Method Article
Quantitative Analysis of Mitochondria-Associated Endoplasmic Reticulum Membrane (MAM) Stabilization in a Neural Model of Alzheimer's Disease (AD)
In This Article
Summary
Here, we describe measuring the axonal transport rate of constitutive stabilizers of mitochondria-associated endoplasmic reticulum (ER) membranes (MAMs) by increasing or maintaining neurotoxic β-amyloid (Aβ) generation from Alzheimer's disease (AD) neurons in real-time to serve as a direct and quantitative metric to measure MAM stabilization and aid the development of AD therapeutics.
Abstract
A method to quantitate the stabilization of Mitochondria-Associated endoplasmic reticulum Membranes (MAMs) in a 3-dimensional (3D) neural model of Alzheimer's disease (AD) is presented here. To begin, fresh human neuro progenitor ReN cells expressing β-amyloid precursor protein (APP) containing familial Alzheimer's disease (FAD) or naïve ReN cells are grown in thin (1:100) Matrigel-coated tissue culture plates. After the cells reach confluency, these are electroporated with expression plasmids encoding red fluorescence protein (RFP)-conjugated mitochondria-binding sequence of AKAP1(34-63) (Mito-RFP) that detects mitochondria or constitutive MAM stabilizers MAM 1X or MAM 9X that stabilize tight (6 nm ± 1 nm gap width) or loose (24 nm ± 3 nm gap width) MAMs, respectively. After 16-24 h, the cells are harvested and enriched by a fluorescence-activated cell sorter (FACS). An equal number of FACS-enriched cells are seeded in the 3-dimensional matrix (1:1 Matrigel) and allowed to differentiate into mature neurons for 10 days. Live cell images of the 10-day differentiated cells expressing the RFP-conjugated MAM stabilizers are captured under a fluorescent microscope equipped with a live-cell imaging culture chamber maintaining the CO2 (5%), temperature (37 °C), and humidity (~90%). Toward this end, we performed live-cell imaging and kymographic analyses to measure the motility of free mitochondria labeled with Mito-RFP or ER-bound mitochondria of tight or loose gap widths stabilized by MAM 1X or MAM 9X, respectively, in the most extended neuronal process of each ReN GA neuron which is at least 500 nm long, considering these as axons.
Introduction
Emerging evidence suggests that the specialized Mitochondria-associated Endoplasmic Reticulum Contacts (MERCs), biochemically harvested as Mitochondria-Associated ER Membranes, often referred to as MAMs1,2 play a role in several neurodegenerative diseases, including AD3,4. These MAMs are composed of cholesterol-rich lipid raft-like microdomains in the ER and the outer membrane of mitochondria tethered by a series of proteins that create structural and functional diversities among the MAMs5,6,7. The recently coined MAM hypothesis posits that the increase of MAMs leads to enhanced Aβ production and the pathogenic cascade of AD, including neurofibrillary tangle (NFT) formation, calcium dyshomeostasis, and neuroinflammation3,8. About 5%-20% of mitochondria make physical contact with the ER to form MAMs9. The gap width of MAMs is determined by the smooth and rough ER (sER and rER, respectively). The variable gap width between sER-mitochondria (10-50 nm) and rER-mitochondria (50-80 nm) suggests that the gap width of MAMs has a long spectrum that ranges between tight (~10 nm) to loose (~80 nm)10,11,12,13. MAM gap width determines MAM functions, such as calcium homeostasis and lipid transport1,14. A recent report has shown that the MAMs formed between tightly (~10 nm) connected ER and mitochondria, called full MAMs, are apoptotic. In contrast, MAMs formed between loosely connected (~25 nm) ER and mitochondria, termed defective or medium MAMs, are anti-apoptotic14,15,16. Stabilization of MAMs with a gap width of 6 nm ± 1 nm increased Aβ generation from a novel 3-dimensional (3D) neural culture model of AD. In contrast, the stabilization of MAMs with a gap width 24 nm ± 3 nm has no effect on Aβ generation17. This finding suggests for the first time that regulating the degree of MAM stabilization, but not destabilizing MAMs, is the key to regulating Aβ generation. An attempt to completely destabilize MAMs may have unwanted consequences because MAMs maintain several cellular events critical for cell survival12.
The modulation of MAMs is an emerging area of research with potential implications for various disorders, including cancer, metabolic disorders, and neurodegenerative diseases18. Despite the availability of many MAM modulators, no major attempt has so far been taken to test their abilities to destabilize MAMs and lower AD pathology, primarily because the structural diversities of MAMs make them a highly complex system to target for drug discovery. But, the newly developed structural systems pharmacology, which considers the specific properties of the drug targets and their environment18,19 should overcome the difficulties and develop highly potent drugs targeting MAMs or MAM-associated proteins in AD. However, the search for an effective modulator of MAM stabilization requires methods to quantify the degree of MAM stabilization precisely. Traditional techniques like electron microscopy (EM) or super-resolution microscopy have limitations in determining MAM stabilization. Overcoming these challenges would likely require the development of novel, more dynamic imaging techniques or biochemical assays that can provide quantitative measures of MAM stabilization in living cells. Focused Ion Beam-Scanning Electron Microscopy (FIB-SEM) of primary neurons revealed that the ER tends to form a network around mitochondria likely to limit mitochondrial motility20,21. The disruption of mitochondrial transport systems, either retrograde, anterograde, or both, had a profound impact on synaptic and neuronal function22. Thus, the novel live-cell imaging and kymography-based analysis of axonal velocity of ER-bound mitochondria described here as a metric to quantitatively measure MAM stabilization will facilitate the identification of MAM modulator(s) that can switch the MAM stabilization threshold to one that maintains or possibly lowers as opposed to increases Aβ generation.
Protocol
AD neural culture models: This study used neurons derived from human neural progenitor ReN cells [naïve ReN (Millipore)] or ReN cells expressing familial AD (fAD) mutations in the amyloid precursor protein (APP) gene (APPSwe/Lon), ReN GA cells. ReN-GA three-dimensional (3D) culture system recapitulates AD pathology, namely Aβ oligomer- driven neurofibrillary tangles (NFTs) 23,24. Naïve ReN cells are commercially available. ReN GA lines were obtained from Dr. Doo Y. Kim, Associate Professor, Massachusetts General Hospital (MGH)23,24,25.
Expression plasmids: AKP1 (34-63) and ER-targeting sequence of Ubc 6 (283-303) proteins linked directly with RFP (Mito-RFP-ER denoted as MAM 1X) or contain a 9 amino acid linker (Mito-9X-RFP-ER denoted as MAM 9X) designed to stabilize MAMs of 6 nm ± 1 nm or 24 nm + 3 nm gap widths, respectively15,26 (Figure 1A).
1. Electroporation
- Transfecting the cells with MAM stabilizers (1-2 h)
NOTE: Follow the protocol described by the nucleofector kit's manufacturer (Table of Materials). Before beginning, prepare 6-well glass-bottom culture dishes pre-coated with DMEM/F12 containing 1% Matrigel (henceforth referred to as basement membrane matrix [BMM]). Use 2 mL of BMM to coat each well. Incubate for at least 1 h at 37 °C. BMM, media, pipette tips, and pipettes should all be prechilled before mixing to prevent BMM from solidifying.- Aspirate the BMM mixture. Replace with 2 mL of room temperature (RT) DMEM/F12 in each well.
- Combine Nucleofector with Supplement 1 in a 4.5:1 ratio (82 µL:18 µL of Nucleofector/Supplement ratio for 100 µL of solution). For each desired transfection, 100 µL of Nucleofector is needed.
- Vortex DNA. Then, add 1-5 µg of DNA (per treatment) in 1 mL microcentrifuge tubes for each transfection.
- Use acustase to harvest a plate of healthy ReN-GA cells.
NOTE: Plate cells in expansion media (Table 1) in 100 mm tissue culture dishes and wait until the confluency reaches 70-80%.- Aspirate the existing media and wash once with 10 mL of PBS.
- Add 1 mL of acutase directly to cells and incubate for 5-10 min at 37 °C.
- Dislodge cells from the dish by gently tapping the side of the dish. Check under a microscope to make sure cells are loosened and freely flowing.
- Neutralize acutase with 10 mL of DMEM/F12 and transfer to a clean 15 mL tube.
- Centrifuge the required number of cells at 274 x g for 5 min and aspirate the supernatant to remove dead cells. Then, resuspend the pellet in 10 mL of DMEM/F12.
- Count the cells to determine density.
NOTE: In this study, an automated cell counter was used to count the number of cells. Per electroporation, 3-5 X 106 cells are needed (e.g., 5 treatments would require 15-25 X 106 cells).- Take 10 µL of the suspended cells and add to side A of a cell counting chamber. Then add 10 µL to side B.
NOTE: Add the cell suspension by angling the pipette to the side. Avoid bubbles by not pushing past the first stop on the plunger of the pipette. - Take the filled counting chamber to the cell counter and insert side A into the main slot on the front.
- After pressing Measure, note the number of cells per mL.
- Take out the chamber, flip to side B, and repeat steps 1.1.5.2-1.1.5.3.
- Add these two numbers together, then divide by 4 (if not using Trypan Blue), then multiply the number by the milliliters of liquid that the cells are suspended in to get the total number of cells in the suspension.
- Take 10 µL of the suspended cells and add to side A of a cell counting chamber. Then add 10 µL to side B.
- Centrifuge the required number of cells at 274 x g for 5 min and aspirate the supernatant.
- Resuspend pellet in 100 µL of Nucleofector mix per electroporation. (e.g., 500 µL for 5 treatments).
NOTE: Leaving the cells in the Nucleofector solution for longer than 15 min could reduce cell viability and overall efficacy. - Add 100 µL of the cell suspension to one of the tubes containing DNA and mix by pipetting.
- Transfer the DNA mixture into an electroporation cuvette and seal with the provided lid. To avoid creating bubbles, angle the cuvette downward and pipette slowly.
- Select the Nucleofector program appropriate for the device being used. For the device used in this study, use Program A-033 for transfection. To optimize, try all 5 Nucleofector programs to determine the most appropriate one for each cell type.
NOTE: A confirmation of a successful electroporation is visible foam at the top of the mixture. - Immediately, using the provided sterile pipettes, add ~500 µL of the DMEM from the prefilled 6-well plate into the cuvette. Gently mix once and then transfer the electroporated cells and medium to the corresponding well.
NOTE: Following electroporation, the cells are extremely sensitive, so transferring the media quickly and pipetting carefully is essential. - Repeat steps 1.1.8-1.1.11 for all remaining DNA treatments.
- Incubate the cells at 37 °C in the presence of CO2 (5%) overnight.
- The next day, exchange media with fresh differentiation media (Table 2) and allow the cells to differentiate for 10 days. Every 2-3 days, replace with fresh media.
2. Live cell imaging
- Preparation of cells for live cell microscopy (30 min)
- Live cell chamber preparation (before moving cells into the chamber)
- Ensure the CO2 tank and humidifier are attached to the chamber, the valves are open, and the tanks are filled.
- Set the temperature to 37 °C, the CO2 to 5%, and the humidity to 95%. (This may take some time for the levels to equilibrate).
- Place a 6-well plate containing cells in the chamber and adjust the focus of the microscope until the cells become visible.
- Turn on the laser (Table of Materials).
- To capture the RFP signal, excite the fluorophore using a 594 nm laser and use 570-640 nm emission. For GFP, use a 488 nm laser for excitation and 510-540 nm emission.
- Using the built-in fluorescent filter, adjust the signal intensity until the background signal has dissipated (should be nearly solid black).
- Capturing live video of axons (10 h-15 h in total)
NOTE: A Nikon C2 Eclipse Ti2 inverted confocal microscope was used to capture fluorescent images using NIS Element AR software. Use 60x magnification at a resolution of 512 pixels, taking videos at 1 frame per second for 3 min produced cleaner kymographs.- Find a cell expressing RFP biosensors. Export an image of the cell for later reference before taking a video.
- Crop the scanning area to fit around the axon. Using a smaller scanning area reduces processing time of the microscope and makes kymography generation much easier.
- Switch off all lasers to help the software run more smoothly. Please note, when the program is run with all lasers active, not all of the frames are captured.
NOTE: The red fluorescent signal from the soma is much brighter than in the axon. Therefore, the soma is excluded to increase the overall signal intensity in the axon. Move to the Time Measurement tab. - Set the interval to be 1 frame per second and set the overall time at 181 s.
- Click Run.
- Save this file as a .nds file (properly labeled) and repeat the process ~10 times per MAM stabilizers (MAM 1X or MAM 9X).
NOTE: The strength of the laser sometimes produced a noticeable bleaching effect on the RFP signal if scanned for too long. Working quickly to capture videos is important to consider.
3. Post-processing (7 days)
NOTE: To analyze transport and generate kymographs, Fiji ImageJ macros were utilized. Vesicles that moved less than 0.1 mm/s were categorized as stationary. The frequency of particle movement was calculated by dividing the number of particles moving in a given direction (anterograde, retrograde) or not moving (stationary) by the total number of particles analyzed in the kymograph. The time each vesicle spent pausing or moving was calculated by averaging the percentage of time spent in each condition for all vesicles in each neuron analyzed. The frequency distribution for velocity and run length was calculated using only moving vesicles for each experimental condition. The analysis was performed on 100 mm axonal tracts for 3 min.
- Generating a kymograph
- Open the .nds file in Fiji ImageJ.
- Go to the Image tab and click Properties. Record the pixel-to-micron ratio. This is needed for calculation later.
- Click File > Save As > Tiff to save the file as a .tiff into its folder (The macro will automatically save everything generated to this folder).
- Drag the Kymo macro into ImageJ. The code is provided in Supplementary Coding File 1. Click Run.
- Do not hit OK. Switch to the MAX_raw window. Manually left-click along the axon and double-click to end tracking.
NOTE: Make sure to track the axon from the soma so the axon terminal. This way the anterograde and retrograde calculations will be correct. - Press command T or Add on the ROI manager.
- Now click OK. A kymograph will be generated in the folder made previously. (The X axis is axon length in microns, and the Y axis is time in seconds).
- Tracking the kymograph
- Drag the kymograph into Fiji Image J (It should be automatically placed in the folder made previously).
- Drag the Track macro into Image J.
- On the following prompt, click Ok. If resuming work from before, input the number point to continue from and then press Ok.
- Using the Rectangle tool on the Select panel, select an area near the middle of the kymograph that is 60 pixels in height and always 100 µm across. To do this, divide 100 by the pixel-to-micro ratio that was recorded in step 3.1.2.
- After selecting an area, press Ctrl T to add i t to the ROI and save the ROI to the folder being worked in (press More in the ROI menu and then Save).
NOTE: Inverting the selected area by pressing Ctrl+Shift+I will make it easier to track. - To track an individual vesicle's movement, hold Ctrl while left clicking from top to bottom.
- To be done, hold Ctrl while right clicking and a window will pop up showing where the axons are tracked. If this looks good, press Ok.
- To continue and select another vesicle, click Yes and repeat the process from steps 3.2.6-3.2.8. Once every visible vesicle has been tracked, press No. This will automatically save the hand tracked overlay to the same folder.
- Measuring the kymograph data
- Create a new folder. Drag every generated text file into the folder.
- Open the kymograph file and record the dimensions.
- Drag the Measure macro into Image J.
- Enter the micron-to-pixel ratio recorded earlier into PixelScale.
- Change the Speed Limit Low to 0.1. (Vesicles moving below this limit are considered stationary).
- Enter the kymograph dimensions previously recorded. The summary file will automatically be generated in the created text folder. Each summary fill will contain %Time Traveled, Overall Speed (µm/s), Total Distance, Average Segment Traveled, Number of Times Stopped, and Number of Times Reversed. The code for generating, tracking and measuring the kymograph data is provided as Supplementary Coding File 1.
Results
Live-cell imaging and kymographic analyses were performed to measure the motility of free mitochondria labeled with Mito-RFP or ER-bound mitochondria of tight (6 nm ± 1 nm) or loose (24 nm ± 3 nm) contact widths stabilized by MAM 1X or MAM 9X, respectively, in the longest neuronal process of each ReN GA (AD) or ReN (naïve) neuron which is at least 500 nm long, considering this as an axon (Figure 1 and Figure 2). Frequencies of movements (overall, retrograde, and anterograde) were calculated by dividing the number of moving or stationary RFP-labeled puncta (MAMs) by the total number in the kymographs (Figure 1A-E). The overall axonal velocity of the MAM 1X-labeled ER-bound mitochondria was dramatically decreased by ~50% compared to the Mito-RFP-labeled ER-free mitochondria or MAM 9X-labeled ER-bound mitochondria (Figure 1B). Quantitative analysis also revealed dramatic differences between the overall and retrograde movements of the MAM 1X-stabilized ER-bound mitochondria compared to the free (Mito-RFP) or MAM 9X-stabilized ER-bound mitochondria. While 53.82% ± 3.3% of the ER-free mitochondria (Mito-RFP) were mobile, only 26.6% ± 3.4% MAM 1X-labeled ER-bound mitochondria were mobile, suggesting that the stabilization of the MAMs significantly reduced overall axonal mobility of mitochondria, tightly associated with ER, compared to mitochondria unbound or loosely bound to the ER (44.79% ± 2.6% of MAM 9X versus 53.82% ± 3.3% of Mito RFP, respectively) (Figure 1C). Consistently, both the retrograde and anterograde movements of MAM 1X-labeled ER-bound mitochondria were significantly lower compared to MAM 9X-labeled or free mitochondria (Mito-RFP) (retrograde: 12.33% ± 2.55% for MAM 1X versus 25.78% ± 2.31% for Mito RFP; anterograde: 14.27% ± 2.81% for MAM 1X versus 28.04% ± 2.48% for Mito RFP) (Figure 1D and E). Table 3 provides the precise axonal velocities of the free Mitochondria or those either tightly or loosely bound to the ER. These values can be used as a remarkable quantitative means to assess the degree of MAM stabilization ranging between the tight and loose MAMs, leading to the reduction of Ab generation. The mitochondrial axonal transport rates upon stabilization of the tight and loose MAMs in naïve ReN cells mirrored the transport patterns observed in ReN GA neurons (Figure 1F-G). The consistent outcomes between naïve ReN neurons and APPSwe/Lon-expressing ReN GA AD neurons suggest that the effect on axonal transport is predominantly attributed to the state of MAM stabilization, independent of the presence of APPSwe/Lon or resultant Aβ production.
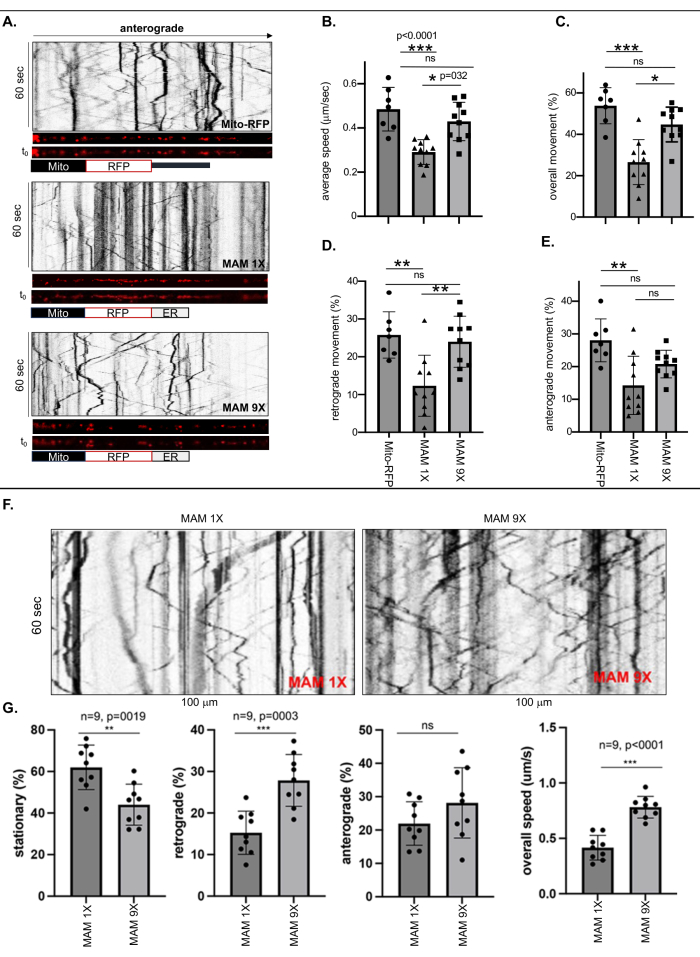
Figure 1: Stabilization of MAMs by MAM 1X reduced the average speed and movement (overall, retrograde, and anterograde) of ER-bound mitochondria in the axons of differentiated ReN GA and naïve ReN cells. (A) Representative kymographs of the RFP-labeled puncta representing free mitochondria (Mito-RFP) or ER-bound mitochondria stabilized by MAM 1X (tight MAMs, 6 nm ± 1 nm contact width) or MAM 9X (loose MAMs, 24 nm ± 3 nm contact width) inside axons (~100 nm). (B-E) Quantitative analysis of the (B) average speed and movement [(C) overall, (D) retrograde, and (E) anterograde] of Mito-RFP, MAM 1X, or MAM 9X inside axons of 10-day differentiated Ren-GA cells. n>7; Two-way ANOVA was performed. *p < 0.05, **p < 0.001. Representative of three independent experiments. (F) Representative kymographs of the movement of MAM 1X or MAM 9X inside axons of 10-day differentiated naïve ReN cells. (G) Quantitative analysis of percent (%) movement (stationary, retrograde, and anterograde) and overall speed (micrometer/second; μm/s) of MAM 1X- or MAM 9X-stabilized MAMs inside the axons of naïve ReN cells. n = 9; Two-way ANOVA was performed. ***p < 0.0001. This figure has been adapted with permission from Zellmer et al.17. Please click here to view a larger version of this figure.

Figure 2: Live cell images of axons of naïve ReN cells expressing MAM 1X or MAM 9X. Representative live-cell video images exhibiting the movements of the MAMs stabilized by MAM 1X or MAM 9X inside 100 μm long axons of 10-day differentiated GFP-expressing ReN cells. n > 10 images from duplicate experiments. The arrows indicate anterograde transport. Scale bar: 100 μm. Please click here to view a larger version of this figure.
| Reagent | Final Concentration | Amount |
| DMEM with L-glutamine | N/A | 500 mL |
| Heparin | 2 µg/mL | 0.5 mL |
| B27 | 1x | 10 mL |
| bFGF | 20 ng/mL | 0.4 mL |
| EGF | 20 ng/mL | 0.5 mL |
| Penicillin/Streptomycin | 100 units/mL | 5 mL |
| Total | 516.4 mL | |
| Filter media before adding penicillin/streptomycin. Store at 4 °C for up to 1 month. | ||
Table 1: Composition of expansion media.
| Reagent | Final Concentration | Amount |
| DMEM with L-glutamine | N/A | 500 mL |
| Heparin | 2 µg/mL | 0.5 mL |
| B27 | 1x | 10 mL |
| Penicilliion/Streptomycin | 100 units/mL | 5 mL |
| Total | 515.5 mL | |
| Filter media before adding penicillin/streptomycin. Store at 4 °C for up to 1 month | ||
Table 2: Composition of differentiation media.
| ReN GA | ReN (naïve) | ReN GA (3D) | |||||
| Overall (%) | Retrograde (%) | Anterograde (%) | Average speed (mm/s) | Ab40 (pM) | Ab42 (pM) | ||
| Mito-RFP | 53.82 ± 3.3% | 25.78 ± 2.31% | 28.04 ± 2.48% | 0.66 ± 0.03 | 0.69 ± 0.07 | 241.7 ± 26.74 | 13.77 ± 1.52 |
| MAM 1X | 26.6 ± 3.4% *** | 12.33 ± 2.5% *** | 14.27 ± 2.81% *** | 0.3 ± 0.02*** | 0.43 ± 0.04*** | 377.2 ± 76.87* | 26.62 ± 3.86* |
| MAM 9X | 44.79 ± 2.6% ns | 23.99 ± 2.17%ns | 20.80 ± 1.33%ns | 0.59 ± 0.02 ns | 0.62 ± 0.02 ns | 158.8 ± 3.27* | 17.01 ± 2.02* |
Table 3: Quantitative analysis. Live-cell imaging and kymography-based quantitative analysis of the average speed (speed) and axonal movements (overall, retrograde, and anterograde) of Mito-RFP, MAM 9X, and MAM 1X. Two-way ANOVA was performed for axonal speed or movement (%). n = 9. For Aβ, ordinary one-way ANOVA was performed; n = 3, three independent experiments. The significance is measured against the un-transfected (control) ReN GA cells. **p < 0.0001; *p < 0.05; not significant (ns). This table has been adapted with permission from Zellmer et al.17.
Supplementary Coding File 1: The code for generating, tracking and measuring the kymograph data. Please click here to download this File.
Discussion
Inhibition of sigma-1 receptor (S1R) downregulated MAM stabilization in the neuronal processes and dramatically reduced (~90%) Aβ generation from axons but not from soma of a three-dimensional (3D) culture system of human neural progenitor (ReN) cells expressing familial AD [FAD] mutations in the amyloid precursor protein [APP] gene (ReN GA)23,24,25,27. RFP-labeled constitutive MAM stabilizers (MAM 1X and MAM 9X) designed to stabilize tight (6 nm ± 1 nm) and loose (24 nm ± 3 nm) MAMs15,26 are remarkable tools to quantitatively measure MAM stabilization. Both stabilizers not only exhibit equal and stable expression in ReN GA cells differentiated in 3D matrix for ~10 days but also detected MAMs in discrete puncta in soma and axons. Most importantly, while the stable expression of MAM 1X in FACS-enriched 3D ReN GA significantly increased Aβ generation, MAM 9X-expression had no effect17. We also tested the effect of a constitutive MAM stabilizer containing 18 amino acid linker (MAM 18X) that detects and stabilizes MAMs >25 nm. Unlike MAM 1X or MAM 9X, MAM 18X exclusively labeled somal MAMs. FACS-enriched MAM 18X-expressing ReN GA neurons, reduced Aβ generation17. These findings suggested the possibility of a MAM stability threshold determined by their gap width that ranges between pathogenic (increasing Aβ generation) tight MAMs to non-pathogenic (maintaining or reducing Aβ generation) loose MAMs.Finding an effective MAM modulator and its optimal concentration that can achieve the optimal MAM stabilization required to cross the threshold from pathogenic and non-pathogenic MAMs will reveal a remarkable therapeutic avenue to lower axonal or neuronal Aβ generation in the brain.
Three different approaches have been employed to develop MAM modulators: (1) Modulators that target MAM tethering proteins, (2) Modulators that alter expression levels of MAM-resident proteins, and (3) Modulators of MAM structures18. Despite these approaches, the major obstacle of finding effective modulators of MAM stabilization is the lack of methods to quantitatively measure the degree of MAM stabilization. Traditional techniques like electron microscopy (EM) or super-resolution microscopy have limitations in capturing real-time changes or providing sufficient detail to assess the stabilization of MAM (reviewed in28).
The method described here will overcome the obstacle and provide key insights into the relationship between MAM stabilization and Aβ production. The results show that MAMs with a thickness of 6 nm ± 1 nm, displaying an overall movement of 26.6% ± 3.4% (Table 3), are associated with Aβ generation. Contrastingly, MAMs with a thickness of 24 nm ± 3 nm, which exhibit an overall movement of 44.79% ± 2.6% (Table 3), do not influence Aβ generation. The overall movement of mitochondria (Mito-RFP) was 53.82% ± 3.3%. Given that MAM thickness typically varies between 6 nm and 80 nm, these findings delineate the upper and lower bounds of MAM stabilization in relation to Aβ production. Consequently, this method can guide the identification and optimization of a modulator(s) for MAM stabilization. The goal would be to alter the overall movement of MAMs from 26.6% ± 3.4% to 53.82% ± 3.3%, or their average speed from  0. 4 μm/s to
0. 4 μm/s to  0. 7 μm/s (Table 3), positioning such modulator(s) as potential therapeutic agents against Aβ production.
0. 7 μm/s (Table 3), positioning such modulator(s) as potential therapeutic agents against Aβ production.
The use of constitutive MAM modulators containing synthetic linkers of increasing lengths (0-18 amino acids) is a powerful method to quantitatively determine the MAM stabilization threshold to switch the MAM stabilization to one that maintains or possibly lowers as opposed to increases Aβ generation. However, to assess the efficiency or efficacy of MAM modulators, inducible MAM stabilizers will be required. Inducible Förster resonance energy transfer/fluorescence-lifetime imaging microscopy (FRET/FLIM)-based MAM stabilizers are available that are expression plasmids encoding YFP-fused OMM-targeting sequence of mAKAP1 (34-63) and CFP-fused ER-targeting Sac1 phosphatase (521-587). Moreover, the constitutive stabilizers may not represent the physiological MAMs, while the FRET/FLIM MAM stabilizers, on the other hand, will detect the physiological MAMs. The split GFP probes where GFP is split into two non-fluorescent fragments tethered to either resident ER or mitochondrial proteins ER-GFP (1-10) and Mito-GFP11 that generate biomolecular fluorescence complementation (BiFC) upon the formation of MAMs27, may also be used. Although, the GFP fragments are prone to spontaneous assembly, BiFC has the simplest readout, clearest signal, and least noise-associated analysis. Moreover, the interaction between the split GFP is highly reversible28, thus their advantages outweigh the drawbacks and make the BiFC method suitable for identifying modulators of MAM stabilization.
Acknowledgements
We thank Dr. György Hajnóczky, Professor, Thomas Jefferson University, Philadelphia for generously providing us with expression plasmids encoding RFP-Mito, MAM 1X, MAM 9X, and MAM 18X. A special thanks to Dr. Lai Ding, Senior Imaging Scientist, Brigham and Women’s Hospital for helping us write the code for generating, tracking and measuring the kymograph data. This study was supported by the Cure Alzheimer's Fund to RB and NIH grant 5R01NS045860-20 to RET.
Materials
| Name | Company | Catalog Number | Comments |
| 6 Well Glass Bottom Plate | Cellvis | P06-1.5H-N | |
| B-27 Supplement (50X), serum free | Gibco/Thermo Fisher Scientific | 17504044 | |
| bFGF | R&D System | 233-FB | |
| BSA | Fisher Scientific | 501781532 | |
| Countess Cell Counting Chamber Slides | Invitrogen | C10283 | |
| DMEM/F12 with L-glutamine | Gibco/Thermo Fisher Scientific | 11320-033 | |
| EDTA | Life Technologies | 41116134 | |
| EGF | Sigma-Aldrich | 92090408 | |
| Falcon 6 Well Plates | VWR International | 41122107 | |
| GAPDH Polyclonal Antibody | Thermo Fisher Scientific | PA1-988 | |
| Gelatin | VWR International | 9000-70-8 | |
| Graphpad Prism N/A | Prism 9, version 9.5.0 | N/A | |
| Heparin | Sigma-Aldrich | H0200000 | |
| ImageJ Software | ImageJ 1.53a | N/A | |
| Matrigel Basement Membrane Matrix | Corning | 356234 | |
| mCherry Polyclonal Antibody | Invitrogen | PA5-34974 | |
| MS Excel | Microsoft Excel, version 2302 | N/A | |
| Multi-array electrochemiluminescence assay kit | Meso Scale Diagnostics (MSD) | K15200E-2 | V-PLEX Aβ Peptide Panel 1 (6E10) kit |
| NaCl | Fisher Scientific | 7647145 | |
| NuPAGE 4–12% Bis-Tris gel | Invitrogen | NP0321BOX | |
| Penicillin/Streptomycin/Amphotericin B | Lonza | 17-745E | |
| Photoshop | Adobe Photoshop CC 20.0.10 | N/A | |
| Rat Neuron Nucleofector Kit | Lonza | VPG-1003 | |
| StemPro Accutase | Gibco | A1110501 | |
| Tris-HCL, pH 7.6 | Boston BioProducts | 42000000 | |
| Triton X-100 | Sigma-Aldrich | T8787 | |
| Tween 20 | Fisher Scientific | 501657287 |
References
- Giacomello, M., Pellegrini, L. The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ. 23 (9), 1417-1427 (2016).
- Degechisa, S. T., Dabi, Y. T., Gizaw, S. T. The mitochondrial associated endoplasmic reticulum membranes: A platform for the pathogenesis of inflammation-mediated metabolic diseases. Immun Inflamm Dis. 10 (7), e647 (2022).
- Schon, E. A., Area-Gomez, E. Mitochondria-associated ER membranes in Alzheimer disease. Mol Cell Neurosci. 55, 26-36 (2013).
- Erpapazoglou, Z., Mouton-Liger, F., Corti, O. From dysfunctional endoplasmic reticulum-mitochondria coupling to neurodegeneration. Neurochem Int. 109, 171-183 (2017).
- Sala-Vila, A., et al. Interplay between hepatic mitochondria-associated membranes, lipid metabolism and caveolin-1 in mice. Sci Rep. 6, 27351 (2016).
- Fujimoto, M., Hayashi, T. New insights into the role of mitochondria-associated endoplasmic reticulum membrane. Int Rev Cell Mol Biol. 292, 73-117 (2011).
- Hung, V., et al. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. eLife. 6, e24463 (2017).
- Area-Gomez, E., Schon, E. A. On the pathogenesis of Alzheimer's disease: The MAM hypothesis. FASEB J. 31 (3), 864-867 (2017).
- Rizzuto, R., et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 280 (5370), 1763-1766 (1998).
- Sukhorukov, V. S., et al. Molecular mechanisms of interactions between mitochondria and the endoplasmic reticulum: A new look at how important cell functions are supported. Mol Biol. 56 (1), 59-71 (2022).
- Zhang, P., Konja, D., Zhang, Y., Wang, Y. Communications between Mitochondria and endoplasmic reticulum in the regulation of metabolic homeostasis. Cells. 10 (9), 2195 (2021).
- Ziegler, D. V., Martin, N., Bernard, D. Cellular senescence links mitochondria-ER contacts and aging. Commun Biol. 4 (1), 1323 (2021).
- Csordas, G., et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 174 (7), 915-921 (2006).
- Cieri, D., et al. SPLICS: a split green fluorescent protein-based contact site sensor for narrow and wide heterotypic organelle juxtaposition. Cell Death Differ. 25 (6), 1131-1145 (2018).
- Carpio, M. A., et al. BOK controls apoptosis by Ca(2+) transfer through ER-mitochondrial contact sites. Cell Rep. 34 (10), 108827 (2021).
- Prudent, J., et al. MAPL SUMOylation of Drp1 stabilizes an ER/mitochondrial platform required for cell death. Mol Cell. 59 (6), 941-955 (2015).
- Zellmer, J. C., Tarantino, M. B., et al. Stabilization of mitochondria-associated endoplasmic reticulum membranes regulates Abeta generation in a three-dimensional neural model of Alzheimer’s disease. Alzheimer’s Dement. , 1-20 (2024).
- Magalhaes Rebelo, A. P., et al. Chemical modulation of mitochondria-endoplasmic reticulum contact sites. Cells. 9 (7), 1637 (2020).
- Berger, S. I., Iyengar, R. Role of systems pharmacology in understanding drug adverse events. Wiley Interdiscip Rev Syst Biol Med. 3 (2), 129-135 (2011).
- Friedman, J. R., Webster, B. M., Mastronarde, D. N., Verhey, K. J., Voeltz, G. K. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 190 (3), 363-375 (2010).
- Wu, Y., et al. Contacts between the endoplasmic reticulum and other membranes in neurons. Proc Natl Acad Sci U S A. 114 (24), E4859-E4867 (2017).
- Cagin, U., et al. Mitochondrial retrograde signaling regulates neuronal function. Proc Natl Acad Sci U S A. 112 (44), E6000-E6009 (2015).
- Choi, S. H., et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature. 515 (7526), 274-278 (2014).
- Kim, Y. H., et al. A 3D human neural cell culture system for modeling Alzheimer's disease. Nat Protoc. 10 (7), 985-1006 (2015).
- Kwak, S. S., et al. Amyloid-beta42/40 ratio drives tau pathology in 3D human neural cell culture models of Alzheimer's disease. Nat Commun. 11 (1), 1377 (2020).
- Csordas, G., et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell. 39 (1), 121-132 (2010).
- Bhattacharyya, R., et al. Axonal generation of amyloid-beta from palmitoylated APP in mitochondria-associated endoplasmic reticulum membranes. Cell Rep. 35 (7), 109134 (2021).
- Tebo, A. G., Gautier, A. A split fluorescent reporter with rapid and reversible complementation. Nat Commun. 10 (1), 2822 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved