Method Article
Generation of Three-Dimensional Spheroids/Organoids from Two-Dimensional Cell Cultures Using a Novel Stamp Device
In This Article
Summary
This study presents a cost-effective and efficient methodology for generating 3D cell structures using a stamp-based system to create microwells in agarose molds. The system promotes the formation of uniform spheroids/organoids, thus improving cell interactions. This approach reduces experimental variability and supports applications in drug testing and tissue engineering.
Abstract
Three-dimensional (3D) cell cultures provide a more accurate representation of the in vivo microenvironment than conventional two-dimensional (2D) cultures, since they promote enhanced interactions among cells and the extracellular matrix. This study aimed to develop an efficient, cost-effective, and reproducible methodology to generate 3D cell structures (spheroids/organoids) using an innovative stamp-based system to create microwells in agarose molds.A novel stamp was used to produce 663 microwells per well of a 6-well plate, providing an ideal environment for cell aggregation. Primary porcine pancreatic islet cells were seeded into these microwells, where they aggregated to form spheroids/organoids. The cultures were incubated at 37 °C under 5% CO2, and the medium was replaced every 3 days. Spheroid formation was periodically monitored, and samples were collected for characterization. The method successfully generated uniform and high-quality spheroids, reducing experimental variability, minimizing manipulation, and enhancing cell interactions. The use of agarose-based micropattern molds provided a simplified, controlled environment for 3D cultures, offering a standardized and cost-effective solution.This methodology supports applications for drug testing and tissue engineering, offering a practical and scalable platform for 3D cell culture models that can be easily implemented in various laboratory settings.
Introduction
Over the past 50 years, numerous cell biology investigations have demonstrated that two-dimensional (2D) cultures fail to accurately replicate the in vivo conditions observed in animal models1. Structurally, 2D cell cultures do not allow cells to organize three-dimensionally and replicate the situation observed in in vivo systems. Furthermore, cellular signaling pathways are altered in 2D cultures compared to three-dimensional (3D) cultures, which could likely explain why certain types of drug screening using 2D cultures are so discrepant2. A significant advancement in cell culture techniques emerged with the introduction of 3D culture systems. 3D systems vary considerably in complexity depending on cellular composition and cytoarchitecture. Generally, two types of structures are generated, namely: spheroids and organoids. Spheroids are described as simple clusters of cells obtained from normal or tumor tissues, embryoid bodies, and cell lines. The formation of 3D structures is influenced by various factors, including cell-cell interactions and signaling pathways mediated by components of the extracellular matrix (ECM), which provide structural support and biochemical cues. These elements regulate interactions that contribute to tissue organization and function3. The spheroid culture system was first described in the early 1970s, using V79 Chinese hamster lung cell lines as a model of nodular carcinomas, growing under non-adherent conditions and forming perfect spheres4. Organoids are described as clusters of organ-specific cell types derived from stem or progenitor cells, which self-organize through processes, such as cell sorting and lineage specification, in a spatially confined manner, mirroring the in vivo development5.
Several available methods and materials are available to culture cells under 3D conditions. The main methods currently employed for generating 3D cultures are: 1) hanging drops; 2) rotating cell culture and low-attachment plastics; 3) pyramid plates containing conical wells; 4) macroporous scaffolds; 5) magnetic beads; and 6) scaffold-free hydrogels.
Hanging drops is the method used to obtain scaffold-free 3D cultures. This method presents certain limitations, including the need for extensive handling, low production efficiency, spherical geometry, and exposure to high shear forces. Moreover, specific procedures, such as medium replacement or compound addition, can be challenging and may result in material loss. Furthermore, literature reports indicate that some cell lines fail to produce tightly packed spheroids when employing this approach6.
Rotating cell culture and low-attachment plastics are used to prevent cells from attaching to the substrate, causing them to aggregate and form spheroids. This process requires specific flasks and/or agitation/rotation. Although this is one of the most straightforward approaches for large-scale spheroids or organoids production, it is not without drawbacks, such as the need for specific equipment, low culture longevity, size variation in spheroids, mechanical damage to cells, and low efficiency6.
Pyramid plates containing conical wells are commercially available plates impacting costs, in addition to the fact that some manipulations may hinder the formation of spheroids/organoids7.
Macroporous scaffolds are also employed for 3D culturing; however, a major obstacle lies in achieving effective cell seeding and uniform distribution. This issue arises because the pore sizes may either be too small for cell penetration or too large to securely retain the cells. To address this issue, several strategies have been explored8, which directly impact the complexity and cost of this technique.
The magnetic beads methodology generates a small number of spheroids/organoids, has a high cost, and may leave nanoparticle residues inside the cells9.
Among the systems for cultivating spheroids, non-adhesive agarose hydrogels are available, representing a scaffold-free hydrogel. This approach offers notable benefits, such as precise control over the size of the 3D structures and the capacity to generate a substantial number of these structures per plate. In this method, cells are introduced into a hydrogel with preformed wells, in which they sink and self-assemble into 3D spheroids10.
In this study, we present a device and methodology for generating agarose microwells using a micropattern mold in a simple, efficient, reproducible, and low-cost manner.
The use of this stamp as a mold to generate microwells in agarose, aided by gravity, aims to enhance cell interaction within the microwell and cellular organization, generating 3D structures (spheroids/organoids) in vitro in a simple, efficient, reproducible, and low-cost manner, thus saving research time and laboratory resources.
Protocol
This protocol follows the guidelines of the Human Research Ethics Committee of our Institution CEUA-FMUSP: 1699/2021, approved on September 8, 2021 -"Isolation and Encapsulation of Porcine Pancreatic islets" and is part of the Thematic Project of our Cell and Molecular Therapy NUCEL Group (www.usp.br/nucel), FAPESP Grant No. 2016/05311-2, entitled: “Regenerative Medicine aiming at therapy of chronic-degenerative diseases (cancer and diabetes)”.
1. Fabrication of the stamp device
NOTE: This Stamp is custom-made by the NUCEL group (https://w3nucel.webhostusp.sti.usp.br/). The stamp device was developed using the referenced software, widely recognized for its precision and advanced three-dimensional modeling capabilities.
- Ensure that the designed prototypes feature 663 micropins arranged to enable the generation of an equivalent number of three-dimensional cultures, such as spheroids or organoids. To ensure the stability of the stamp on T6 tray surfaces, position five support points strategically along the device's circumference.
- Ensure that the micropins have a conical design with adjustable dimensions, ranging from 1 to 3 mm in length. The base diameter of the cone varies between 0.7 and 1.5 mm, while the taper angle ranges from 5° to 10°.
- Make sure that the "stop" structure of the device, which defines the depth of the micropins, has an adjustable length of 10 to 19 mm and a diameter of 20 to 40 mm, with micropins uniformly distributed across its surface.
- Additionally, design the device to have an ergonomic handle, suitable for one-handed operation, to enhance practicality and precision during use.
NOTE: To facilitate large-scale production of the device, the digital file in .stl format is provided, being essential for the 3D printing process. - Simply slice it using appropriate software and print it with a compatible 3D printer. Use the following printing dimensions: X - 68 mm; Y - 120 mm; Z - 150 mm, ensuring an ideal balance between functionality and structural quality.
- For the device's fabrication, use a photocurable 3D printer, which is compatible with the UV-curable polymer resin that must have high resolution and uniform surface finish, essential characteristics for optimal device's performance.
- Slice the digital model for printing using software compatible with the selected 3D printer.
2. Preparation of agarose microwells
- Prepare the agarose solution.
- Weigh the pure agarose powder and dissolve it in 1x phosphate buffered saline (PBS) to achieve a final concentration of 1-2% (w/v).
- Heat the solution in a microwave or water bath until fully dissolved, ensuring it is homogeneous and transparent.
NOTE: Avoid overheating or excessive reheating, as this can degrade the agarose. Do not prepare large volumes of stock solution. - Allow the solution to cool to ~40 °C, the ideal temperature for pipetting .
- Prepare the custom-made stamp.
- Wash the stamp with a soft-bristle sponge and distilled water to remove residues.
- Expose the cleaned stamp to UV light for 5-10 min to ensure sterility.
- Form the microwells.
- Pour/pipette ~3 mL of the 40 °C agarose solution into each well of a 6-well plate, ensuring a uniform depth of 2-3 mm.
- Position the custom-made stamp in the center of the well while the agarose is still warm.
NOTE: The stamp has lateral supports that ensure alignment and stability, eliminating the need for manual support. Each stamp creates ~663 microwells (~2 mm in height) per well. Ensure there are no bubbles on the micropins; if bubbles form, gently move the stamp to eliminate them. - Allow the agarose to solidify for 5-10 min at room temperature without moving the plate.
NOTE: Do not disturb the agarose during solidification to avoid variations in the microwell structure. - Remove the stamp with gentle back-and-forth motions to release the vacuum without damaging the microwells.
NOTE: This movement facilitates air entry, allowing smooth stamp removal without deforming the gel. The microwells must remain intact. - Wash the microwells 3x with PBS solution to remove residues.
- Expose the plate to UV light for 10 min to eliminate microbial contamination.
- Add 3 mL of culture medium and incubate the plate in a CO2 incubator at 37 °C overnight.
NOTE: This incubation verifies the absence of contamination before cell seeding.
3. Cell seeding in microwells
- Prepare the cell suspension.
- Collect the cells via trypsinization or another dissociation method to obtain a homogeneous suspension.
- Count the cells and adjust the concentration according to the desired number per microwell.
NOTE: To seed 5,000 cells per microwell in 663 microwells, prepare a suspension containing at least 3.315 × 106 total cells. Optimize the cell concentration to prevent central necrosis and to ensure adequate nutrient and oxygen diffusion.
- Seed the cells.
- Pipette ~3 mL of the prepared cell suspension over the microwells, ensuring even distribution by gently swirling the plate.
- Allow the cells to settle by gravity (~10-15 min) or perform gentle centrifugation at 100 × g for 5 min.
NOTE: Centrifugation can be used to ensure the cells concentrate in the microwells. - Transfer the plate to a CO2 incubator at 37 °C with a humidified atmosphere.
4. Maintenance of 3D cell cultures
- Replace the culture medium.
- Change the medium every 2-3 days, removing 50% of the old medium and adding fresh medium.
NOTE: Ensure the microwells remain submerged throughout the culture period to prevent dehydration.
- Change the medium every 2-3 days, removing 50% of the old medium and adding fresh medium.
- Monitor the formation of 3D cultures.
- Continue culturing until the 3D structures are fully formed and compact (e.g., 2-3 days for primary pancreatic islet cultures).
NOTE: For cultures requiring a longer formation time, perform partial medium changes every 2-3 days.
- Continue culturing until the 3D structures are fully formed and compact (e.g., 2-3 days for primary pancreatic islet cultures).
5. Collection and characterization of 3D structures
- Collect the spheroids/organoids.
- Remove the old medium and collect the 3D structures via vigorous pipetting using a wide-diameter or cut pipette tip to prevent compression or disaggregation.
NOTE: Vigorous pipetting must be controlled to dislodge the aggregates without deforming them.
- Remove the old medium and collect the 3D structures via vigorous pipetting using a wide-diameter or cut pipette tip to prevent compression or disaggregation.
- Prepare for analysis.
- Use the collected spheroids/organoids immediately or fix them for future analysis.
NOTE: The association and stability of spheroids depend on cell type, formation time, and culture conditions. Take care is essential to avoid dissociation.
- Use the collected spheroids/organoids immediately or fix them for future analysis.
Results
The cell culture used in this study was derived from porcine pancreatic islets. The islet preparation used in this study had an 80 ± 5% purity based on dithizone staining, and >80% islet cell viability based on the detection of fluorescein diacetate in live cells or propidium iodide in dead cells (the Live/Dead fluorescent method). Ensure that the porcine pancreatic islet preparation is at least 80% pure (e.g., by dithizone staining) and >80% viable. Upon isolation, maintain adherent cultures in CMRL 1066 medium, supplemented with 1 mM L-glutamine, 0.2% ciprofloxacin, and 10% fetal calf serum (FCS), at 37 °C and 5% CO2, as previously described11,12.
In the present study, the effectiveness of a stamp-based system for generating microwells in agarose molds was thoroughly evaluated (Figure 1). The stamp design, shown in Figure 2, demonstrates the precision of the micropins and their placement, facilitating consistent alignment and handling during the microwell formation process. This ensured uniform microwell patterns across the entire plate, a critical feature for achieving reproducible 3D cell cultures.
The process of microwell formation using the stamp is detailed in Figure 3, in which the stamp's ability to create uniform microwells is visualized. The results confirm that multiple stamps can be used simultaneously, streamlining the preparation process. Microscopic analysis of the agarose molds reveals concave, standardized microwell shapes, a crucial factor in controlling the aggregation of cells into 3D structures. The uniformity of the microwells significantly contributes to the reliability and reproducibility of 3D culture formation, as demonstrated by the consistent results obtained across various experiments.
Figure 4 illustrates the time-dependent development of 3D structures from the time of cell inoculation (0 h) throughout several time points (6 h, 24 h, 48 h, and 72 h). Cell compaction into aggregated/compact 3D structures occurred gradually, with significant aggregation observed by the 48 h mark. This timeline may vary depending on the cell type, as evidenced by the use of primary porcine pancreatic islets in this study, which formed compact and functional 3D structures within 48 h. This demonstrates the effectiveness of the protocol in generating organized 3D cultures.
Figure 5 highlights the stability and viability of the 3D structures after being removed from the agarose microwells. The 3D structures retained their conformation post removal (Figure 5A), confirming the robustness of the methodology. Furthermore, the live/dead assay (Figure 5B,C) indicated that the cells within the structures remained viable, as shown by the green staining of live cells. This finding confirms that the protocol not only facilitates the formation of well-organized 3D structures, but also preserves cell viability, rendering it a reliable and efficient approach for 3D cell culture applications.
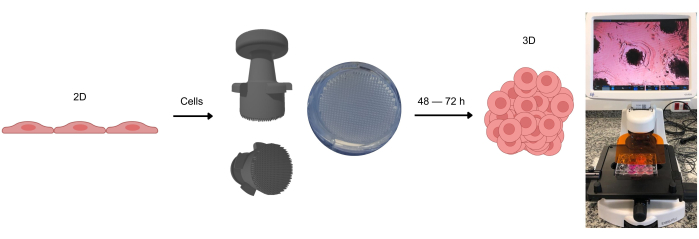
Figure 1: The process of forming 3D cell cultures. Cells initially cultivated in 2D are detached from the adherent surface and placed into agarose microwells. These agarose microwells are created using a stamp device, which allows the formation of these microwells after the agarose gel solidifies at room temperature. When observed under optical microscopy, the cells can be seen distributing homogeneously across the microwells. After approximately 2-3 days (depending on the cell type), the formation of spheroids or organoids can be observed. Please click here to view a larger version of this figure.

Figure 2: Stamp device. Representative images of the stamp device from (A-C) from three different perspectives, illustrating its main features, namely: 663 micropins, plate supports, and manual handling support. Please click here to view a larger version of this figure.

Figure 3: Step-by-step representation of the microwell-mold production process. (A) Stamp device; (B) positioning of the stamp device in the center of the well containing liquid agarose; (C) agarose polymerization; (D) agarose microwells molded by the stamp device; (E) demonstration of the production using three stamp devices in the same plate (P6); (F) optical microscopy images of the microwells at 4x magnification (scale bar = 1,000 µm); (G) optical microscopy images of the microwells at 10x magnification (scale bar = 400 µm). Please click here to view a larger version of this figure.
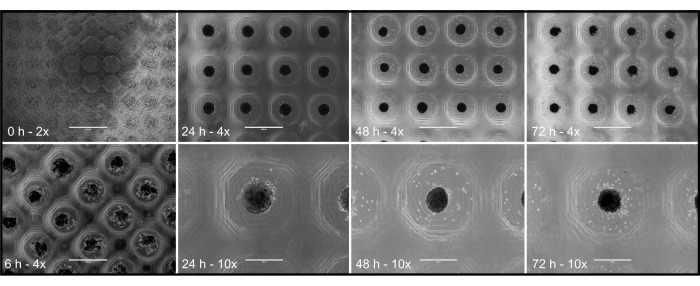
Figure 4: Optical microscopy images of primary pancreatic islet porcine culture. Images at 2x (scale bar = 2,000 µm), 4x (scale bar = 1,000 µm), and 10x (scale bar = 400 µm) magnification, at the following time points: 0 h, 6 h, 24 h, 48 h, 72 h. Please click here to view a larger version of this figure.

Figure 5: Live/Dead assay. Representative images showing the viability of spheroids from primary cultures of porcine pancreatic islet cells that were generated in agarose microwells and collected after 72 h (3 days) of 3D cultivation using the improved stamp device. Fluorescence optical microscopy images were obtained. Green staining represents viable cells labeled with fluorescein diacetate, while red staining indicates non-viable cells labeled with propidium iodide. The images confirm that the spheroidal structure of the 3D culture is maintained and remains viable after removal from the microwells. Scale bar = 400 µm. Please click here to view a larger version of this figure.
Discussion
Although various 3D culture protocols exist in the literature, a study conducted by Wassmer et al.13 tested several methodologies for generating 3D structures using pancreatic islets. The authors observed that native islets and self-aggregated spheroids exhibited considerable heterogeneity with respect to size and shape and were larger than those obtained using other methods. Based on their findings, they concluded that spheroids can be generated using different techniques, each with its own advantages and disadvantages. For islet cell aggregation, the recommended methods are the hanging drop technique, agarose microwell plates, or the Sphericalplate 5D.
There are devices for generating agarose microwells that have been successfully employed in the literature. For example, Stuart et al.14 showed promising results using a device, but it presented certain disadvantages that need to be addressed. These include the limited number of microwells it can produce, a reduced working volume due to the small mold area, and its design as a negative mold, which complicates handling and increases the risk of breakage and distortion of the microwells.
A stamp-like device for generating spheroids was developed by Charelli and colleagues15 using 3D printing technology. Capable of producing up to 4,716 spheroids per 6-well plate, the device was created through stereolithography with photocurable resin. The resulting device featured cylindrical micropins, each 1.3 mm in height and 650 µm in width. This method enabled the rapid formation of uniform spheroids, including co-cultured spheroids, with consistent shape and size. Despite its advantages, several challenges must be addressed to improve its usability and performance. A significant issue is the lack of manual support, which makes the device difficult to handle and prone to instability during the mold formation process. This instability often leads to breakage or cracking of the agarose gel, compromising its structural integrity. Additionally, the device suffers from the lack of plate support, meaning it cannot be securely anchored to the well plate. As a result, the stamp must be manually suspended until the agarose solidifies, introducing variability and affecting the uniformity of the microwell formation. These limitations reduce the reproducibility and overall quality of the resulting spheroids.
Our improved stamp/protocol allows for an efficient and low-cost method, generating 3D spheroids and organoids in agarose microwells, being notable for its simplicity and reproducibility. Inspired by the work of Decarli and colleagues16, our group used the non-adhesive agarose hydrogel methodology to develop a new stamp device. As a result, we addressed issues such as handling difficulties and inconsistencies in microwell formation. The stamp developed through our methodology incorporates these improvements, making it more user-friendly, efficient, and highly reproducible.
Regarding modifications, cell density can be adjusted for different cell types and experimental purposes. A higher cell seeding density may be required for cells with low proliferation rates or limited cell interaction, while a lower density might favor better structural organization for certain cell types. If issues arise with spheroid formation, small modifications such as swift centrifugation of the plates may help cells settle properly at the bottom of the microwells.
One of the critical steps of the protocol is the accurate and uniform seeding of cells into the microwells. Homogeneous cell distribution is essential to ensure that the formed structures have consistent sizes and uniform properties, directly impacting the quality of the results. The collection of 3D cultures is another crucial point. This step requires extreme care to avoid destroying the structures during retrieval, requiring appropriate pipetting techniques to preserve the integrity of the spheroids and organoids.
The size of the spheroid will vary depending on the cell type used. Additionally, the number of cells per spheroid also varies and must be optimized based on the specific cell line or primary cells used. It is important to note that excessively high cell density within a spheroid has been shown to cause the development of a necrotic core, as reported in the literature. This necrotic core results from inadequate oxygen and nutrient diffusion to the innermost cells, which may compromise the overall viability and function of the spheroid17.
Despite the advantages of simplicity and low cost, a significant limitation of the method is the potential variability in spheroid collection, especially in experiments requiring larger and more complex structures. In such cases, the use of more specialized collection tools or even methodological adaptations may be necessary to optimize the process and reduce material loss. Obtaining very small spheroids may also complicate manipulation and collection, requiring strict protocol control.
The association and dissociation of spheroids/organoid vary significantly depending on the cell type used, as well as the number of days required for structure formation, the culture medium employed, and the need for possible agitation. These factors are intrinsic to the specific characteristics of each cell type. However, to minimize cell dissociation, it is essential to strictly follow the precautions outlined in the methodology, such as gentle handling during medium changes and proper maintenance of culture conditions.
Compared to other methods, such as rotary cultures or magnetic beads, this protocol stands out for its greater simplicity, eliminating the need for specialized equipment and ensuring a more reproducible production of 3D spheroids and organoids, rendering it a practical alternative for a wide range of research purposes. According to the results previously described in the literature13, agarose microwells facilitate the generation of 3D spheroid structures of insulin-producing cells with higher viability and insulin secretion rates compared to other commercial models, such as the Sphericalplate 5D.
The versatility of this method, combined with its low cost, makes it ideal for various research areas, such as cell biology, oncology, drug development studies, and toxicology. It offers an effective solution for in vitro 3D modeling, facilitating more precise studies on cell behavior, cell interaction, drug response, and therapy development.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
We are especially grateful to the excellent technical assistance provided by Zizi de Mendonça (School of Medicine, University of São Paulo, Brazil). This work was supported by grants from the following Brazilian research agencies: BNDES 09.2.1066.1, CAPES (PVE process number 88881.068070/2014-01), CNPq (grant numbers 457601/2013-2, 401430/2013-8, and INCT-Regenera number 465656/2014-5), FAPESP (Thematic project number 2016/05311-2), FINEP 01.08.06.05 and the Ministries of Science and Technology (MCTI) and Health (MS-DECIT).
Materials
| Name | Company | Catalog Number | Comments |
| 31L Microwave | Electrolux | 78965840 6699 9 | Equipment used to heat the agarose solution, facilitating its dissolution and ensuring greater homogeneity. It allows the solution to reach the ideal liquid state for the formation of the wells. |
| 3DFila Gray Opaque Photosensitive 3D Resin | UV-curable polymer resin | ||
| 3D Printer - Creality Halot One | Creality | N/A | 3D printer used for printing the stamp device |
| Agarose | UNISCIENCE | UNI-R10111 | To form the gel, dissolve 1 to 2% in Saline Phosphate Buffer (PBS) or appropriate medium. |
| Autodesk Fusion 360 | 3D modeling | ||
| BB15 CO2 Incubator | Thermo Fisher | 51023121 | Equipment used to incubate cultured cells in a suitable and controlled environment. |
| Chitubox | Chitubox | N/A | Software used for slicing the part for printing |
| Class II Biological Safety Cabinet | Grupo VECO | N/A | Ensures a sterile environment for performing cell culture within established parameters and protocols. |
| Culture medium | USBiological/Life Sciences | C5900-03A | Contains additives for proper cell cultivation. |
| Culture plates (P6) | SARSTEDT | 1023221 | Used to shape the agarose and culture the cells. |
| Erlenmeyer Flask (25 mL) | Laborglas | 91 216 14 | A container used for dissolving 1–2% agarose in Phosphate Buffered Saline (PBS) or another suitable medium, typically heated in a microwave. |
| Falcon 15 mL Polystyrene Centrifuge Tube | Corning | 352099 | Used to keep cells in suspension and perform possible dilutions. |
| Fetal bovine serum (FBS) | Vitrocell Embriolife | 005/19 | Additive for culture medium. |
| PBS solution (Saline Phosphate Buffer) | Lab made | N/A | Diluted 1x with MiliQ ultrapure water. Used to dissolve agarose 1 to 2% and to wash wells already produced. |
| Reagent bottle with blue cap - Schott | Laborglas | 21801545 | Used for preparing and storing culture medium. |
| Stamp device | NUCEL Group | N/A | Link- This link provides access to the .stl file of the stamp device. Simply slice it using appropriate software and print it with a compatible 3D printer. https://drive.google.com/drive/folders/1gTYComnJWzHpN6ZKOyK EChKS3Qns0rOA?usp=sharing |
| Treated culture flask with filter 25 cm² | Corning | 430639 | Used for the cultivation and maintenance of adherent cells. |
| Trypsin | Merck | 07-07-9002 | For dissociation of cells before seeding. |
| Ultra violet light (UV) | N/A | N/A | Used to sterilize the stamp and plates. |
References
- Lian, J., Yue, Y., Yu, W., Zhang, Y. Immunosenescence: a key player in cancer development. J Hematol Oncol. 13 (1), 151 (2020).
- Wang, F., et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 95 (25), 14821-14826 (1998).
- Dzobo, K., Dandara, C. The extracellular matrix: its composition, function, remodeling, and role in tumorigenesis. Biomimetics. 8 (2), 146 (2023).
- Sutherland, R. M., McCredie, J. A., Inch, W. R. Growth of multicell spheroids in tissue culture as a model of nodular carcinomas. J Natl Cancer Inst. 46 (1), 113-120 (1971).
- Lancaster, M. A., Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 345 (6194), 1247125 (2014).
- Bialkowska, K., Komorowski, P., Bryszewska, M., Milowska, K. Spheroids as a type of three-dimensional cell cultures-examples of methods of preparation and the most important application. Int J Mol Sci. 21 (17), 6225 (2020).
- Razian, G., Yu, Y., Ungrin, M. Production of large numbers of size-controlled tumor spheroids using microwell plates. J Vis Exp. (81), e50665 (2013).
- Andersen, T., Auk-Emblem, P., Dornish, M. 3D Cell culture in alginate hydrogels. Microarrays (Basel). 4 (2), 133-161 (2015).
- Hou, S., et al. Advanced development of primary pancreatic organoid tumor models for high-throughput phenotypic drug screening. SLAS Discov. 23 (6), 574-584 (2018).
- Napolitano, A. P., et al. Scaffold-free three-dimensional cell culture utilizing micromolded nonadhesive hydrogels. Biotechniques. 43 (4), 494-500 (2007).
- Maria-Engler, S. S., et al. Co-localization of nestin and insulin and expression of islet cell markers in long-term human pancreatic nestin-positive cell cultures. J Endocrinol. 183 (3), 455-467 (2004).
- Mantovani, M. C., et al. Immobilization of primary cultures of insulin-releasing human pancreatic cells. Islets. 1 (3), 224-231 (2009).
- Wassmer, C. H., et al. Engineering of primary pancreatic islet cell spheroids for three-dimensional culture or transplantation: a methodological comparative study. Cell Transplant. 29, 963689720937292 (2020).
- Stuart, M. P., et al. Successful low-cost scaffold-free cartilage tissue engineering using human cartilage progenitor cell spheroids formed by micromolded nonadhesive hydrogel. Stem Cells Int. 2017, 7053465 (2017).
- Charelli, L. E., Dernowsek, J. A., Balbino, T. A. Generation of tissue spheroids via a 3D printed stamp-like device. J Vis Exp. (188), e63814 (2022).
- Decarli, M. C. Micromold for the production of cellular spheroids and use. , (2019).
- Riffle, S., Pandey, R. N., Albert, M., Hegde, R. S. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer. 17, 338 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved