 with as little liquid as possible into the previously made injection plate. Align the embryos in the grooves of the plate.
- Inject 1 nL of a 50 µM mix of both Morpholinos into the cell or the yolk sac just beneath the cell during the one-cell stage29. Inject sufficient eggs for subsequent procedures (e.g., for one 96-well plate, inject up to 200 eggs to have enough backup for potential dropout before transplantation).
- Transfer injected eggs into Petri dishes containing E3/P/S. Incubate the injected eggs at 28 °C. Retain un-injected eggs as control fish for flow cytometry analysis at 5 dpf. Store the remaining E3/P/S medium at 4 °C.
3. Day 3: De-chorionation
- De-chorionation of zebrafish embryos: Manually de-chorionate embryos when they are older than 24 hpf using two precision forceps29.
- Remove any dead embryos that show no heartbeat or movement and appear opaque or embryos with irregular shapes from the dishes with a Pasteur pipette.
- Pinch the chorion with precision forceps to hold it in place. Pinch right next to the tip of the precision forceps, holding the embryo in place with the second forceps, and carefully pull the chorion apart to release the embryo
NOTE: To dechorionate embryos younger than 24 hpf, they should be kept on an agarose-coated dish to prevent them from sticking to the plastic. Manual de-chorionation is preferred as it is gentler for the embryos. An alternative enzymatic de-chorionation method is described elsewhere30.
- Incubate de-chorionated embryos at 28 °C overnight.
4. Day 4: Xenotransplantation and drug treatment
- Preparation of host embryos: Remove the prepared agarose plates and E3/P/S from the fridge and allow them to reach RT. Screen embryos for the appropriate developmental stage at 48 hpf using a stereo microscope. Include only properly staged and morphologically typical embryos in the workflow, as described elsewhere29. Count all healthy embryos and plan further treatment. Keep no more than 100 embryos per 10 cm Petri dish at 28 °C to avoid uneven developmental speeds caused by oxygen shortage.
NOTE: Follow Step 4.2. for cell lines. For fresh/frozen material, proceed directly to Step 4.3. - Preparation of cell lines
- To prepare fluorescently labeled BCP-ALL cells for flow cytometry and transplantation, wash cells (from step 1.5.) with 1x PBS: centrifuge at 350 x g for 5 min, and resuspend in 20 mL of PBS.
- Count cells and transfer 3 x 105 unstained cells into a FACS tube for day 0 (0 days post-injection, dpi) flow cytometry analysis. Store on ice.
- Plate 3 x 105 cells in 3 mL of RPMI-complete in one well of a 6-well plate and maintain at 37 °C for 3 dpi control.
- Transfer 1 x 107 cells into a 15 mL centrifuge tube for CTV labeling. Centrifuge at 350 x g for 5 min at RT, pour out the supernatant (use a pipette for the rest), and resuspend the pellet in 2.5 mL of PBS (RT) with 1 µL of CTV stock solution.
- Incubate for 5 min in the dark at 37 °C, stop the reaction with 12.5 mL of RPMI-complete, then incubate for 10 min in the dark at 37 °C.
- Centrifuge at 350 x g for 5 min at RT and wash once with 10 mL of RPMI-complete. Centrifuge again and resuspend in 10 mL of RPMI-complete.
- Filter the cells using a 10 µm filter by centrifuging at 350 x g for 5 min. Resuspend the pellet, count the cells, and transfer 3 x 105 CTV-labeled cells into a FACS tube. Plate 3x 10^5 cells with 3 mL of RPMI-complete in one well of a 6-well plate and maintain at 37°C for 3 dpi proliferation control. Store remaining cells in 1 mL of PBS on ice until transplantation.
- Preparation of fresh/frozen patient material
- Pre-warm two 15 mL centrifuge tubes with 10 mL of RPMI-complete each at 37 °C. Thaw a vial containing 5-10 x 106 cells in a 37 °C water bath.
- Once only a small amount of ice remains, transfer the cells to the centrifuge tube with the pre-warmed medium. Centrifuge at 350 x g for 5 min at RT, discard the supernatant and resuspend the pellet in the second tube of pre-warmed RPMI-complete.
- Count viable cells using trypan blue. Aliquot 1x 10^5 cells into a 1,5 mL centrifuge tube and centrifuge this aliquot of cells in a FACS tube at 350 x g for 5 min at RT, discard the supernatant and resuspend the pellet in 300 µL of PBS.
- Store the resuspended aliquot on ice as an untreated control for flow cytometry analysis.
NOTE: If sufficient sample size allows, maintain an untreated control in culture for flow cytometry measurement on day 7. - Add 1 µL of the CTV stock solution to 2.5 mL of PBS (RT) for every 1 x 106 cells. Adjust the volume of the CTV stock solution if fewer cells are available.
- Incubate cells with CTV for 5 min at 37 °C in the dark, stop the reaction by adding 12.5 mL of pre-warmed (37 °C) RPMI-complete and incubate for 10 min in the dark at 37 °C.
- Centrifuge the cells at 350 x g for 5 min at RT and wash the cells once with 10 mL of RPMI-complete. Centrifuge again at 350 x g for 5 min at RT and resuspend the cells in 10 mL of RPMI-complete.
- Filter the cell suspension into a fresh 50 mL centrifuge tube using a 10 µm filter and centrifuge at 350 x g for 5 min.
- Do not discard the supernatant. Resuspend the pellet and count the cells. Transfer 3 x 105 CTV-labelled cells into one FACS tube.
- Centrifuge the remaining cells at 350 x g for 5 min at RT and discard the supernatant. Resuspend the remaining cells in 1 mL of PBS and store on ice until use in transplantation.
NOTE: If sufficient CTV-positive cells remain, they can be maintained in 2D culture parallel to the xenografts for proliferation rate comparison.
- Flow cytometry measurement
NOTE: Flow cytometry at 0 dpi should be performed by a second person to minimize the time cells remain on ice before transplantation. Before the first flow cytometry measurement using the appointed staining panel (CTV, CD19-Alexa488, APC-Annexin V, and 7AAD), perform a compensation test following the manufacturer's instructions.- Prepare two FACS tubes as follows: Tube 1: Unstained control cells; Tube 2: Cells stained with CTV, CD19, 7AAD, and Annexin.
NOTE: Staining and Labels: CellTrace Violet (CTV) identifies labeled graft cells versus host fish cells and assesses the proliferation rate after 3 days. CD19 antibody, a cell surface marker, serves as an additional marker for identifying human BCP-ALL graft cells. For other cancer types, alternate marker antibodies may be required. Annexin V marks early-stage apoptosis for cell viability assessment. 7AAD marks late-stage apoptosis or necrosis for cell viability assessment. - Centrifuge tubes (one containing CTV-stained cells and the other unstained cells) at 350 x g for 5 min at RT and discard the supernatant from Tube 1. Resuspend the pellet in 310 µL of Annexin Binding Buffer (ABB).
- Tube 2: Perform antibody and viability staining as described below.
NOTE: This staining protocol is optimized for CD19 B-cell staining. For other human antibodies used for labeling different cell types, the protocol may need adaptation.- Add 98 µL of ABB to Tube 2 (containing CTV-labeled cells). Add 2 µL of CD19-Alexa488 antibody (1:50 dilution) to the 98 µL of ABB added. Mix well.
- Incubate the mixture at 4 °C for 30 min and add 500 µL of ABB to stop the reaction. Centrifuge the tube at 350 x g for 5 min at 4 °C. Remove the supernatant and repeat washing step.
- Resuspend the cell pellet in 100 µL of ABB and proceed to 7AAD and APC-Annexin V staining. Perform 7AAD and APC Annexin V staining and flow cytometry measurement as described below.
- Add 5 µL of 7AAD and 5 µL of APC-Annexin V to the resuspended cells. Vortex gently to mix. Incubate the tube for 15 min in the dark at RT. Add 200 µL of ABB to reach a final volume of 310 µL.
- Perform flow cytometry analysis on the day of transplantation in the following order to avoid cross-contamination: Unstained control cells (Tube 1), CTV-labeled cells stained with Alexa488-CD19, APC-Annexin V, and 7AAD (Tube 2). Record at least 10,000 events per sample for sufficient analysis.
- Prepare two FACS tubes as follows: Tube 1: Unstained control cells; Tube 2: Cells stained with CTV, CD19, 7AAD, and Annexin.
5. Transplantation
- Transplantation plate: Prepare two 10 cm Petri dishes filled with E3/P/S and place in the incubator at 28 °C for at least 30 min to prewarm.
- Cell preparation: Centrifuge the cells at 350 x g for 5 min at RT, discard the supernatant and remove any remaining liquid using a micro-pipette. Add PBS to achieve a final volume of 20 µL. Keep the concentrated cell suspension on ice.
NOTE: Cells should not remain on ice for more than 2 h during the transplantation procedure. - Transplantation needle and host embryo preparation
- Transfer 25-30 embryos to one of the prewarmed Petri dishes containing E3/P/S using a glass Pasteur pipette to serve as control. Load 4 µL of the cell suspension into the transplantation needle using a micro loader tip.
NOTE: Loading should proceed smoothly within 1-2 min. If this is not the case, carefully add more PBS to the cell suspension. - Calibrate the injection pressure and pulse length by adjusting the microinjector. Adjust until one injection expels approximately 1,000 ALL cells or 2 nL of cell suspension (for 1x 107 cells in 20 µL). To estimate 1,000 cells, expel a volume of the suspension onto an agarose surface covered with medium. Count 100 cells in a small area, extrapolate their distribution to the total cell population, and estimate the total number.
- Prepare 50 mL of E3 containing tricaine (final concentration: 80 mg/L). Transfer the host embryos into the tricaine solution and incubate for at least 2 min to ensure proper anesthesia. Embryo is properly anesthetized when no motor response is observable
NOTE: A micro loader tip or forceps can be used to carefully approach and/or touch the embryo. - Transfer 15-20 dechorionated embryos onto an agarose-coated injection dish (see earlier preparation instructions) using as little liquid as possible to prevent embryos from slipping.
- Arrange the embryos as illustrated in Figure 2A. Inject approximately 1,000 CTV-positive BCP-ALL cells into the pericardial cavity. Introduce the needle at a 45° angle from the dorso-caudal direction, as shown in Figure 2B to inject the cells.
NOTE: Use commercially available pre-pulled, blunt-ended needles with a 20 µm opening diameter, specifically designed for small leukemia cells (see materials list). If the needle becomes blocked, trim it as necessary, but recalibrate the injection volume and cell count afterward. - Once all 15-20 embryos are injected, transfer them to the prewarmed Petri dish filled with E3/P/S and maintain at 28 °C.
- Repeat the steps until approximately 100 - 150 embryos are transplanted for one drug treatment assay, which should include three drug concentrations and one control.
- Incubate both the transplanted embryos and non-transplanted controls at 28 °C for 1-3 h before starting drug treatment.
- Transfer 25-30 embryos to one of the prewarmed Petri dishes containing E3/P/S using a glass Pasteur pipette to serve as control. Load 4 µL of the cell suspension into the transplantation needle using a micro loader tip.
- In vivo drug treatment (96-well plate)
- Using fluorescent stereomicroscopy, screen embryos to confirm successful engraftment (Figure 2C). Ensure that the yolk is intact, as the yolk environment might be toxic to graft cells21. Discard embryos with cells in the yolk.
- Prepare 2.5 mL of a 2x concentrated solution for each drug condition to be tested in E3/P/S with 0.5% DMSO. Additionally, prepare a 5 mL vehicle control solution containing 0.5% DMSO in E3/P/S.
- Add 100 µL of E3/P/S + 0.5% DMSO to each well of a 96-well plate. This step prevents the unintended transfer of minimal drug amounts between wells.
- Carefully transfer one engrafted embryo into each well. Use a glass Pasteur pipette to pick up each embryo in as little E3 medium as possible.
- Allow the embryo to sink to the bottom of the pipette tip by gently tilting the pipette and release it into the well using capillary forces. Avoid touching the medium in the well during transfer.
- Add drug solutions to the plate: Fill 24 wells (2 rows) of the 96-well plate with 100 µL of either the vehicle control solution or one of the three 2x concentrated drug solutions.
NOTE: Effective drug concentrations must be determined in prior experiments specific to each drug being tested. - Maintain embryos at 35 °C for 72 h, including the non-transplanted embryos, which will serve as controls for flow cytometry analysis on day 7.
6. Day 7
- Embryo/graft dissociation
- Screen the 96-well plate using stereomicroscopy to identify and select healthy zebrafish embryos. Randomly pool 10 healthy host embryos from each condition into a 1.5 mL microcentrifuge tube (ideally resulting in 2 tubes per condition).
- Remove as much liquid as possible from each embryo-containing tube and sacrifice the embryos by hypothermal shock by incubating the tubes on ice for 1 h.
- Add 500 µL of calcium- and magnesium-free Hank's balanced salt solution (HBSS) to each tube. Mechanically dissociate the embryos and graft cells by trituration using a 200 µL micropipette tip, pipetting up and down approximately 15x.
- Pellet the tissue fragments by centrifugation at 350 x g for 5 min at RT. In the mean time, prepare FACS tubes with a 35 µm fine mesh filter strainer cap containing 2 mL of PBS per condition (4 tubes total).
- Resuspend each pellet in 500 µL of enzyme mix (0.01% papain, 0.1% dispase II, 0.01% deoxyribonuclease I, and 12.4 mM MgSO4 in calcium- and magnesium-free HBSS) for enzymatic dissociation. Incubate at RT for 15 min.
- During the incubation, pipette the mixture up and down every 5 min repeatedly using the same pipette tip for each individual tube to avoid tissue loss.
- Flow cytometry measurement
- Transfer the dissociated cells into the 35 µm fine mesh filter strainer cap of the FACS tubes and centrifuge at 350 x g for 5 min.
- While centrifuging, prepare a master mix for CD19 B-cell surface staining. Combine 98 µL of ABB with 2 µL of Alexa Fluor 488 anti-human CD19 antibody for each condition.
- Discard the supernatant from the dissociated cell pellet and resuspend the pellet in 100 µL of the CD19 staining master mix.
- Perform the CD19, 7AAD, and APC Annexin V staining protocol for all ZefiX samples, including 3 x 105 CTV-positive cells from parallel cell culture if available.
- Conduct flow cytometry analysis following the order and record the number of events outlined in Table 2. Run all samples containing host and graft cells as completely as possible to evaluate the total graft cell numbers.
- Analysis of results using commercial software.
- Gating strategy for cell culture sample: Open the software and load the FCS files into the workspace. Create a dot plot with CD19 and CTV to ensure the signal is overlapping and to confirm the presence of the cancer cells. Next, create a dot plot with FSC-A (x-axis) and SSC-A (y-axis) to distinguish intact cells (Q2) from debris (Q4; Figure 3A').
- Use the Intact Cells population to create a new plot with SSC-H (x-axis) and SSC-A (y-axis) to identify single cells (Figure 3A'').
- Create another dot plot with Annexin V (x-axis) and 7AAD (y-axis) using the Single Cells population (Figure 3A'''). Distinguish cells into four populations: Viable cells: Annexin V negative, 7AAD negative (Q4); Early apoptotic cells: Annexin V positive, 7AAD negative (Q3); Late apoptotic/necrotic cells: Annexin V positive, 7AAD positive (Q2); Necrotic cells: Annexin V negative, 7AAD positive (Q1).
- Repeat these steps for 3 dpi samples (Figure 3B).
- Gating strategy for graft and host embryo cells
- Separate human graft cells from host cells using a dot plot with CTV (x-axis) and CD19 (y-axis). Identify and isolate CTV/CD19 double-positive graft cells (Figure 3C).
- Apply the same gating strategy described for 3 dpi culture cells (Figure 3B' - B''') to the graft cell population. Copy the gating strategy for consistency.
- Merge all Annexin V- and 7AAD-negative cell populations into a histogram with CTV values on the x-axis (Figure 3D). Calculate the geometric mean for all five samples to determine proliferation rates.
- Calculations for treatment response evaluation
- To determine the number of cell divisions after 3 days, use the formula:
n = log2 (I0/I)
Where, I0 = Initial CTV fluorescence intensity (geometric mean, 0 dpi), I = CTV fluorescence intensity at 72 h, n = Number of cell divisions.
Example: Fresh-frozen patient cells divided 2.6x in ZefiX log2(88317/14644) = 2.6
and 2.8x in 2D culture log2(88317/12992) = 2.8 - To determine the total number of graft cells per fish after 3 days divide the total number of cancer cells (including apoptotic cells but excluding debris) by the number of fish pooled in the sample (typically n = 10).
- To determine the viability of CTV-positive graft cells after 3 days, use the formula:
V = (C/100) x A
Where: V = Viability, C = fraction of intact single cells without debris (as percentage), A = Fraction of Annexin V- and 7AAD-negative cells (as a percentage).
- To determine the number of cell divisions after 3 days, use the formula:
Results
For a detailed scientific evaluation of the ZefiX protocol, including the xenograft and drug treatment of fresh-frozen, primary BCP-ALL cell samples, please refer to the previously published manuscript21. Approval for the use of patient samples in research for preclinical drug testing was granted as part of add-on studies to the ALL-REZ BFM 2002 trial (NCT00114348) and the ALL-REZ BFM registry and biobank (EA2/055/12) by the local medical research ethics committees, as well as to the IntReALL SR 2010 international trial (NCT01802814) by the national authority. Informed consent was obtained from patients and/or their guardians through the respective trial or registry in which they were enrolled.
Figure 2 illustrates an example of embryo alignment in an agarose dish prior to injection, which helps streamline the injection process. The injection should be performed at the angle depicted to precisely target the cavity surrounding the developing heart. Additionally, Figure 2C provides a reference of successfully injected 2 dpf embryos containing human graft cells (blue), which were labeled with CTV before injection. Embryos with injection outcomes differing from those shown in Figure 2C were excluded, with a particular focus on avoiding yolk sac perforation to ensure graft cell viability during the subsequent three-day incubation.
Following the three-day incubation period, host embryos are processed in pools of 10 for flow cytometry analysis. After enzymatic dissociation, cell suspensions are stained with an anti-human-CD19 antibody and two viability markers: Annexin V for early apoptotic cells and 7AAD for late apoptotic and necrotic cells.
Figure 3 presents flow cytometry data of ZefiX-expanded BCP-ALL cells from a patient with BCP-ALL. Panels A, A', A'', and A''' show data collected at 0 dpi on the day of transplantation. Figure 3A displays CTV and CD19 fluorescence values for a total of 10,000 cells as a reference for the gating strategy applied to host-graft cell suspensions at 3 dpi (Figure 3C). In Figure 3A', debris is excluded through regular gating of cells using Forward Scatter Area (FSC-A) and Sideward Scatter Area (SSC-A). In Figure 3A'', single cells are separated from doublets using a graph of SSC height (SSC-H) versus SSC-A. This single-cell population is used for viability assessment in Figure 3A''', where viable cells (Q4) are distinguished from early apoptotic cells (Q3, higher Annexin V values) and late apoptotic or necrotic cells (Q2, higher 7AAD levels).
For comparison, patient cells cultured under conventional 2D conditions are also analyzed by flow cytometry after three days (Figures 3B,B',B'',B'''), following the same gating strategy. Viability of patient cells after 72h in 2D-culture is calculated from Q2 in Figure 2B' and Q4 in Figure 2B''': (95.0%/100)*61.9% = 58.8%.
In Figure 3C, the starting material is the cell suspension from host embryos containing graft cells. Unlike in vitro measurements, all cells in the tube are analyzed by flow cytometry. CD19 and CTV-positive graft cells are gated to separate them from CD19 and CTV-negative fish cells. The intact graft cell population is further analyzed in Figure 3C', where debris is excluded. The viability of single graft cells is then assessed using the same gating strategy as in Figures 3B.
The results indicate that the percentage of viable single cells expanded in embryos is 95.2%, which is 1.6 times higher than the viability of cells cultured in a dish (Figure 3B'''). Cell division rates were calculated in vivo and in vitro by analyzing the decrease in CTV fluorescence intensity in each population from 0 dpi to 3 dpi (Figure 3D). The number of cell divisions was determined using the formula in Section 6.4.1 and the geometric mean of each CTV curve (Figure 3D). The calculated division rates (2.59 divisions in vivo and 2.77 divisions in vitro) suggest that viable cells divide at a similar rate under both conditions over three days.
Finally, the average number of intact graft cells per embryo after three days was determined by dividing the number of intact graft cells (excluding debris, Figure 3C') by the number of embryos pooled in a tube21.
In conclusion, fresh BCP-ALL samples engrafted in zebrafish embryos exhibit higher viability after three days compared to conventional culture in a dish and viable cells divide at a comparable rate in both conditions.
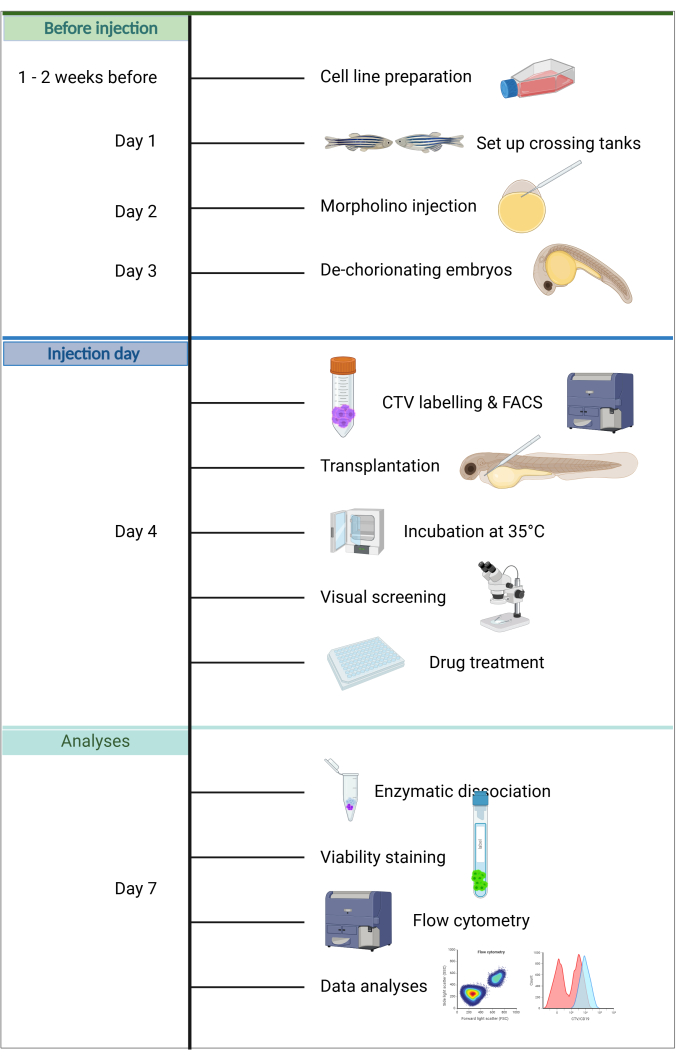
Figure 1. Workflow of the ALL-ZefiX pipeline. Created in https://BioRender.com. Please click here to view a larger version of this figure.
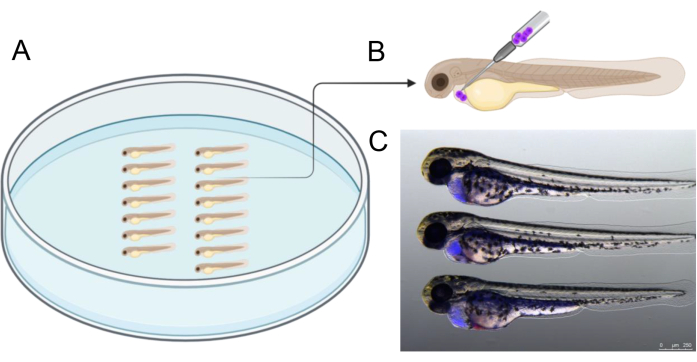
Figure 2: Injection arrangement. (A) Arranging embryos as depicted facilitates injection. Embryos can be arranged using forceps or with a 20 µL microloader pipette tip that was cut to have a tip of 2.5 - 3 cm length. (B) Schematic representation of the recommended injection angle to graft cells into the pericardium of 2 dpf embryos. (C) Visual aids to correctly estimate the amount of transplanted cancer cells. This picture shows a 48 hpf embryo at 3 h post-injection with human cancer cells previously labeled with CellTrace Violet). Created in https://BioRender.com. Please click here to view a larger version of this figure.

Figure 3: Gating strategy and flow cytometry analysis of a patient derived fresh frozen sample of isolated BCP-ALL blast cells following 2D culture of engraftment in zebrafish embryos. (A, B) Patient cells were labeled with CellTrace Violet (CTV) before culture. Cells were cultured on tissue culture plastic at 37 °C for 0h (A) or 72 h (B) before flow cytometry analysis. (C) Patient cells labeled with CTV and grown at 35 °C for 72 h as grafts in host zebrafish embryos. A group of 10 embryos was pooled before single-cell dissociation for flow cytometry analysis. The gating strategy in (B) was applied, and viable graft cell fraction was identified and quantified. To do this, CTV-positive graft cells (Q2) were separated from auto-fluorescent zebrafish cells (Q1/4) to sort out the graft cell population for analysis. CTV labeling intensity was analyzed in this graft cell population. (D) Cell counts and mean intensity of CTV labels from viable cell selection only. Note the shift of CTV intensity after 3 days (3 dpi). Please click here to view a larger version of this figure.
Table 1: Table of solutions used. Please click here to download this Table.
Table 2: Samples measured by flow cytometry. Please click here to download this Table.
Discussion
Zebrafish embryos have become an increasingly popular xenograft model for drug screening and cancer research due to their high throughput capacity and cost-effectiveness. These xenografts hold promise as a critical pillar of translational medicine, aiding preclinical research and decision-making9,21. However, zebrafish xenograft models for human leukemia cell expansion and treatment remain underrepresented compared to the extensive body of work on solid tumor grafts. This protocol offers detailed guidance for leveraging zebrafish xenografts in leukemia research while remaining adaptable for use in solid tumors.
Achieving consistent cancer cell transplantation can be challenging, highlighting the need for standardized analysis and higher statistical reliability. This protocol addresses these issues by presenting a comprehensive pipeline for preparation, transplantation, and downstream flow cytometry analysis, along with troubleshooting recommendations.
Morpholino injection for transient immune suppression
Zebrafish embryos rely on their innate immune system during the first days of development, which defines the timeframe for this experimental pipeline20. Primitive macrophages emerge around 12 hpf, with some differentiating into neutrophils by 33 hpf20,31,32. T-cells enter circulation at approximately 8 days post-fertilization20,33. Macrophages and neutrophils, as part of the innate immune response, have been implicated in the reduced survival of BCP-ALL cells observed three days post-transplantation in previous studies21.
Morpholino-mediated temporal immune suppression, targeting spi1 and csf3r, effectively inhibits macrophage and neutrophil differentiation, leading to improved engraftment of BCP-ALL cells without affecting embryo viability21. Although this method cannot achieve permanent depletion, as complete knockout of spi1 and csf3r is lethal, it remains the best approach for this pipeline.
Calibration of injection volumes using a graticule and precise delivery into the yolk sac at the one-cell stage ensures consistent Morpholino injections with high survival rates. Alternatives like liposomal clodronate injections (Clodrosome) for macrophage depletion have shown promise but require further validation for this pipeline34,35.
Cell preparation
A sufficiently dense and viable cell population is critical for successful BCP-ALL expansion in this protocol. CellTrace Violet (CTV) is used for fluorescent labeling to evaluate implantation success at 0 dpi and to track proliferation rates throughout the experiment. Unlike other labels, CTV does not alter cell behavior, allowing precise single-cell-level proliferation analysis. This offers advantages over Ki-67 antibody staining, which only captures cells during proliferation but not divided cells that have already exited the cell cycle.
CTV also outperforms CellTracker CM-DiI (DiI) in reflecting cell viability. DiI and its derivatives are more stable fluorophores, often persisting beyond cell death, which can confound experimental results2. Additionally, the inclusion of a BCP-ALL-specific antibody against CD19 in flow cytometry enables precise identification of graft cells. Human-specific antibodies such as anti-HLA can serve as alternatives for other cancer cell types36.
Cancer cell transplantation
Consistent engraftment requires optimal dilution and concentration of the cell suspension. The suspension should maintain sufficient density while avoiding viscosity that impairs injection. This protocol prioritizes injection into the pericardial cavity or perivitelline space (PVS) over the yolk sac, as these sites offer better vascularization and less hypoxic conditions37. Yolk sac transplantation, although accessible, often results in high mortality rates and poor cell viability21.
Needle clogging due to microparticles remains a procedural challenge. Filtering the cell suspension and recalibrating injection volumes after trimming blocked needles are essential steps. Only embryos with densely filled pericardia should be used for subsequent drug treatments21,36.
The suggested incubation temperature of 35 °C balances the natural temperature of human cancer cells (37°C) and the standard zebrafish housing temperature (28 °C)21. Zebrafish embryos adapt to this temperature with minimal developmental deformations, and the environment enhances the proliferation and survival of fresh patient-derived cells38.
Drug treatment
Zebrafish xenograft models were developed to facilitate high-throughput drug screening. However, drug treatment remains one of the most challenging aspects of the ZefiX assay. Many standard-of-care drugs and targeted therapies do not effectively reach graft cells in vivo. It might also require the testing of a bigger panel of drug concentrations. Successful examples, such as venetoclax and dasatinib, require significantly higher concentrations than in conventional 2D culture assays21.
Alternatively, pre-treating cells in vitro before transplantation also allows certain systemic and localized effects to be studied. For example, this approach may be suitable for Adeno-associated virus (AAV)-based treatments in glioblastoma39.
If effects of drug treatment are observed in vitro but not in vivo using this pipeline, an alternative could be, for instance, transplanting into the 1k-cell stage (3 hpf) or the blastula stage and starting drug treatment at 24 hpf40,41. This could allow drugs to reach the graft cells that are not successful in 48 h old embryos or co-injection of cells and drugs at the same time25.
Dissociation and flow cytometry analysis
Tissue dissociation is critical for analyzing total graft cell numbers and reliably interpreting experimental results. A combination of mechanical and enzymatic dissociation ensures high-quality single-cell suspension while maintaining cell-surface protein integrity. Adjusting dissociation conditions (e.g., enzyme composition, pipetting, or using a Dounce homogenizer) may be necessary for different cancer types.
Samples should be filtered to prevent clogging of the flow cytometer, and sticky proteins or lipids can be mitigated with EDTA or embryo deyolking prior to dissociation.
Summary
The ZefiX protocol provides a fast and cost-effective experimental pipeline for preclinical cancer research, drug resistance studies, and personalized treatment evaluations. While zebrafish xenograft models have limitations and cannot accommodate all drug types, this standardized protocol allows the in vivo expansion of fresh patient leukemia cells and cell lines. Adaptable for other cancer types, it offers a promising tool for rapid, personalized drug response prediction within the clinical decision-making time frame.
Disclosures
All authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) within the Collaborative Research Center CRC1588, project number 493872418 and the Dr. Kleist Stiftung, Berlin, as well as by the Deutsche José Carreras Leukämie Stiftung (R03/2016), the Berliner Krebsgesellschaft (HEFF201633KK) and the German Cancer Consortium (DKTK, Joint Funding Call 2016). We thank Julia Köppke and Mareike Wolff for their critical reading of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Petri dish (10 cm) | Greiner | P7237 | |
| 7-AAD viability staining solution | Invitrogen | 00-6993-50 | |
| Agarose (LE, analytic grade) | Biozym | 840004 | |
| Air pressure injector | Narishige | IM400 | with external gas supply |
| Alexa Fluor 488 anti-human CD19 antibody | Biolegend | 302219 | |
| Annexin binding buffer | Biolegend | 422201 | Or see solutions for preparation |
| APC annexin V | Biolegend | 640941 | |
| Capillaries (10 cm, OD 1.0 mm, with filaments) | WPIINC | TW100F-4 | 1.0 OD; 0.75 ID |
| Cell culture flask (T-175) | Sarstedt | 83,39,12,002 | |
| CellTrace Violet | Invitrogen | C34557 | |
| Dimethyl sulphoxide (DMSO) | Roth | A994.1 | |
| Dispase II | Sigma Aldrich | D4693-1g | |
| DNase I | AppliChem GmbH | A3778 | |
| Eppendorf tubes (1.5 ml) | Eppendorf | 30120086 | |
| FACS tube (Polystyrene round botton Tube with Cell strainer Cap, 5 ml) | Falcon | 352235 | |
| Falcon tubes (50 ml) | Falcon | 352070 | |
| Fetal calf serum (FCS) | Sigma Aldrich | C8056 | |
| Fine mesh filter (10 µm) | PluriStrainer | 435001050 | |
| Fine mesh filter (20 µm) | PluriStrainer | 431002040 | |
| Flow cytometer | Becton Dickinson | BD LSRFortessa X-20 | |
| Fluorescent stereomicroscope | Leica | ||
| Fluorescent stereomicroscope with camera | Leica | M165 FC | Camera: DFC7000 T |
| Hank’s Balanced Salt Solution (HBSS, Calcium and Magnesium free ) | Sigma Aldrich | 88284 | |
| Injection mold (Zebrafish MI/Transplant KIT) | World Precision Instruments | Z-MOLDS | |
| Injection needles (without filament) | Biomedical instruments | VZIPbl-20-10-55 | Zebrafish injection pipette, blunt, OD: 20μm ± 1, TL:~10mm, PL: 55mm, Glass: BM100T-10P |
| Macro-centrifuge | Eppendorf | ||
| Micro-centrifuge | |||
| Morpholino (csf3r) | Gene Tools LLC | csf3r (GAAGCACAAGCGA GACGGATGCCA) | |
| Morpholino (spi1) | Gene Tools LLC | spi1(GATATACTGATAC TCCATTGGTGGT) | |
| Papain | Sigma Aldrich | P3125 | |
| Penicillin-Streptomycin (Penstrep; 10.000 U/ml) | Gibco | 15140122 | |
| Plates (4-well) | Greiner Bio one | 657160 | |
| Plates (96-well) | Greiner Bio one | 657180 | |
| Roswell Park Memorial Institute (RPMI) 1640 Medium | Gibco | 21875-034 | |
| Tricaine (MS-222) | Sigma Aldrich | E10521-50G | Ethy-3 aminobenzoate methanesulfenate |
References
- Fontana, C. M., Van Doan, H. Zebrafish xenograft as a tool for the study of colorectal cancer: a review. Cell Death Dis. 15, 1-12 (2024).
- Sturtzel, C., et al. Refined high-content imaging-based phenotypic drug screening in zebrafish xenografts. NPJ Precis Oncol. 7 (1), 1-16 (2023).
- Al-Hamaly, M. A., Turner, L. T., Rivera-Martinez, A., Rodriguez, A., Blackburn, J. S. Zebrafish cancer avatars: A translational platform for analyzing tumor heterogeneity and predicting patient outcomes. Int J Mol Sci. 24, 2288 (2023).
- Gamble, J. T., Elson, D. J., Greenwood, J. A., Tanguay, R. L., Kolluri, S. K. The zebrafish xenograft models for investigating cancer and cancer therapeutics. Biology. 10 (4), 252 (2021).
- Costa, B., Estrada, M. F., Mendes, R. V., Fior, R. Zebrafish avatars towards personalized medicine-A comparative review between avatar models. Cells. 9 (2), 293 (2020).
- Wang, W., et al. Progress in building clinically relevant patient-derived tumor xenograft models for cancer research. Animal Model Exp Med. 6 (5), 381-398 (2023).
- Xiao, J., Glasgow, E., Agarwal, S. Zebrafish xenografts for drug discovery and personalized medicine. Trend Cancer. 6 (7), 569-579 (2020).
- Fazio, M., Ablain, J., Chuan, Y., Langenau, D. M., Zon, L. I. Zebrafish patient avatars in cancer biology and precision cancer therapy. Nat Rev Cancer. 20 (5), 263-273 (2020).
- Costa, B., et al. Zebrafish avatar-test forecasts clinical response to chemotherapy in patients with colorectal cancer. Nat Comm. 15 (1), 4771 (2024).
- Grissenberger, S., et al. Chapter 8 - Preclinical testing of CAR T cells in zebrafish xenografts. Method Cell Biol. 167, 133-147 (2022).
- Yan, C., et al. Single-cell imaging of T cell immunotherapy responses in vivo. J Exp Med. 218 (10), 20210314 (2021).
- Pascoal, S., et al. A preclinical embryonic zebrafish xenograft model to investigate CAR T cells in vivo. Cancers. 12 (3), 567 (2020).
- Pal, D., et al. Long-term in vitro maintenance of clonal abundance and leukaemia-initiating potential in acute lymphoblastic leukaemia. Leukemia. 30 (8), 1691-1700 (2016).
- Beneduce, G., et al. Blinatumomab in children and adolescents with relapsed/refractory B cell precursor acute lymphoblastic leukemia: A real-life multicenter retrospective study in seven AIEOP (Associazione Italiana di Ematologia e Oncologia Pediatrica) Centers. Cancers. 14 (2), 426 (2022).
- Xie, J., et al. Short-course blinatumomab for refractory/relapse precursor B acute lymphoblastic leukemia in children. Front Pediatr. 11, 1187607 (2023).
- Mengxuan, S., Fen, Z., Runming, J. Novel treatments for pediatric relapsed or refractory acute B-cell lineage lymphoblastic leukemia: Precision medicine era. Front Pediatr. 10, 923419 (2022).
- Howe, K. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496 (7446), 498-503 (2013).
- Lee, H. C., Lin, C. Y., Tsai, H. J. Zebrafish, an in vivo platform to screen drugs and proteins for biomedical use. Pharmaceuticals. 14 (6), 500 (2021).
- Santoriello, C., Zon, L. I. Hooked! Modeling human disease in zebrafish. J Clin Investigation. 122 (7), 2337-2343 (2012).
- Miao, K. Z., Kim, G. Y., Meara, G. K., Qin, X., Feng, H. Tipping the scales with zebrafish to understand adaptive tumor immunity. Front Cell Dev Biol. 9, 660969 (2021).
- Gauert, A., et al. Fast, in vivo model for drug-response prediction in patients with B-cell precursor acute lymphoblastic leukemia. Cancers. 12 (7), 1883 (2020).
- Ellett, F., Pase, L., Hayman, J. W., Andrianopoulos, A., Lieschke, G. J. Mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood. 117 (4), e49-e56 (2011).
- Pase, L., et al. Neutrophil-delivered myeloperoxidase dampens the hydrogen peroxide burst after tissue wounding in zebrafish. Current Biol. 22 (19), 1818-1824 (2012).
- Wijk, R. C. V., et al. Mechanistic and quantitative understanding of pharmacokinetics in Zebrafish larvae through nanoscale blood sampling and metabolite modeling of paracetamol. J Pharmacol Exp Ther. 371 (1), 15-24 (2019).
- Lázaro-Navarro, J., et al. Inhibiting casein kinase 2 sensitizes acute lymphoblastic leukemia cells to venetoclax via MCL1 degradation. Blood Advances. 5 (24), 5501 (2021).
- Rhodes, J., et al. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Developmental Cell. 8 (1), 97-108 (2005).
- Zakaria, Z. Z., Eisa-Beygi, S., Benslimane, F. M., Ramchandran, R., Yalcin, H. C. Design and microinjection of Morpholino antisense oligonucleotides and mRNA into zebrafish embryos to elucidate specific gene function in heart dvelopment. J Vis Exp. (186), e63324 (2022).
- . ZFIN: Zebrafish Book: Contents Available from: https://zfin.org/zf_info/zfbook/cont.html (2025)
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., Schilling, T. F. Stages of embryonic development of the zebrafish. Dev Dyn. 203 (3), 253-310 (1995).
- Hasegawa, E. H., Gist H Farr, I. I. I., Maves, L. Comparison of pronase versus manual dechorionation of zebrafish embryos for small molecule treatments. J Dev Biol. 11 (2), 16 (2023).
- Wattrus, S. J., Zon, L. I. Stem cell safe harbor: the hematopoietic stem cell niche in zebrafish. Blood Adv. 2 (21), 3063-3069 (2018).
- Harvie, E. A., Huttenlocher, A. Neutrophils in host defense: new insights from zebrafish. J Leukocyte Biol. 98 (4), 523-537 (2015).
- Page, D. M., et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood. 122 (8), e1-e11 (2013).
- Nguyen-Chi, M., et al. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell Death Dis. 8 (8), e2979-e2979 (2017).
- Rosowski, E. E. Determining macrophage versus neutrophil contributions to innate immunity using larval zebrafish. Dis Models Mech. 13 (1), dmm041889 (2020).
- Rebelo de Almeida, C., et al. Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy. Comm Biol. 3 (1), 1-13 (2020).
- Pringle, E. S., et al. The zebrafish xenograft platform-A novel tool for modeling KSHV-associated diseases. Viruses. 12 (1), 12 (2020).
- Pype, C., et al. Incubation at 32.5 °C and above causes malformations in the zebrafish embryo. Reprod Toxicol. 56, 56-63 (2015).
- Xu, X., et al. Adeno-associated virus (AAV)-based gene therapy for glioblastoma. Cancer Cell Int. 21 (1), 76 (2021).
- Siebert, J., et al. Rhabdomyosarcoma xenotransplants in zebrafish embryos. Pediat Blood Cancer. 70 (1), e30053 (2023).
- van Bree, N., et al. Development of an orthotopic medulloblastoma zebrafish model for rapid drug testing. Neuro-Oncol. noae210, (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved