Method Article
Isolation and Analysis of Aortic Arch and Root Lesions in an Atherosclerotic Mouse Model
* These authors contributed equally
In This Article
Summary
This protocol provides a comprehensive methodology for the quantitative analysis of atherosclerotic plaques to facilitate more in-depth research into the mechanisms and progression of atherosclerosis.
Abstract
Atherosclerosis, a leading cause of cardiovascular diseases, necessitates a detailed examination of lesion development and progression. This study introduces a comprehensive protocol for the isolation and histological analysis of aortic arch and root lesions in a widely used atherosclerotic mouse model, low-density lipoprotein receptor knock-out (Ldlr-/-) mice. The aortic arch and root are key sites for atherosclerotic lesions, and their examination is critical for assessing the onset, progression, or regression of atherosclerosis, predicting cardiovascular event risks, and identifying potential therapeutic targets. This protocol outlines methods for quantifying atherosclerotic burden in the aortic arch and root, including tissue isolation, fixation, Oil Red O staining, aortic root sectioning, Hematoxylin and Eosin (HE) staining, Verhoeff-Van Gieson (VVG) staining, and image analysis. Oil Red O staining measures plaque area in the aortic arch, evaluating the severity of atherosclerosis, while HE staining of the aortic root reveals plaque components such as the lipid core and fibrous cap, facilitating the assessment of plaque stability and rupture risk. VVG staining can stain collagen fibers within tissues, providing further insights into plaque composition and related information. This thorough analysis offers valuable insights into the mechanisms of lesion development and may guide the creation of novel therapeutic strategies for preventing and treating atherosclerosis.
Introduction
Cardiovascular diseases, particularly atherosclerosis, have emerged as a significant health burden and a primary cause of death worldwide1,2. Atherosclerosis is a chronic progressive inflammatory disease characterized by the gradual accumulation of lipids and the formation of plaques in the arterial wall, ultimately leading to the narrowing of the arterial lumen and potentially to the rupture of plaques, triggering acute cardiovascular events such as myocardial infarction and stroke1,2,3. Given its profound impact on human health, there is a pressing need to understand the mechanisms underlying atherosclerosis and develop effective therapeutic strategies.
In recent years, animal models have played a crucial role in advancing our understanding of atherosclerosis. Among various species, mice have emerged as a preferred model due to their rapid reproduction, low maintenance costs, and the availability of advanced genetic manipulation techniques4,5. In particular, LDL receptor knockout (Ldlr-/-) mice and ApoE-/- mice have been extensively used to mimic human atherosclerosis, as they exhibit similar pathophysiological characteristics4,5,6,7,8,9.
Atherosclerotic lesions in mice can occur in various locations of the aorta, but they are particularly prone to develop in areas closely associated with hemodynamics, such as the aortic root, aortic arch, and brachiocephalic trunk, while the descending aorta is relatively less affected10. To accurately evaluate the atherosclerotic lesion burden in mouse models, evaluate the presence, size, and stage of plaques, and thereby investigate the impact of different drugs or factors on the onset, progression, and regression of atherosclerosis, a combination of histological staining techniques and imaging analysis is essential11. Oil Red O staining, a well-established method, specifically stains neutral lipids and lipoproteins12, providing a direct visualization of plaque formation in the aortic arch13. Meanwhile, Hematoxylin-Eosin (HE) staining of the aortic root not only delineates the plaque area but also provides detailed structural features, such as the fibrous cap and lipid necrotic core. These details are crucial for assessing plaque stability and predicting the risk of plaque rupture11. Together, these techniques facilitate a comprehensive assessment of atherosclerotic lesion severity and progression.
This protocol took C57BL6/J Ldlr-/- mice fed with Chow diet and Western diet as examples, aiming to provide a detailed step-by-step guide for assessing the atherosclerotic lesion burden in mice using Oil Red O staining of the aortic arch and HE staining of paraffin-embedded aortic root sections, followed by image analysis. The protocol covers all aspects of aortic isolation and fixation, paraffin embedding and sectioning, staining procedures, and image analysis while incorporating operational details and considerations for key steps, ensuring the reproducibility and reliability of the results. By following this protocol, researchers can accurately and efficiently evaluate the efficacy of therapeutic interventions and gain insights into the mechanisms underlying atherosclerosis.
Protocol
All animal protocols used in this study were approved by the Ethics Review Committee for Animal Experimentation of the Shanghai University of Sport.
1. Preparation of reagents and dissection tools
- Sterilize dissection tools, including fine scissors, straight forceps, curved forceps, spring scissors, and pins, by autoclave in advance.
- 75% ethanol: Mix 75 mL of Anhydrous ethanol with 25 mL of ddH2O.
- 1x Phosphate buffered saline (PBS): Dissolve 0.01 M PBS powder in 2 L of ddH2O.
- 4% paraformaldehyde (PFA) solution: Heat 800 mL of 1x PBS to approximately 60 °C on a magnetic stirrer in a fume hood. Slowly add 40 g of paraformaldehyde powder, stirring continuously. Titrate with 1N NaOH until fully dissolved. Cool to room temperature, adjust the volume to 1 L with 1x PBS, filter, and adjust the pH to 7.2-7.4.
CAUTION: PFA is a hazardous chemical that requires careful handling and strict adherence to safety protocols. - Stocking solution of Oil Red O: Dissolve 2.5 g of Oil Red O powder in 500 mL of 100% isopropanol, seal in a brown bottle, and store in a refrigerator at 4 °C for long-term storage.
CAUTION: Isopropanol is a toxic and harmful chemical; therefore, to ensure health and safety, it is essential to wear the necessary personal protective equipment (PPE), including gloves. - Working solution of Oil Red O: Mix the stocking solution and sterile ddH2O at a ratio of 3:2. Prepare fresh for each use and filter the solution with a 0.22 µm sterile syringe filter or large filter paper.
- Victoria Blue'B staining solution: Dissolve 0.5 g Victoria Blue'B in 100 mL of 70% ethanol.
2. Isolation of the aorta and heart
- House all mice at 3-5 animals per cage, with a 12 h light/dark cycle, in a temperature-controlled environment and feed either a standard chow diet (chow) or a western-type diet (WTD) containing 0.2% cholesterol and 21.2% fat. Select C57BL6/J LDLr-/- male mice that are 12 weeks old and weigh between 25 and 30 g. Sacrifice mice in a CO2 chamber.
- Before dissection, place the mice in the supine position (ventral side up) on a foam board covered with 1-2 layers of absorbent paper and fix the limbs with pins.
- Spray 75% ethanol over the abdomen of the mice to clean and moisten the fur.
- Use forceps to grasp the ventral skin of the mice and use fine scissors to cut the skin from the base of the abdomen to the top of the neck.
- Open the abdominal wall, then use forceps to lift the sternum and cut through both the diaphragm and ribs to expose the thoracic cavity.
- Remove the esophagus and trachea, which will enable easier cleaning of the carotid arteries. Remove organs, including the liver, lung, spleen, gastrointestinal tract, and pancreas, while preserving the kidneys located behind the peritoneum and the abdominal aorta beside the spine. When dissecting the mesenteric arteries, be sure to lift the intestines and make incisions away from the aorta to avoid damaging the abdominal aorta.
- To provide a clear observation field, place the mice under a stereomicroscope and align the cold light source with the dissection area.
- Slowly inject a 10 mL syringe filled with PBS with a 0.45 x 15 (II) RWLB needle into the left ventricular apex and observe the dilation of the aorta.
- Gently wipe away the blood and fluid in the dissection area with tissue paper to fully expose the field of view.
- The thoracic aorta is easy to expose. Use it as a breakthrough by using forceps to grasp the diaphragmatic segment attached to the end of the thoracic aorta and spring scissors to cut through the connective tissue between the aorta and the thoracic muscle wall.
- Gently pull the heart with forceps and lift the thoracic aorta, observing the branches of the aortic arch, and dissect the adventitial fat and connective tissue around the aortic arch and its branches upwards along the thoracic aorta.
NOTE: Be careful, keep the aorta intact, avoid tearing or injuring it, and thoroughly remove the surrounding fat and connective tissue. This step requires repeated practice to achieve proficient manipulation. - Dissect the abdominal aorta and common iliac arteries downwards along the thoracic aorta.
- Cut the common iliac arteries 1-2 mm below their bifurcation, trim the small branches of the aorta along the spine to free it, detach the renal arteries near the kidneys, cut off the three branch vessels in the neck from their distal ends, and finally cut the ascending aorta near the heart.
- Before dissecting the aortic arch, place a small, dark-colored gasket with high contrast to the blood vessels underneath it and photograph the aortic arch area.
- Trim the heart tissue and cut the lower half of the heart along a plane parallel to the atria. Fix the trimmed heart (Figure 1A).
3. Fixation and pretreatment of the aortic arch
- Prepare 1 mL of 4% PFA solution in a 1.5 mL centrifuge tube in advance.
- Place the aorta into the tube and fix it for at least 24 h at room temperature.
NOTE: The aorta can be preserved in 4%PFA for up to 1 month without affecting the quality of Oil Red O staining. To maintain its normal configuration, placing the tissue onto a rubber prior to fixation can be a suitable option. - Transfer the aorta to a Petri dish containing PBS to prevent it from drying out. Under a stereomicroscope, use forceps and spring scissors to carefully remove any remaining adventitial fat that was not completely stripped during dissection in order to reduce interference with the quantification of plaques within the aorta.
- Use spring scissors to carefully cut open the aorta along its inner longitudinal axis and then sequentially cut open the three branches of the aortic arch along the lateral side to the level of the curvature of the aortic arch, allowing it to spread out fully (Figure 1C).
- At this stage either use for oil red O staining or store in 4% PFA.
4. Oil red O staining of the aortic arch
- Place the cut-open aorta into a 12-well plate. Add 1 mL of sterile ddH2O to each well, and wash for 5 min on a shaker, repeat 2x.
- Place the 12-well plate with the removed solution in a fume hood and air-dry for 20 min until there are no visible watermarks.
- Add 1 mL of freshly prepared Oil Red O working solution to each well and shake for 20 min on the shaker, then remove the Oil Red O working solution.
- Add 2 mL of sterile ddH2O to each well and wash for 5 min, repeat this step 3x. After that, keep the aorta in ddH2O.
- Place the aorta onto a slide and spread it out under a stereomicroscope. To enhance contrast, place a black rubber pad underneath the slide.
- Place the slide on a white paper to further enhance contrast, with a ruler placed beside it, and take a photo using a camera. To keep the aorta moist, store the treated aorta in a 1.5 mL centrifuge tube containing 1 mL of PBS.
5. Image analysis of the aortic arch
- Open the captured aortic image on a computer equipped with Image J software.
- Select the area with a rectangular box, such as 450 pixels x 900 pixels, which contains the whole aorta, and save it as a new .tiff picture.
- Open the newly saved .tiff picture in Image J. Click Edit > Invert, then select Image and click Type > RGB Stack.
- Go to Image, click Stacks > Stack to Images, and then choose the Green one with the best contrast.
- Go to Image, click Adjust > Brightness/Contrast. Change the minimum value to about 200, minimize the background while maintaining similar backgrounds for each image, and then click Apply.
- Go to Analyze and select Set Measurement. Select Area, Area Fraction, Limit to Threshold and Display Label, then click OK.
- Go to Analyze, select Measurement and copy the result to a spreadsheet.
- Go to Analyze, click Tool > ROI Manager, then choose the Arch part of the aorta with a rectangular box. Go to ROI Manager and select Add [t] and then Measure. Copy the measured results into a spreadsheet.
- Analyze the area measured in the groups of mice based on normality testing. A p < 0.05 indicates that the difference is statistically significant.
NOTE: Select the appropriate statistical method according to the design of the experiment.
6. Paraffin embedding of heart
- Remove the apex of the heart and ensure that the incision is straight and aligned with the direction of the root of the valve (Figure 1B).
- Place each heart sample individually into a paraffin embedding box and mark the box with a pencil.
- In the fume hood, place the paraffin-embedded cassette with tissue in an empty container and rinse the embedding box with running water for 3 min.
- Perform tissue dehydration using ethanol with increasing concentration gradients as follows: 50% ethanol for 2 h, 75% ethanol for 30 min, 85% ethanol for 30 min, 95% ethanol for 30 min, and 100% ethanol for 10 min. Repeat steps by changing ethanol at each step.
- Perform tissue transparency with xylene as follows: 1:1 mixture of xylene and ethanol for 20 min, xylene for 15 min, repeat this 1x by changing the xylene.
- Melt the paraffin wax in the oven at 60 °C in advance. Treat samples with a mixture of xylene and paraffin for 30 min, followed by paraffin soft wax for 2 h and then paraffin hard wax for 1 h.
CAUTION: Ethanol is a flammable and volatile organic solvent. Xylene is moderately toxic and requires protection when used. - Paraffin embedding
- Select the appropriate-sized mold, turn it on, and warm the embedding machine beforehand.
- After adding molten paraffin wax to the mold, extract the tissue from the embedding cassette and position it, with its cut surface facing downwards, at the central bottom of the mold using forceps that have been heated beforehand.
- After the mold has cooled on a freezer table and the paraffin within has solidified, place the indicated embedding cassette lid on top of the mold and fill it with the necessary amount of paraffin.
- Once the paraffin wax has cooled and set, remove the block from the mold and keep it in a refrigerator at 4 °C (Figure 1D).
7. Paraffin sections of aortic root
- Clean off any excess paraffin surrounding the embedding cassette lid, and then securely affix the paraffin block to the clamp seat located on the head of the paraffin slicer. Adjust the block to a slightly offset position from where the slice will be cut, ensuring that the tissue section within the paraffin block is parallel to the cutting edge of the blade. For the first sectioning, set the slice thickness to 10 µm in order to reveal the tissue location.
- When the first incision is found under the microscope, adjust the slice thickness to 6 µm and perform serial sectioning. Cut, slice, and pick sections in the slicer until three intact aortic valves are visible under the microscope, at which point preparation for sectioning begins. Leave one slice at 6 µm intervals for each mouse, and place different slice positions on different glass carriers, for example, section 1-11-21-31, to get a proper overview.
- Float the sections on a spreader containing 37 °C warm water to spread the tissue flat and then pick up the tissue with a slide. After waiting for the water to dry, place the slices on a toaster set to 42 °C overnight.
- The mean plaque area of these 8 sections represents the value of the aortic root plaque area in each mouse. After staining these sections, measure the mean plaque area and plaque area.
8. Hematoxylin Eosin staining
- Place the slices in a dryer at 60 °C for 30 min. In a fume hood, using a staining rack, place sections sequentially in xylene for 10 min (repeat this step once, changing the xylene), 100% ethanol for 5 min (repeat this step, changing the ethanol), 95% ethanol for 5 min, 85% ethanol for 5 min and 75% ethanol for 5 min.
- Rinse with running water for 5 min. Immerse the sections in hematoxylin stain for 8 min and rinse under running water.
- Differentiate the sections with 1% hydrochloric acid alcohol for a few seconds and rinse with running water and observe that the sections turn blue purple under the microscope.
- Immerse the sections in an eosin staining solution for 5 min. Place the sections in 95% ethanol for 7 dips and then in 100% ethanol for 30 s.
- Clear in xylene for at least 30 s. Seal the slides using neutral gum, photograph under a microscope, and save high-resolution images, preferably in .tiff format.
9. Verhoeff-Van Gieson (VVG) staining
- Perform routine dewaxing and rehydration on the paraffin sections, following the same steps as outlined in sections 8.1 and 8.2.
- After rinsing briefly in 70% ethanol, immerse the sections in Victoria Blue'B staining solution for 15 min.
- Differentiate the sections for a few seconds in 95% ethanol. Wash the sections 2x with distilled water.
- Stain the sections with Ponceau staining solution for 5 min by droplet application. Differentiate and dehydrate the sections using 100% ethanol.
NOTE: After Ponceau staining, avoid any contact with water. - Clear in xylene for at least 30 s. Mount the slides with neutral gum and photograph them under a microscope.
10. Image analysis of the aortic root plaque
- Open the image on a computer equipped with Image J software. Select any box tool to box the plaque.
- Go to Image, click Overlay > Add Selection, then Measure to measure the area of the plaque. Copy the measured results into a spreadsheet.
- Analyze the Area measured in the groups of mice based on normality testing. A p < 0.05 indicates that the difference is statistically significant.
Results
The representative results demonstrate the application of the isolation and analysis technique for aortic arch and root lesions in an atherosclerotic mouse model. These results provide clear evidence of the technique's ability to identify and characterize atherosclerotic lesions. For example, histological images with specific stains (e.g., Oil Red O) highlight lipid accumulation, while hematoxylin and eosin (H&E) staining reveals the overall morphology of the atherosclerotic lesions, including the structure of the arterial wall, the presence of lipid cores, and areas of necrosis. By analyzing H&E-stained sections, we can assess the extent of lesion development and structural changes in the aorta, providing an overview of plaque progression and tissue remodeling.
In this protocol, we analyzed the extent of atherosclerotic lesions in 12-week-old C57 Ldlr knockout mice fed a Western-style diet (WTD) for 4 months and Ldlr-/- mice fed a Chow diet as controls.
After 12 weeks of Chow diet and Western diet feeding, Ldlr knockout mice in the Western diet group had significantly elevated body weights compared to the control group. In addition, the lipid levels in the Western diet group were also changed, with significantly higher Plasma triglycerides (TG) and Total cholesterol (TC) levels, where TG and TC were detected by Enzyme-Linked Immunosorbent Assay taken from the tail blood of the mice (Figure 2).
The results of aortic Oil Red O staining in Ldlr knockout mice fed with the western diet, which exhibited severe lipid accumulation and atherosclerotic lesions compared with mice fed with the chow diet, indicate that more lipid deposits on the arteries correspond to more severe atherosclerotic lesions (Figure 3).
In aortic root sections, a larger area of plaque and necrotic cores implies a more severe atherosclerotic lesion. Here, it was shown that the aortic root lesional area and necrotic core are much bigger in Western diet-fed mice than in Chow-diet-fed mice (Figure 4A). VVG staining of paraffin sections of the aortic root was performed to assess the diseased area of the aortic root; elastic fibers were bluish-purple, and collagen fibers usually proliferated to form a fibrous cap and appeared red. VVG staining helps to help us see if there is destruction or reduction of elastic fibers, thickening of the vessel wall, and deposition of abnormal collagen fibers, which may be a sign of inflammation or pathological remodeling. Here, it showed the trends of increasing collagen fibers in the fibrous cap in the aortic root of Western diet-fed mice compared to that in Chow-diet-fed mice (Figure 4B). The quantification of the aortic root lesional area and the necrotic area did show that the Western diet worsens the atherosclerotic status in Ldlr knockout mice (Figure 4C).

Figure 1: Steps for isolation and analysis of aortic arch and root lesions. (A) Isolation of the aorta and heart. (B) Preparation of the aortic root. The heart tissue between the two dotted lines was sliced. (C) Fixation and pretreatment of the aortic arch. (D) Paraffin embedding of the heart. Please click here to view a larger version of this figure.

Figure 2: Changes in body weight and blood lipid levels of Control and WTD group mice fed for 12 weeks. (A) Body weight curve on CD and WTD feeding. (B) Plasma triglycerides (TG). (C) Total cholesterol (TC) (n=4). Results are expressed as mean ± SEM, significance was assessed by Student t-test. ***p < 0.001, **** p < 0.0001. Please click here to view a larger version of this figure.
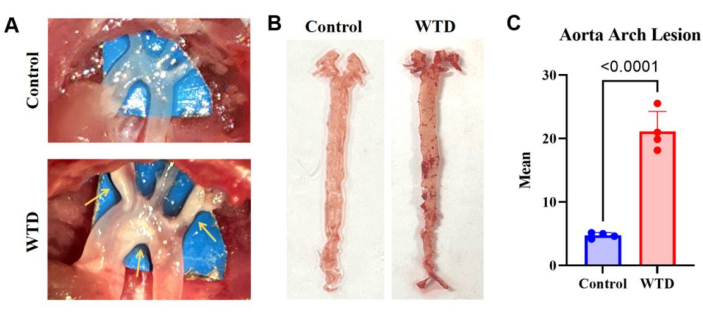
Figure 3: The aortic arch and its Oil Red O staining and analysis. (A) Representative images of atherosclerotic plaques in the aortic arch. The aortic arches of Ldlr-/- mice fed a Chow Diet were observed microscopically with no visible plaques, whereas the aortic arches of Ldlr-/- mice fed a Western diet were observed microscopically with visible plaques. (B) Representative images of aortic oil red O staining. Whole aorta from Ldlr-/- mice fed a Chow diet without lipid accumulation, and whole aorta from Ldlr-/- mice fed a Western diet for 4 months had plaques visible in the aortic arch. (n=4). (C) Mean number for aorta arch lesions. Results are expressed as mean ± SEM, and significance was assessed by Student t-test. ***p < 0.001, **** p < 0.0001. Please click here to view a larger version of this figure.
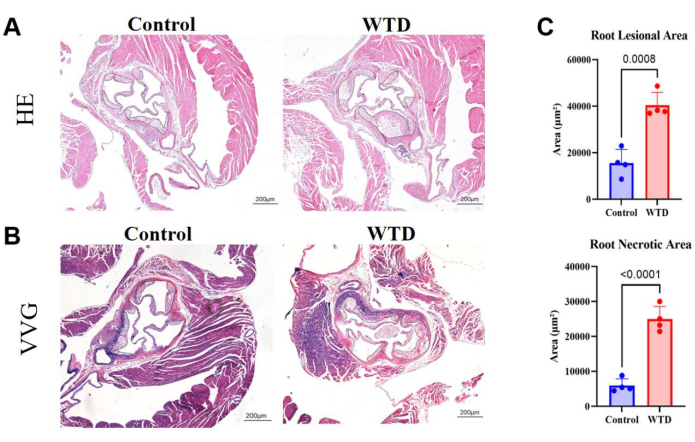
Figure 4: Aortic root sections stained with HE and VVG, with quantification of plaque area and necrotic area. (A) Representative hematoxylin and eosin-stained aortic root sections. Aortic roots of Ldlr-/- mice subjected to Chow diet and Western diet interventions, respectively. (B) Representative Verhoeff-Van Gieson-stained aortic root sections. Aortic roots of Ldlr-/- mice subjected to Chow diet and Western diet interventions, respectively, are circled in black and are atherosclerotic lesions in the mice. (C) The quantification of aortic root lesional area and necrotic area (n=4). Results are expressed as mean ± SEM, and significance was assessed by Student t-test. ***p < 0.001, **** p < 0.0001. Please click here to view a larger version of this figure.
Discussion
Here, we provide detailed information on the methods of aortic sampling in Ldlr knockout mice and quantitative analysis of plaques.
The precision of the dissection procedure is the biggest technical challenge to in vivo aortic stripping in the mouse model of atherosclerosis. Based on our experience, the key points are as follows: (1) use PBS to wash out all the blood in the artery to increase the comparison between aortic arch branches and perivascular fat. (2) Be patient. Due to the small size of the mouse aorta, the procedure must be performed under a body microscope and using high-quality microscopic clippers and forceps to minimize errors in the operation. (3) it is imperative to guarantee that the stripping is initiated precisely and in the right direction to prevent any cuts or damage to the vessel.
Regarding aortic oil red O staining in mice, the peripheral fat of the isolated mouse aorta should be removed microscopically before formal staining, and the integrity of the tissue must be maintained to avoid affecting the staining results. The oil red dyeing process demands precise control over dyeing time; both excessive and insufficient durations can compromise the dyeing effect, resulting in inaccurate outcomes.
In the embedding of mouse aortic roots, the dehydration time of the tissue needs to be controlled to avoid tissue cracking during sectioning. Sections of the aortic root need to be of uniform thickness to ensure uniform staining, and sections that are too thin or too thick can interfere with observation. At the same time, it is necessary to accurately determine where the tricuspid valve appears when viewed under the microscope. The hematoxylin eosin staining process requires precise control of the staining time and the concentration of the staining solution in order to avoid nonspecific staining.
Compared to the existing excellent methods of evaluating atherosclerosis, this protocol provided step-by-step details to dissect both the aortic arch and root, which can help comprehensively evaluate the atherosclerotic status11,13Both hematoxylin-eosin staining and VVG staining can help evaluate the aortic root lesional area, which increases the quantification accuracy of atherosclerosis.
However, there are still some limitations of aortic oil red O staining. For example, oil red staining is primarily used to detect lipids but has poor specificity for other types of cell or tissue components11, which may lead to misclassification. On the other hand, the process of sample fixation and dissection may lead to loss of lipids, thereby affecting the reliability of the staining results. As for hematoxylin-eosin staining of the aortic root, it is limited in differentiating cellular components and may be difficult to identify specific areas of the lesion in cases of complex tissue structure. When performing quantitative analyses, the statistical results of the patch area may also be influenced by the subjective judgment of the observer. In addition, hematoxylin-eosin staining provides primarily morphological information and does not provide insight into cellular function or molecular characterization.
Apart from standard oil red staining and hematoxylin-eosin staining, other techniques for analyzing atherosclerotic plaques include Masson staining, immunohistochemical staining for various antigens, and other methods that can be employed to examine the plaque's composition and dimensions. Oil red O staining of aortic root sections is also widely used to analyze the lipids deposition in the aortic root14. Alizarin Red S staining is a commonly used stain for detecting calcium deposits; it forms a red complex with calcium ions, making it ideal for visualizing calcium deposits15. Furthermore, lipid metabolism can be inferred from serum biochemical markers such as levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), and total triacylglycerol (TG). One of the key contributing factors to the development of atherosclerosis is abnormal lipid metabolism, particularly the relationship between atherosclerosis and cardiovascular disease (ASCVD) and low-density lipoprotein cholesterol (LDL) levels.
As research on atherosclerosis deepens, the future development of quantitative analysis methods will become more precise and diversified. First, the integration of advanced imaging technologies, such as high-resolution MRI, optical coherence tomography (OCT), and ultrasound imaging, will make the quantitative analysis of atherosclerosis more accurate, enabling dynamic monitoring of live mice. This will help in the real-time assessment of plaque progression, stability, and response to treatment. With the advancement of molecular imaging technologies, quantitative analysis will allow for in-depth analysis of inflammatory responses, lipid accumulation, and cellular components within plaques, providing more comprehensive data for the early diagnosis and treatment of atherosclerosis16.
Disclosures
Nothing to declare.
Acknowledgements
This work was supported by Shanghai Frontiers Science Research Base of Exercise and Metabolic Health, the research program of exercise and public health (0831) in Shanghai University of Sport, Shanghai higher education young teachers training funding program (A2-0213-22-0058-5), and Shanghai Municipal Science and Technology Committee of Shanghai outstanding academic leaders plan (21XD1403200) for Longhua Liu.
Materials
| Name | Company | Catalog Number | Comments |
| Adhesion microscope slides(25×75mm) | CITOTEST | Cat# 80312-3161 | |
| Embedding cassette | CITOTEST | Cat# 80106-1100-16 | |
| Eosin Staining Solution | Beyotime | Cat# C0109 | |
| Ethanol | Sinopharm Chemical Reagent Co. | Cat# 10009218 | |
| Hematoxylin Staining Solution | Beyotime | Cat# C0107 | |
| Low-profile disposable blades | Leica | Cat# 14035838925 | |
| Microscope cover glass(24×50mm) | CITOTEST | Cat# 10212450C | |
| Neutral Balsam Mounting Medium | Sango Biotech | Cat# E675007-0100 | |
| Oil red o powder | Sigma-Aldrich | Cat# 1320-06-5 | |
| paraffin with ceresin | Sinopharm Chemical Reagent Co. | Cat# 69019461 | |
| Paraformaldehyde | Servicebio | Cat# G1101 | |
| Phosphate Buffered Saline (PBS, Powder) | Servicebio | Cat# G0002-2L | |
| Ponceau S Staining Solution | EveryLab | Cat# FM024 | |
| Victoria Blue’B | Aladdin | Cat# 2580-56-5 | |
| Xylene | Sigma-Aldrich | Cat# 104-81-4 |
References
- Libby, P., et al. Atherosclerosis. Nat Rev Dis Primers. 5 (1), 56 (2019).
- Herrington, W., Lacey, B., Sherliker, P., Armitage, J., Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 118 (4), 535-546 (2016).
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 11, 117 (2013).
- Gisterå, A., Ketelhuth, D. F. J., Malin, S. G., Hansson, G. K. Animal models of atherosclerosis-supportive notes and tricks of the trade. Circ Res. 130 (12), 1869-1887 (2022).
- Getz, G. S., Reardon, C. A. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 32 (5), 1104-1115 (2012).
- Ilyas, I., et al. Mouse models of atherosclerosis in translational research. Trends Pharmacol Sci. 43 (11), 920-939 (2022).
- Liu, L., Chan, M., Yu, L., Wang, W., Qiang, L. Adipsin deficiency does not impact atherosclerosis development in ldlr(-/-) mice. Am J Physiol Endocrinol Metab. 320 (1), E87-E92 (2021).
- Liu, L., et al. Pparγ deacetylation confers the antiatherogenic effect and improves endothelial function in diabetes treatment. Diabetes. 69 (8), 1793-1803 (2020).
- Zahr, T., et al. Pparγ (peroxisome proliferator-activated receptor γ) deacetylation suppresses aging-associated atherosclerosis and hypercholesterolemia. Arterioscler Thromb Vasc Biol. 43 (1), 30-44 (2023).
- Tang, C., et al. Endothelial ccrl2 induced by disturbed flow promotes atherosclerosis via chemerin-dependent β2 integrin activation in monocytes. Cardiovasc Res. 119 (9), 1811-1824 (2023).
- Andrés-Manzano, M. J., Andrés, V., Dorado, B. Oil red o and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods Mol Biol. 1339, 85-99 (2015).
- Mehlem, A., Hagberg, C. E., Muhl, L., Eriksson, U., Falkevall, A. Imaging of neutral lipids by oil red o for analyzing the metabolic status in health and disease. Nat Protoc. 8 (6), 1149-1154 (2013).
- Chen, P. Y., Qin, L., Simons, M. Imaging and analysis of oil red o-stained whole aorta lesions in an aneurysm hyperlipidemia mouse model. J Vis Exp. (183), e61277 (2022).
- Lin, Y., et al. Practical assessment of the quantification of atherosclerotic lesions in apoe-/- mice. Mol Med Rep. 12 (4), 5298-5306 (2015).
- Bozycki, L., Łukasiewicz, K., Matryba, P., Pikula, S. Whole-body clearing, staining and screening of calcium deposits in the mdx mouse model of duchenne muscular dystrophy. Skelet Muscle. 8 (1), 21 (2018).
- Glaudemans, A. W., et al. Molecular imaging in atherosclerosis. Eur J Nucl Med Mol Imaging. 37 (12), 2381-2397 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved