Method Article
إنشاء 50 نيوتن متر الفرعية علم الموائع النانوي المفارق في PDMS ميكروفلويديك رقاقة عن طريق عملية التجميع الذاتي من الغروية الجسيمات
In This Article
Summary
We propose a simple self-assembly technique of silica colloidal nanoparticles to create a nanofluidic junction between two microchannels in polydimethylsiloxane (PDMS). Using this technique, a nanoporous bead membrane with a pore size down to ~45 nm was built inside a microchannel and applied to electrokinetic preconcentration of DNA samples.
Abstract
ثنائي ميثيل بولي سيلوكسان (PDMS) هي مواد البناء السائدة لجعل أجهزة ميكروفلويديك نظرا لسهولة صب والترابط وكذلك شفافيتها. بسبب ليونة من المواد PDMS، ومع ذلك، فإنه يشكل تحديا لاستخدام PDMS لبناء nanochannels. القنوات تميل إلى الانهيار بسهولة خلال الترابط البلازما. في هذه الورقة، نقدم طريقة التجميع الذاتي يحركها تبخر النانوية السيليكا الغروية إلى إنشاء تقاطعات علم الموائع النانوي مع الفرعي 50 نانومتر المسام بين اثنين microchannels. حجم المسام وكذلك تهمة سطح تقاطع علم الموائع النانوي هو الانضباطي ببساطة عن طريق تغيير الغروية السيليكا حبة حجم والسطحية functionalization خارج الجهاز ميكروفلويديك تجميعها في قارورة قبل عملية التجميع الذاتي. باستخدام التجميع الذاتي للجزيئات النانوية مع حجم حبة من 300 نانومتر و 500 نانومتر، و 900 نانومتر، وكان من الممكن أن يصنع غشاء مسامي مع حجم المسام من ~ 45 نانومتر، ~ 75 نانومتر، و~ 135 نانومتر، على التوالي. تحت الكهربائيةآل المحتملة، وهذا nanoporous غشاء بدأ تركيز أيون الاستقطاب (ICP) بصفتها غشاء الموجبة انتقائي للتركيز الحمض النووي التي كتبها ~ 1700 مرات في غضون 15 دقيقة. تفتح هذه العملية nanofabrication غير معدني يصل فرصة جديدة لبناء تقاطع علم الموائع النانوي الانضباطي لدراسة عمليات النقل النانو الأيونات والجزيئات داخل شريحة ميكروفلويديك PDMS.
Introduction
علم الموائع النانوي هو المجال الناشئ البحوث μ TAS (مايكرو إجمالي تحليل النظم) لدراسة العمليات البيولوجية أو الظواهر نقل الأيونات والجزيئات في نطاق طول 10 يناير - 10 فبراير نانومتر. مع ظهور أدوات علم الموائع النانوي مثل nanochannels، يمكن رصد عمليات نقل الجزيئات والأيونات بدقة لم يسبق لها مثيل والتلاعب بها، إذا لزم الأمر، من خلال استغلال الميزات التي لا تتوفر إلا في هذا النطاق طول لفصل وكشف 1،2 أحد هذه الميزات النانو مميزة هي نسبة عالية من السطح إلى المسؤول الأكبر (أو عدد Dukhin) في nanochannels التي يمكن أن تسبب خلل الاتهام وبدء الاستقطاب تركيز أيون (ICP) بين نانوية ومتناهية 3
منصة جهاز مشتركة لدراسة الظواهر علم الموائع النانوي يتكون من نظام ثنائي متناهية متصلة بواسطة مجموعة من nanochannels بمثابة مفترق 4-6 المواد من خيار لبناء مثل هذا الجهاز علم الموائع النانوي هي السيليكون بسبب صلابة العالية التي تمنع القناة من الانهيار أثناء عمليات الترابط. 7 ومع ذلك، تصنيع الجهاز السيليكون يتطلب أقنعة مكلفة وكمية كبيرة من المعالجة في منشأة غرف الأبحاث. 8- 10 نظرا لسهولة تصنيع الجهاز من خلال (PDMS) الصب، وارتباط البلازما، ثنائي ميثيل بولي سيلوكسان تمت على نطاق واسع تم قبول كمادة بناء لعلى microfluidics وسيكون مثاليا للمادة علم الموائع النانوي كذلك. ومع ذلك، معامل منخفضة يونغ لها في جميع أنحاء 360-870 الجيش الشعبي الكوري، يجعل قناة PDMS للطي بسهولة خلال الترابط البلازما. الحد الأدنى لنسبة أبعاد نانوية (العرض إلى العمق) يجب أن تكون أقل من 10: 1 وهذا يعني أن تصنيع الأجهزة PDMS عبر ضوئيه القياسية ستصبح صعبة للغاية إذا كان عمق نانوية يجب أن يكون أقل من 100 نانومتر، والتي تتطلب عرض القناة أقل من الحد الحالي من photolithography في حوالي 1 ميكرون. للتغلب على هذا القيد، وكانت هناك محاولات لخلق nanochannels في PDMS باستخدام أساليب غير الزخرفية مثل تمتد إلى الشروع في الشقوق مع عمق متوسط من 78 نانومتر 11 أو لتشكيل التجاعيد بعد العلاج البلازما 12 تنهار قناة PDMS مع الضغط الميكانيكي سمحت ارتفاع نانوية منخفضة تصل إلى 60 نانومتر. 13
على الرغم من أن هذه الأساليب المبتكرة للغاية غير معدني سمحت nanochannels بناء أقل من 100 نانومتر في العمق، والتحكم الأبعاد للتصنيع نانوية لا يزال يشكل عقبة أمام قبول واسع من PDMS كمادة بناء لأجهزة علم الموائع النانوي. مشكلة خطيرة أخرى من nanochannels، سواء في السيليكون أو PDMS، هو functionalization السطح في حالة وجود حاجة لتغيير تهمة السطحية على الجدار قناة للتلاعب من الأيونات أو الجزيئات. بعد تجميع الجهاز من خلال الترابط، وnanochannels من الصعب للغايةوصول إلى functionalization السطح بسبب نقل محدودة الانتشار. لإنشاء قناة النانو مع الإخلاص الأبعاد عالية وfunctionalization سطح سطحي، وطريقة التجميع الذاتي للجزيئات الغروية الناجم عن تبخر 14-16 في أجهزة ميكروفلويديك يمكن أن يكون واحدا من النهج الواعدة. بالإضافة إلى إمكانية التحكم في حجم المسام والممتلكات السطح، بل هناك إمكانية لضبط حجم المسام في الموقع عند استخدام الجسيمات الغروية المغلفة مع polyelectrolytes عن طريق التحكم في درجة الحرارة، درجة الحموضة 17، 18،19 والقوة الأيونية. 18 وبسبب هذه تطبيقات من المزايا، وطريقة التجميع الذاتي للجزيئات الغروية قد وجدت بالفعل لاستشراب كهربي، 20 أجهزة الاستشعار، وتركيز البروتين 21 22 و الفصل بين البروتينات والحمض النووي في على microfluidics. 14،23 في هذه الدراسة، قمنا بنشر هذه الطريقة التجميع الذاتي لبناء جهاز preconcentration حركي كهربي فيPDMS يتطلب تقاطع علم الموائع النانوي بين اثنين microchannels 24 آلية الأساسية وراء تركيز حركي كهربي يقوم على الاستقطاب تركيز أيون (ICP). وضمت 25 وصفا مفصلا لتصنيع وتجميع الخطوات في البروتوكول التالي.
Protocol
1. إعداد الخرزة المعلقات السيليكا الغروية
- إعداد 300 نانومتر و 500 نانومتر تعليق حبة السيليكا
- دوامة تعليق السيليكا الأسهم حبة (10٪ ث / ت في الماء) لمدة 30 ثانية. للحصول على تعليق متجانسة. ماصة ما مجموعه 600 تعليق الأسهم ميكرولتر في أنبوب 1.5 مل وأجهزة الطرد المركزي أنه في 2600 x ج لمدة 1 دقيقة.
- استبدال طاف مع 400 ميكرولتر من العازلة 1 ملم فوسفات الصوديوم (PB، ودرجة الحموضة 7.0).
- تعليق الخرز السيليكا إلى تركيز النهائي من 15٪ في 1 ملي حل فوسفات الصوديوم في 7.0 درجة الحموضة من خلال vortexing ل.
- functionalize سطح الخرز 500 نانومتر السيليكا الكربوكسيل مع بولي (آليلامين هيدروكلوريد، الهيئة العامة للإسكان)، ومع بولي polyelectrolytes (الصوديوم الستايرين سلفونات، PSS)
- تعليق 0.1 غرام من 500 حبات السيليكا نانومتر مع مجموعة الكربوكسيل مع 10 مل 1 M كلوريد الصوديوم (7.0 درجة الحموضة) للفترة من 1٪ (ث / ت) تعليق حبة.
- تحضير 0.4٪ الهيئة العامة للإسكان (MW 65K) في 1 M بريدال عن طريق تذويب 300 ميكرولتر من محلول المخزون (20٪ ث / ت في الماء) في 15 مل من 1 M كلوريد الصوديوم. تحضير 0.9٪ PSS (MW 70K) في 1 الحل M كلوريد الصوديوم عن طريق إذابة 0.18 ز جهاز الأمن الوقائي في 20 مل محلول 1 M كلوريد الصوديوم. دوامة كلا حلول لمدة 1 دقيقة. حل polyelectrolytes تماما.
- إضافة 200 ميكرولتر من حل الهيئة العامة للإسكان إلى 9.8 مل من 1٪ حبات السيليكا الكربوكسيل في أنبوب 15 مل لإيداع طبقة متضاعف الكتروليتي موجبة على حبات السيليكا مع مجموعة وظيفية الكربوكسيل. دوامة تعليق حبة لمدة 1 دقيقة. واحتضان على محور دوار أنبوب لمدة 60 دقيقة. في RT.
- الطرد المركزي تعليق حبة في 1801 x ج لمدة 1 دقيقة. ويغسل غير منضم الهيئة العامة للإسكان خمس مرات بالماء DI 10 مل. بعد كل الطرد المركزي وإزالة طاف، كانت معبأة في حبات كثيفة في الجزء السفلي من الأنبوب. تعطيل أجمة حبة من قبل pipetting قوية مع 2 مل من الماء DI قبل إضافة 8 مل من الماء DI بحيث حبات يمكن إعادة علقت وغسله قبل خطوة الطرد المركزي المقبلة.
- إتبعالخطوات في 1.2.3 و1.2.4 لطلاء PSS لإيداع طبقة مشحونة سلبا على الخرز. إعادة تعليق الخرز في 9.8 مل من 1 M كلوريد الصوديوم قبل ترسب PSS بعد إزالة طاف المياه DI من الخطوة غسل الموافق 5 من 1.2.4.
- استخدام نفس الخطوة pipetting لنشطة باستخدام 2 مل من 1 M كلوريد الصوديوم لتفريق أجمة حبة في الجزء السفلي من أنبوب 15 مل ثم قم بإضافة 8 مل من 1 M كلوريد الصوديوم. إضافة 200 ميكرولتر من حل جهاز الأمن الوقائي إلى 9.8 مل من الخرز السيليكا المودعة لدى طبقة الهيئة العامة للإسكان واحدة. بعد vortexing لمدة 1 دقيقة. والحضانة لمدة 60 دقيقة. على محور دوار أنبوب، كرر 5 خطوات الغسيل بالماء DI.
- قياس إمكانات زيتا من الخرز قبل وبعد كل طلاء متضاعف الكتروليتي باستخدام نظام تشتت الضوء الحيوي وفقا لبروتوكول الشركة المصنعة للتحقق من إجراءات متضاعف الكتروليتي ترسب تم تنفيذها بشكل صحيح (انظر الجدول 1).
- كرر خمس خطوات الغسل بالماء DI بعد طبقة واحدة PSSترسب وإعادة تعليق الخرز في 650 ميكرولتر من 1 ملي العازلة فوسفات الصوديوم مع 0.05٪ توين 20 (15٪ ث / ت) قبل استخدامها في الجهاز ميكروفلويديك لتعزيز سيولتها لها.
- اتبع الإجراء الموضح أعلاه من 1.2.5 إلى 1.2.6 عن 500 نانومتر حبات السيليكا مع مجموعة وظيفية أمين لإيداع طبقة واحدة من جهاز الأمن الوقائي.
2. تلفيق من PDMS ميكروفلويديك رقاقة
- التصنيع الدقيق للسيد السيليكون
- افتعال سيد السيليكون لقالب PDMS باستخدام تقنيات التصنيع الدقيق على النحو التالي.
- تدور معطف 1 ميكرون مقاومة للضوء رقيقة في 4000 دورة في الدقيقة على رقاقة السيليكون. نمط طبقة باستخدام الطباعة الحجرية الإسقاط (زمن التعرض 170 ميللي ثانية) وحفر 700 نانومتر nanochannels مستو عميق و 2 ميكرون واسع (بدور nanotraps لحبات السيليكا) مع النقش ايون على رد الفعل.
- استخدام المعلمات النقش التالية لتحقيق معدل حفر من 3.5 نانومتر / ثانية: CHF 3 (45 SCCM)، CF 4 (15 SCCM)، هارون (100 SCCM)، الضغط 100 mTorr، والطاقة RF 200 W.
- تدور معطف الثانية 1 ميكرون طبقة سميكة مقاومة للضوء في 2000 دورة في الدقيقة وإجراء محاذاة إلى nanotraps نمط سابقا. نمط و microchannels عن طريق الطباعة الحجرية الاتصال والحفر العميق رد الفعل أيون (DRIE) من السيليكون. استخدام DRIE المعلمات 26 في الجدول 2.
- افتعال سيد السيليكون لقالب PDMS باستخدام تقنيات التصنيع الدقيق على النحو التالي.
- تلفيق PDMS العفن
- Silanize سيد السيليكون مع trichlorosilane (50 ميكرولتر) في فراغ جرة O / N.
تنبيه: Tricholorosilane هو مادة سامة وقابلة للتآكل. دائما استخدامها في غطاء الكيميائية مع المعدات المناسبة الحماية الشخصية. - مزيج القاعدة إلى وكيل المعالجة في 10: 1 نسبة ويلقي PDMS على سيد السيليكون silanized وعلاجه عند 70 درجة مئوية لمدة 2 ساعة في الفرن الحراري.
- إزالة بلاطة PDMS من سيد السيليكون بسكين والبلازما السندات على رقاقة فارغة باستخدام نظافة البلازما بعد ررالعلاج أسماء في نظافة البلازما لمدة 1 دقيقة. نعلق الأشرطة على طول الحافة للاحتفال خط التقسيم لPDMS التالية الصب خطوة.
- Silanize القالب PDMS في جرة فراغ مع trichlorosilane (50 ميكرولتر) O / N.
- PDMS المصبوب (قاعدة: وكيل علاج في 10: 1 نسبة) على قالب PDMS silanized وعلاجه عند 70 درجة مئوية لمدة 2 ساعة في الفرن الحراري.
- Silanize سيد السيليكون مع trichlorosilane (50 ميكرولتر) في فراغ جرة O / N.
- تصنيع الجهاز PDMS
- انزع بلاطة PDMS شفي من العفن PDMS على طول خط التقسيم ملحوظ مع الشريط.
- لكمة ثقوب الخزان مع 1.5 مم لكمة خزعة ونظيفة مع شريط، شطف مع ايزوبروبيل (IPA) وجافة مع النيتروجين.
- البلازما السندات الجهاز PDMS على 25 ملم × 75 ملم شريحة ميكروسكوب بعد العلاج البلازما في نظافة البلازما لمدة 1 دقيقة.
- Ultrasonicate تعليق حبة لمدة 60 دقيقة. في حمام بالموجات فوق الصوتية قبل التعبئة. ماصة تعليق حبة 10 ميكرولتر (300 نانومتر السيليكا غير functionalized يكونالإعلانات، أو 500 الخرز نانومتر السيليكا الكربوكسيل مع طبقات الهيئة العامة للإسكان، جهاز الأمن الوقائي، أو 500 حبات الأمينات نانومتر السيليكا مع طبقة PSS) في مداخل 4 و 6 لكل (انظر الشكل 1 A، B) مباشرة بعد الربط البلازما من الشريحة PDMS ل الركيزة الزجاج. اضغط بلطف على الرقاقة PDMS مع طرف ماصة لتعزيز التعبئة حبة.
- بعد ملء قنوات التوزيع حبة، تغطية جميع المنافذ ما عدا 1 و 9 مع الشريط. الهواء الجاف الجهاز لمدة 3 ساعة وتخزينها في +4 درجة مئوية قبل استخدامها. الشكل 2 يعطي التخطيطي خطوة بخطوة عملية التجميع الذاتي الغروية.
3. تجربة للحركي كهربي تركيز الحمض النووي
- ملء الخزانات 3 و 7 مع حل العازلة (10 ميكروليتر من 1 ملم PB) وخزان 5 مع عينة الحمض النووي (10 ميكرولتر 10 نانومتر في 1 ملم PB) وتطبيق الضغط السلبي لطيف مع تلميح ماصة مقلوب على الخزانات 2 و 8 و 10 لملء قنوات مع حلول دون فقاعات (انظر الشكل 1B).
- إضافة 10 ميكرولتر من 1 ملم PB إلى الخزانات 2 و 8 و 10 ميكرولتر من 10 نانومتر الحمض النووي للخزان 10 لتحقيق التوازن في الضغط والانتظار لمدة 5 دقائق. للوصول إلى التوازن.
- إدراج الأسلاك حزب العمال في مكامن 3، 5، 7، 10.
- تطبيق الجهد عبر تقاطع علم الموائع النانوي باستخدام مقسم الجهد متصلة متر مصدر وأسلاك حزب العمال. تطبيق أول 30 V على الخزانات 5 و 10 و GND على الخزانات 3 و 7.
- تقليل الجهد إلى 25 V على خزان 10 بعد ~ 30 ثانية.
- استخدام مفتاح ميكانيكي مع افتتاح الدوري في كل 5 ثوان لتقليل photobleaching من العينة عند تسجيل الاشارات مضان من الحمض النووي.
النتائج
An electrokinetic concentrator chip in PDMS that contains a self-assembled nanofluidic junction between two microchannels is shown in Figure 1A). The channel in the middle of the device is filled with a DNA sample solution and flanked by two buffer solution channels on each side via a 50 µm wide bead delivery channel (Figure 1B). The silica colloidal suspension is flown into the bead delivery channel immediately after plasma bonding to create a nanofluidic junction between the sample and the buffer solution channel. The nanotrap array consisting of 700 nm deep and 2 μm wide nanochannels is used to trap the colloidal particles. Its scanned image obtained with a non-contact surface profiler is shown in Figure 1C). The colloidal bead membranes after evaporation are shown in Figure 1D). The SEM in Figure 1E) shows the silica beads trapped at the planar nanotrap array separating the sample channel from the bead delivery channel. The 300 nm silica bead packing shows highly ordered hexagonal packing with some minor defects that could cause a variation in the concentration behavior (Figure 1F). The design of the PDMS concentrator chip with its dimensions can be found here and in the Supplemental Files.

Figure 1. Microfluidic concentrator in PDMS with an integrated sub-50 nm nanoporous junction. (A) Photo of the PDMS concentrator device. (B) Schematic of the micro-nanofluidic device with a bead delivery channel between the sample and buffer solution channel. The voltage is applied across the bead membranes between the sample channel and the buffer solution channels. (C) Surface profile of the nanotrap array in PDMS with a width of 2 μm and a depth of 700 nm. (D) Micrograph of the device with a colloidal particle assembly inside the bead delivery channel after evaporation. (E) Scanning electron micrograph of the self-assembled 300 nm silica colloidal particles with the nanotrap arrays between the sample and buffer channel. The 300 nm beads are trapped at the entrance of the nanotraps due to surface tension. (F) Hexagonally packed 300 nm silica beads inside the bead delivery microchannel after evaporation. (Adapted from Ref. 25 with permission from The Royal Society of Chemistry) Please click here to view a larger version of this figure.
A schematic of the microfabrication steps for the PDMS concentrator device is shown in Figure 2. To make a PDMS device, a double PDMS casting is required. The bead filling process in the PDMS concentrator is shown in Figure 3. The details for the microfabrication and the filling process can be found in the protocol. The zeta potential of the silica beads without and with polyelectrolyte coating is shown in Table 1.
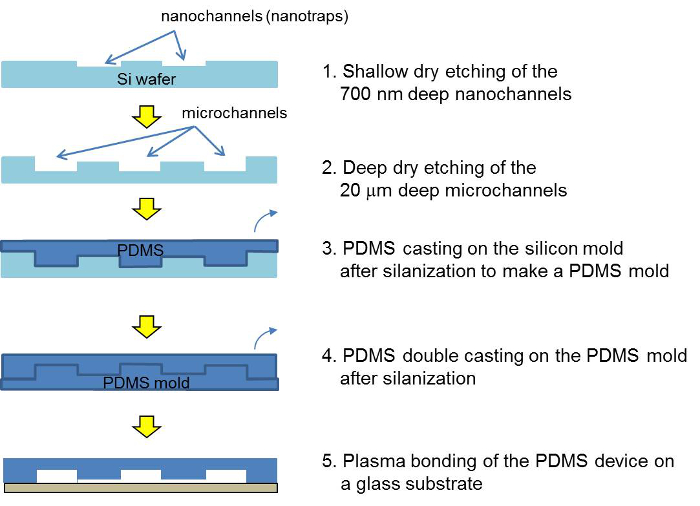
Figure 2. Schematic of the fabrication process for the silicon master, the PDMS master and the PDMS concentrator device. After two photolithographic and etching steps, the silicon master is cast with PDMS. After a double-molding, the PDMS device is assembled via plasma bonding and filled with a bead suspension. Please click here to view a larger version of this figure.

Figure 3. Step-by-step schematic for self-assembly of colloidal silica beads. 10 µl of the bead suspension was pipetted in to the bead delivery channels immediately after plasma treatment. Once the bead delivery channel was filled, all but two inlets 1 and 9 were covered with tape and the devices air dried for 3 hr prior to use. (Reproduced from Ref. 25 with permission from The Royal Society of Chemistry) Please click here to view a larger version of this figure.
| Colloidal particles (500 nm) | Zeta potential (mV) |
| Silica | -2.04 |
| Silica amine | 19.6 |
| Silica carboxyl | -19.73 |
| Silica carboxyl, PAH coated | 31.8 |
| Silica carboxyl, PAH, PSS coated | -28.5 |
| Silica amine, PSS coated | -31.2 |
Table 1. Zeta potential of silica beads at 25 °C. 0.1% (w/v) colloidal solutions were used for the measurements (n=3).
The SEM images taken from the bead packing channel after drying out show a pore size ranging between 60 nm, 91 nm and 170 nm, as shown in Figure 4. The pore size corresponds to approximately 20% of the bead size, 300 nm, 500 nm and 900 nm, respectively (15% of the bead diameter is the theoretical pore size).

Figure 4. SEM images of self-assembled 300 nm (A), 500 nm (B) and 900 nm (C) silica colloidal bead packing. PDMS devices were reversibly bonded to glass slides and beads flown into the channel using negative pressure. After air-drying the devices O/N, the PDMS devices were peeled of the glass carefully and imaged. This pore sizes were estimated to be 60±2, 91±5 and 170±7 nm for 300 nm, 500 nm and 900 nm beads respectively (n=9). These pore sizes were close to the theoretical size, ~15% of the bead diameter. (Adapted from Ref. 25 with permission from The Royal Society of Chemistry) Please click here to view a larger version of this figure.
When applying voltage of 30 V across the 300 nm bead membrane, an ion depletion zone was observed near the colloidal membrane inside a microchannel filled with a fluorescent labeled DNA (Figure 5 A, B). When lowering the voltage to 25 V on the left side, the DNA molecules got accumulated in the form of a plug and its concentration increased due to electroosmotic flow driven by a voltage difference of 30 V- 25 V across the sample channel (Figure 5 C, D).
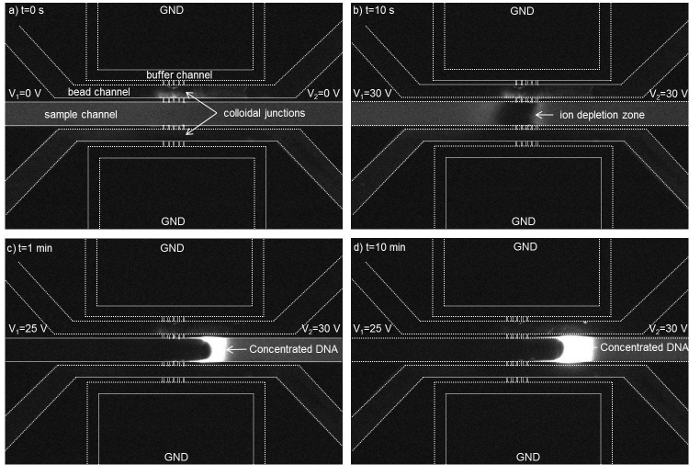
Figure 5. Time-lapse micrographs show the formation of an ion depletion region near the nanofluidic colloidal junctions in the channel filled with DNA (initial concentration of 10 nM). The ion depletion region was initiated at t=10 s and a concentrated DNA plug was generated at V2 = 30 V and V1 = 25 V across the sample channel while the buffer channels were grounded. The dotted lines have been used to highlight the channels walls. A concentration factor of ~1,700 folds was achieved within 15 min. using a 300 nm colloidal membrane. (Reproduced from Ref. 25 with permission from The Royal Society of Chemistry) Please click here to view a larger version of this figure.
The silica membranes with a bead size of 300 nm and 500 nm showed the highest concentration factor at ~1,700 times for the Cy 5 tagged DNA (CAA CCG ATG CCA CAT CAT TAG CTA C) within 15 min. (Figure 6 A, B). The polyelectrolyte-coated silica bead membranes led to a 200- to 1,000-fold increase in the DNA concentration after 15 min. (Figure 6 C, D).
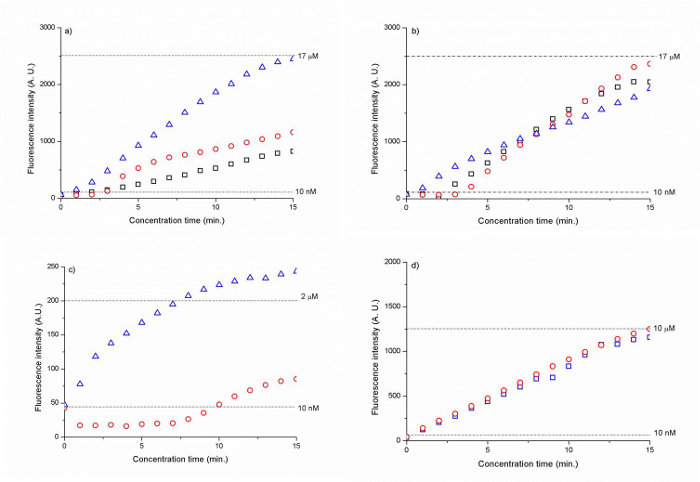
Figure 6. Fluorescence intensity of DNA as a function of time for (A) 300 nm silica beads (B) 500 nm silica beads and (C) 500 nm PSS-coated silica amine beads and (D) 500 nm PAH/PSS coated silica carboxyl beads. The dotted lines represent the fluorescence signal intensity level for 10 nM (A, B , C, D), 17 µM (A, B), 2 µM (C) and 10 µM (D) DNA. The results have been normalized against background fluorescence. (Reproduced from Ref. 25 with permission from The Royal Society of Chemistry) Please click here to view a larger version of this figure.
| Process time | Etch mode | Passivation mode |
| Process time | 6 s | 4.5 s |
| Overrun | 0.5 s | 0 s |
| Platen generator Power | 80 W | 60 W |
| Coil generator Power | 600 W | 600 W |
| Gas | SF6 70 sccm | C4F8 35 sccm |
| Etch rate | 1.47 µm/min | |
Table 2. DRIE parameters.
Discussion
Following the common device design scheme to study nanofluidics, we fabricated a nanofluidic junction between two microfluidic channels by using the evaporation-driven self-assembly of colloidal nanoparticles instead of lithographically patterning an array of nanochannels. When flowing the colloidal particles into the bead delivery channel, an array of nanotraps with a depth of 700 nm and a width of 2 µm on both sides of the bead delivery channel at a total width of 100 μm prevented the bead suspension from flowing into the buffer and sample channel due to the surface tension at the nanotraps. Once trapped, the colloidal particles packed in the bead delivery channel rapidly and formed a nanoporous junction between the sample and buffer channel.
It is important to load the bead suspension immediately after plasma bonding so that the capillary force drives the silica bead suspension up to the entrance of the outlet reservoirs in the temporarily hydrophilic bead delivery channel. In order to prevent an air bubble blocking the flow in the inlet reservoir, it is highly recommended to reach the bottom of the reservoir with a pipette tip and then release the bead suspension into the reservoir. In the case of the surface-functionalized beads with polyelectrolytes, their flowability was drastically reduced compared to the silica beads without surface functionalization and tended to aggregate more easily and adhere to the channel surface during the filling process. In order to prevent a clogging of the channel with the polyelectrolyte-coated beads, we added a surfactant, 0.05% Tween 20, to the bead suspension. In case there was still a clogging problem during filling, a gentle tapping on the PDMS chip with a pipette tip generally helped to resolve it.
Also, it is important that the bead suspension was not completely dried out after evaporation since it would be difficult to infiltrate the bead membrane with the sodium phosphate buffer solution again. Therefore, after 3 hr of partial evaporation, all in- and outlets of the PDMS device were taped and kept at 4 °C for storage prior to use so the bead packing stays moist. During the preconcentration experiments, the self-assembled bead maintained its structural stability for the most part. However, in few instances, we observed a dislocation of the beads which indicated a defective packing of the beads in microchannels. The self-assembled silica beads ranging from a diameter of 900 nm down to 300 nm after the self-assembly can be seen in Figure 4. The theoretical pore size of the bead packing was ~45 nm, approximately ~15% of the colloidal particle diameter. We could confirm the pore size using a SEM analysis and measured a pore size approximately 20% of the bead diameter after packing.
Using the self-assembled 300 nm and 500 nm colloidal particle membranes as an ion-selective nanoporous junction, we could initiate ion depletion region at 30 V and concentrate 10 nM Cy5 tagged DNA (CAA CCG ATG CCA CAT CAT TAG CTA C) in 1 mM sodium phosphate buffer (Figure 5). By continuously flowing the DNA sample towards the ion depletion zone with an electroosmotic flow at a voltage difference V2-V1 =5 V, we could increase the initial DNA concentration by ~1,700 folds within 15 min. (Figure 6a, b). 500 nm beads allowed more robust DNA concentration than 300 nm beads, as shown in Figure 6b). Since the electrokinetic concentration is based on a force balance between the electroosmotic force and the highly nonlinear electrophoretic forces, the resulting concentration factor is determined by the degree to which this force balance can be maintained during electrokinetic concentration.27
Another significant advantage of deploying the colloidal particles for building a nanofluidic junction is the ease with which its surface functionalization can be performed. Instead of creating a nanochannel through bonding first and then performing a surface functionalization on it, we can simply surface functionalize the colloidal particles in a vial outside of the device first and then flow them into the channel for self-assembly. Based on this approach, we could initiate ICP using the 500 nm silica amine particles coated with a single layer of PSS and 500 nm silica carboxyl particles coated with a layer of PAH and PSS (Figure 6 c and d), at lower voltages (8 V and 10 V, respectively) than the colloidal particles without surface functionalization (30 V). This result shows that the surface functionalization of the colloidal particles prior to the self-assembly was effective to increase the surface charge of colloidal particles and resulted in higher ICP. However, in terms of the concentrator factor obtained, the nanofluidic junction of the surface functionalized beads was less effective than the non-functionalized silica beads. The amine/PSS-coated beads enabled a factor of ~200, while the carboxyl/PAH/PSS bead membrane showed a 1,000-fold increase after 15 min. (Figure 6d). This result can be explained by a higher surface charge of the surface functionalized nanopores that led to an increased length of the ion depletion region pushing the sample concentration plug farther away from the bead membrane and therefore, to less stable concentration. We believe that shortening the total width of the nanoporous bead membrane from currently 1 mm (the section of the bead membrane parallel to the sample channel) could mitigate this instability issue. According to our previous study, the width of the nanoporous junction determines the amount of ionic current passing through it.28 As the width increases, the ionic current increases and since more cations can migrate through the membrane, the depletion length increases and the concentration plug is further pushed away from the nanoporous junction. Therefore, the accumulation occurs in a less confined manner and the sample plug becomes less stable. Empirically, the nanoporous junction should be ~100-400 µm in width. Another feature to improve was an insufficient thickness of the PDMS wall of 15 µm between the sample channel and the bead delivery. This thin PDMS section led to an insufficient bonding that enabled an ionic current between the buffer and sample channel. Therefore, the entire bead membrane section parallel to the sample channel (1 mm in width) was acting as a nanoporous junction, even though only 100 μm of the bead was intended as a nanoporous junction membrane according to the total width of the nanotrap array. The PDMS wall thickness should be at least 25 µm or higher.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIH R21 EB008177-01A2 and New York University Abu Dhabi (NYUAD) Research Enhancement Fund 2013. We express our thanks to the technical staff of MIT MTL for their support during microfabrication and James Weston and Nikolas Giakoumidis of NYUAD for their support in taking SEM pictures and building a voltage divider, respectively. The device fabrication in PDMS was conducted in the microfabrication core facility of NYUAD. Lastly, we would like to thank Rebecca Pittam from the NYUAD Center for Digital Scholarship for video shooting and editing.
Materials
| Name | Company | Catalog Number | Comments |
| Poly(Styrenesulfonic Acid) Sodium Salt | Polysciences | 08772 | |
| Poly(allylamine) Solution | Sigma Aldrich | 479144-5G | |
| Silica Microsphere - 300 nm | Polysciences | 24321 | |
| Silica Microsphere - 500 nm | Polysciences | 24323 | |
| Silica Microsphere Carboxyl Functional - 500 nm | Polysciences | 24753 | |
| Silica Microsphere Amine Functional - 500 nm | Polysciences | 24756 | |
| Sylgard 184 Silicone Elastomer kit | Dow Corning | ||
| Trichlorosilane | Sigma Aldrich | 175552 | |
| Ultrasonic Cleaner | Branson | 3510 | |
| Tube Rotator | VWR | 10136-084 | |
| Vortex Mixer | WiseMix | VM-10 | |
| Microcentrifuge | VWR | Micro 1207 | |
| Plasma Cleaner | Harrick Plasma | PDC-001-HP | |
| PDMS Mixer | Thinky | ARE-250 | |
| Oven | Thermo Scientific | PR305220M | |
| Epi-fluorescence Microscope | Nikon | Eclipse Ti | |
| CCD Camera | Andor | Clara | |
| Platinum Electrodes | Alfa Aesar | 43014 | |
| Source Meter | Keithley | 2400 | |
| Digital Multimeter | Extech | 410 | |
| Microscopy Glass Slides | Thermo Scientific | 2951-001 | |
| Tween 20 | Merck Millipore | 822184 | |
| Sodium chloride | Fisher Scientific | 7646-14-5 | |
| Sodium phosphate monobasic | Sigma Aldrich | 71505 | |
| Sodium phosphate dibasic | Sigma Aldrich | S3264 | |
| DNA | IDT | CAA CCG ATG CCA CAT CAT TAG CTA C | |
| B-Phycoerythrin | Life Technologies | P-800 | |
| Dynamic light scattering system for Zeta Potential Measurement | Malvern | Zetasizer Nano S | |
| Photoresist | Shipley | SPR700-1.0 | |
| Projection lithography | Nikon | NSR2005i9 | |
| Reactive Ion Etcher | Applied Materials | AME P5000 | |
| ICP deep reactive ion etcher | STS | STS-6" | |
| Contact lithography | Electronic Visions | EV620 | |
| Photoresist Coater Developer | SSI | SSI 150 | |
| Non-contact surface profiler | Wyko | NT 9800 |
References
- Mawatari, K., Kazoe, Y., Shimizu, H., Pihosh, Y., Kitamori, T. Extended-Nanofluidics: Fundamental Technologies, Unique Liquid Properties, and Application in Chemical and Bio Analysis Methods and Devices. Anal Chem. 86, 4068-4077 (2014).
- Tsukahara, T., Mawatari, K., Kitamori, T. Integrated extended-nano chemical systems on a chip. Chem Soc Rev. 39, 1000-1013 (2010).
- Mani, A., Zangle, T. A., Santiago, J. G. On the Propagation of Concentration Polarization from Microchannel-Nanochannel Interfaces Part I: Analytical Model and Characteristic Analysis. Langmuir. 25, 3898-3908 (2009).
- Aizel, K., et al. Enrichment of nanoparticles and bacteria using electroless and manual actuation modes of a bypass nanofluidic device. Lab Chip. 13, 4476-4485 (2013).
- Wang, Y. C., Stevens, A. L., Han, J. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal Chem. 77, 4293-4299 (2005).
- Karnik, R., et al. Electrostatic control of ions and molecules in nanofluidic transistors. Nano letters. 5, 943-948 (2005).
- Mao, P., Han, J. Y. Fabrication and characterization of 20 nm planar nanofluidic channels by glass-glass and glass-silicon bonding. Lab Chip. 5, 837-844 (2005).
- Mao, P., Han, J. Massively-parallel ultra-high-aspect-ratio nanochannels as mesoporous membranes. Lab Chip. 9, 586-591 (2009).
- Balducci, A., Mao, P., Han, J. Y., Doyle, P. S. Double-stranded DNA diffusion in slitlike nanochannels. Macromolecules. 39, 6273-6281 (2006).
- Yamada, M., Mao, P., Fu, J. P., Han, J. Y. Rapid Quantification of Disease-Marker Proteins Using Continuous-Flow Immunoseparation in a Nanosieve Fluidic Device. Anal Chem. 81, 7067-7074 (2009).
- Huh, D., et al. Tuneable elastomeric nanochannels for nanofluidic manipulation. Nat Mater. 6, 424-428 (2007).
- Chung, S., Lee, J. H., Moon, M. W., Han, J., Kamm, R. D. Non-lithographic wrinkle nanochannels for protein preconcentration. Adv Mater. 20, 3011-3016 (2008).
- Park, S. M., Huh, Y. S., Craighead, H. G., Erickson, D. A method for nanofluidic device prototyping using elastomeric collapse. Proc Natl Acad Sci. 106, 15549-15554 (2009).
- Zeng, Y., Harrison, D. J. Self-assembled colloidal arrays as three-dimensional nanofluidic sieves for separation of biomolecules on microchips. Anal Chem. 79, 2289-2295 (2007).
- Malekpourkoupaei, A., Kostiuk, L. W., Harrison, D. J. Fabrication of Binary Opal Lattices in Microfluidic Devices. Chem Mat. 25, 3808-3815 (2013).
- Merlin, A., Salmon, J. -. B., Leng, J. Microfluidic-assisted growth of colloidal crystals. Soft Matter. 8, 3526-3537 (2012).
- Schepelina, O., Zharov, I. PNIPAAM-modified nanoporous colloidal films with positive and negative temperature gating. Langmuir. 23, 12704-12709 (2007).
- Schepelina, O., Zharov, I. Poly(2-(dimethylamino)ethyl methacrylate)-Modified Nanoporous Colloidal Films with pH and Ion Response. Langmuir. 24, 14188-14194 (2008).
- Smith, J. J., Zharov, I. Ion transport in sulfonated nanoporous colloidal films. Langmuir. 24, 2650-2654 (2008).
- Gaspar, A., Hernandez, L., Stevens, S., Gomez, F. A. Electrochromatography in microchips packed with conventional reversed-phase silica particles. Electrophoresis. 29, 1638-1642 (2008).
- Lee, S. Y., et al. High-Fidelity Optofluidic On-Chip Sensors Using Well-Defined Gold Nanowell Crystals. Anal Chem. 83, 9174-9180 (2011).
- Hu, Y. L., et al. Interconnected ordered nanoporous networks of colloidal crystals integrated on a microfluidic chip for highly efficient protein concentration. Electrophoresis. 32, 3424-3430 (2011).
- Zhang, D. -. W., et al. Microfabrication-free fused silica nanofluidic interface for on chip electrokinetic stacking of DNA. Microfluid Nanofluid. 14, 69-76 (2013).
- Syed, A., Mangano, L., Mao, P., Han, J., Song, Y. A. Creating sub-50 nm nanofluidic junctions in a PDMS microchip via self-assembly process of colloidal silica beads for electrokinetic concentration of biomolecules. Lab Chip. 14, 4455-4460 (2014).
- Kim, S. J., Song, Y. A., Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: theory, fabrication, and applications. Chem Soc Rev. 39, 912-922 (2010).
- Fu, J. P., Mao, P., Han, J. Y. Continuous-flow bioseparation using microfabricated anisotropic nanofluidic sieving structures. Nat Protoc. 4, 1681-1698 (2009).
- Plecis, A., Nanteuil, C., Haghiri-Gosnet, A. M., Chen, Y. Electropreconcentration with Charge-Selective Nanochannels. Anal Chem. 80, 9542-9550 (2008).
- Ko, S. H., et al. Nanofluidic preconcentration device in a straight microchannel using ion concentration polarization. Lab Chip. 12, 4472-4482 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved