Method Article
Isolation of Lung Retinoid-Containing Cells by Cell Sorting
In This Article
Summary
The protocol described below is a simple and effective way to isolate retinoid-containing cells from highly heterogeneous lung cell populations by making use of specific retinoid autofluorescence and by employing fluorescent-activated cell sorting.
Abstract
Retinoids (vitamin A and its metabolites) are an essential lipid component of the alveolar microenvironment, and cell-type specific retinoid metabolism is required to maintain the functional health of the developing and adult lungs. Lung cells utilize specific pathways, allowing for the efficient uptake of circulating retinoids from the blood as retinol (ROH), followed by intracellular stepwise conversion of ROH into the transcriptionally active retinoid species, all-trans-retinoic acid (ATRA). ATRA-mediated (or retinoid-mediated) signaling is crucial for regulating lung alveolarization, surfactant production, angiogenesis, permeability, and immunity. Importantly, specific lung cells, including fibroblasts, can accumulate retinoids in the form of retinyl esters (RE), which can be stored or further mobilized as ROH for transfer to the neighboring cells when needed. Lung retinoid-containing cells can be isolated and collected from the single-cell suspension of digested lungs by making use of retinoid autofluorescence (the emission at 455 nm upon excitation at 350 nm) and by employing fluorescence-activated cell sorting (FACS). Additional cell-specific in vivo labeling of lung cells with red fluorescent protein allows isolating and collecting specific retinoid-containing lung cell populations. The collected cells can be directly analyzed or cultured for further analyses of cell morphology, gene expression, and responsiveness to pharmacological manipulations. This technique of isolation and application is important for animal model studies of lung health and lung injury to gain deeper insight into cellular aspects of retinoid metabolism in the lungs and lipid-mediated cellular communications.
Introduction
Retinoids (vitamin A and its metabolites) are an essential lipid component of the alveolar microenvironment, and cell-type specific retinoid metabolism and signaling are required to maintain the functional health of the developing and adult lung1,2,3,4,5,6,7,8,9,10,11,12,13,14. Lung cells utilize specific pathways, allowing for the efficient uptake of circulating dietary-derived retinoids from the blood as retinol (ROH)15,16,17,18,19, followed by intracellular stepwise conversion of ROH into the transcriptionally active retinoid species, all-trans-retinoic acid (ATRA)20. ATRA-mediated (or retinoid-mediated) signaling is achieved through ATRA interaction with its three distinct cognate nuclear hormone receptors, retinoic acid receptors (RARα, RARβ, and RARγ21,22), and is crucial for regulating lung alveolarization23,24,25,26,27,28,29, surfactant production30,31,32,33,34,35, angiogenesis36, permeability37, and immunity38,39,40. Importantly, specific lung cells, especially lung fibroblasts, can accumulate retinoids in the form of retinyl esters (RE), which can be stored or further mobilized as ROH for transfer to the neighboring cells when needed1.
The complexity of retinoid metabolism and signaling, as well as the cellular complexity of the lung, make studies aimed at exploring retinoid metabolism in the lung in vivo challenging. We have outlined a simple and robust protocol for isolating retinoid-containing cells (Figure 1) from highly heterogeneous lung cell populations by making use of specific retinoid autofluorescence (the emission at 455 nm upon excitation at 350 nm) and by employing fluorescence-activated cell sorting (FACS). The protocol does not require additional cell labeling except for viability staining if the goal of the study is to isolate and characterize primary live lung cells based on their ability to store retinoids. This significantly reduces the preparation time for cell sorting, eliminates the need for additional staining, and allows for isolating high yields of viable primary cells. However, if the goal of the study is to isolate and characterize specific lung cell populations (fibroblasts, endothelial, epithelial, or immune cells), additional cell sorting can be performed after labeling the sorted retinoid-containing cells with cell-specific antibodies.
Retinoid autofluorescence has been used in published studies to establish the identities of retinoid-containing cells and/or to quantify the abundance of these cells in the liver41,42,43,44, pancreas45,46, kidneys41,47, and lung41. Moreover, several research groups reported the use of retinoid fluorescence to isolate by FACS and study primary retinoid-containing cells from living tissues, including liver44,48,49,50,51,52,53 and lung1. In the current protocol, we show how specific cell populations can be labeled in vivo prior to isolating retinoid-containing cells using tdTomato (red fluorescent protein). tdTomato's spectral characteristics (the emission at 581 nm upon excitation at 554 nm54) and brightness do not interfere with retinoid autofluorescence and, therefore, make it convenient to achieve cell specificity during sorting. Given the critical role of uncompromised retinoid metabolism and signaling within the normal alveolar microenvironment1, the described technique of lung cell isolation is a useful tool in animal model studies of lung health and disease to gain deeper insight into cellular aspects of retinoid metabolism in the lungs and lipid-mediated cellular communications in vivo.
Protocol
All described procedures and experiments involving mice were carried out with the approval of the Institutional Animal Care and Use Committee (IACUC) of Rutgers University (IACUC ID: PROTO202200111) according to criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences55.
1. Considerations and preparations for the experiment
- Animal husbandry and manipulations
NOTE: Three-month-old (10-12 weeks-old) male and female mice can be used in the studies. Lecithin:retinol acyltransferase-deficient mice (Lrat-deficient, Lrat-/- mice56) on a C57BL/6J genetic background and age-matched littermates (wild type, Lrat+/+ mice) were used in the described experiments. Mice expressing the tdTomato gene in fibroblasts (F-tdT mice) were generated by crossing mice harboring a tdTomato reporter cassette (B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J, Jackson Lab strain #007914) with Col1a2-CreER mice (B6.Cg-Tg(Col1a2-Cre/ERT,-ALPP)7Cpd/2J, Jackson Lab strain #029567).- Employ the Cre-expressing mouse model, one of the many available options to label the target cell population in vivo.
NOTE: Depending on the choice of the target cell, Cre expression, the efficiency of Cre-mediated recombination, and cell specificity, the investigators may utilize any reliable Cre-expressing mouse model. - Use tdTomato transgene expression (upon tamoxifen treatment as described below) to label Col1a2+ fibroblasts, followed by their isolation from single cell suspensions of the digested lung as described below.
- Employ the Cre-expressing mouse model, one of the many available options to label the target cell population in vivo.
- Cre-mediated recombination and transgene activation in vivo
- Induce Cre expression in F-tdT mice through intraperitoneal (IP) injection of 2 mg of tamoxifen once every 24 h for a total of 5 consecutive days.
- Sanitize the injection site with 70% ethanol prior to injection. Use the mice for experiments 1 month after the final tamoxifen injection.
- Check the effectiveness of Cre-recombination prior to the sorting experiments. Confirm the effective Cre recombination and tdTomato expression by isolating the target cell population of lung fibroblasts from the tamoxifen-treated F-tdT mice using anti-Pdgfrα magnetic beads and magnetic-activated cell sorting (MACS).
- Preparation of solutions, plastic- and glassware
NOTE: Aseptic techniques should be followed for the procedures described below. The procedures involving solution preparations, tissue digestion, and cell isolation should be done under the laminar hood. Solutions should be sterilized by filtration using a 0.2 µm filter; surgical tools as well as labware should be sterilized by autoclaving.- Under the laminar hood, prepare Hanks' balanced salt solution (HBSS), calcium- and magnesium-free (HBSS without Ca2+/Mg2+, containing 5.3 mM KCl, 0.4 mM KH2PO4, 4.2 mM NaHCO3, 137.9 mM NaCl, 0.3 mM Na2HPO4, 5.6 mM D-Glucose) for the lung perfusion (5 mL per mouse). Sterilize the solution using a 0.2 µm filter and fill up the syringe with the solution.
- Under the laminar hood, prepare Hanks' balanced salt solution with calcium- and magnesium (HBSS with Ca2+/Mg2+, containing 1.3 mM CaCl2, 0.5 MgCl2·6H2O, 0.4 mM MgSO4·7H2O, 5.3 mM KCl, 0.4 mM KH2PO4, 4.2 mM NaHCO3, 137.9 mM NaCl, 0.3 mM Na2HPO4, 5.6 mM D-Glucose), containing 0.3 mg/mL of type IV collagenase and 1 mg/mL of dispase for the lung perfusion (10 mL per mouse). Sterilize the solution using a 0.2 µm filter and fill up the syringe with the solution.
- Under the laminar hood, prepare HBSS with Ca2+/Mg2+, containing 0.3 mg/mL of type IV collagenase, 1 mg/mL of dispase, and 5 mg/mL of DNase I for lung digestion (10 mL per mouse). Sterilize the solution using a 0.2 µm filter and fill up the syringe with the solution.
- Under the laminar hood, fill up a cell culture dish (35 mm 10 mm, one dish per collected lung from one mouse) with 2 mL of sterile HBSS with Ca2+/Mg2+. Place the dish(es) containing HBSS with Ca2+/Mg2+ on ice.
2. Lung perfusion, digestion, and collection of single-cell suspension
- Anesthetize the mouse by administering an anesthesia cocktail containing 10 mg/mL ketamine and 2 mg/mL xylazine at a dose of 0.01 mL/g body weight via IP injection.
- Make sure that the mouse is in a deep state of unconsciousness by assessing a toe pinch response. Remove the loose hair and visible dirt/debris from the surgical site, clip and wipe clean the surgical site using 70% alcohol for disinfection.
- Using sterile surgical tools, open the abdominal and chest cavities. Cut the ribs and the diaphragm to expose the lungs and the heart.
NOTE: This will assure an instantaneous death as the thoracotomy will cause a cessation of breathing while the mouse is anesthetized; if properly performed, this part of the procedure will take up to 3 min from the start of the procedure till the thoracotomy, followed by the death of the animal. - Cut the inferior vena cava and apply an absorbent pad to absorb the released blood.
- Perfuse the lungs in situ through the heart's right ventricle using a 10 mL syringe with a 25 G X 1" needle filled with 5 mL of sterile HBSS without Ca2+/Mg2+. If the perfusion is successful, the lung tissue will become white.
- Perfuse the lungs in situ using a 10 mL syringe with a 25 G X 1" needle filled with 10 mL of sterile HBSS with Ca2+/Mg2+, containing type IV collagenase (0.3 mg/mL) and dispase (1 mg/mL).
- Remove the lungs and transfer them into a cell culture dish (35 mm X 10 mm) with 2 mL of sterile HBSS with Ca2+/Mg2+.
- Under the laminar hood, rinse the lungs and mince them into small pieces using a sterile surgical blade (#20, fits handle #4). Transfer the minced lung into a 15 mL tube by adding the first 5 mL of HBSS with Ca2+/Mg2+ containing 0.3 mg/mL of type IV collagenase, 1 mg/mL of dispase, and 5 mg/mL of DNase I to the minced tissue and transferring it using a 10 mL serological pipette. Collect and transfer the remaining minced tissue residues by washing the cell culture dish with the remaining 5 mL of HBSS with Ca2+/Mg2+ containing 0.3 mg/mL of type IV collagenase, 1 mg/mL of dispase, and 5 mg/mL of DNase I.
- Incubate the minced lung tissue in HBSS with Ca2+/Mg2+ containing 0.3 mg/mL of type IV collagenase, 1 mg/mL of dispase, and 5 mg/mL of DNase I on a rotating shaker maintained at 37 °C for 45 min. At each of the three 15-min intervals, under the laminar hood, pass the minced tissue 10 times through a 10 mL serological pipette to better dissociate cells.
- Pass the resultant cell suspension through a 100 µm strainer to collect single cells into a 50 mL tube containing 20 mL of cold live cell imaging solution (physiological saline containing 20 mM HEPES, pH 7.4) containing less than 5% of fetal bovine serum (FBS). Collect the cells by centrifugation at 500 g for 10 min at 4 °C.
- Aspirate the supernatant and resuspend the cell pellet in 1 mL of red blood cell lysing buffer and leave the tube for 5 min at room temperature (RT) to eliminate erythrocytes. Add 20 mL of cold live cell imaging solution containing less than 5% FBS. Collect the cells by centrifugation at 500 g for 10 min at 4 °C.
- Aspirate the supernatant and resuspend the cell pellet in 20 mL of cold live cell imaging solution containing less than 5% FBS. Pass the resultant cell suspension through a 40 µm strainer to collect single cells into a 50 mL tube containing 20 mL of cold live cell imaging solution containing less than 5% FBS. Collect the cells by centrifugation at 500 g for 10 min at 4 °C.
- Aspirate the supernatant and resuspend the cell pellet in 10 mL of cold live cell imaging solution containing less than 5% FBS. Count the cells and adjust cell concentration to ~5-10 x 106 cells/ mL.
3. Isolation of retinoid-containing lung cells using Fluorescence Activated Cell Sorting (FACS)
- Take an aliquot of cell suspension and set it aside to be used as an unstained gating control. Add viability dye SYTOX Green to the remaining cell suspension (1:1000 dilution, final concentration 30 nM).
- Pass cell suspensions through the filter attached to the polystyrene 5 mL (12 x 75 mm) collecting tube.
- Proceed with FACS cell isolation by sorting live, individual retinoid-containing cells based on their emission at 455 nm. Perform sequentially singlet discrimination using a plot for forward scatter (forward scatter height/FSC-H versus forward scatter area/FSC-A). Exclude dead cells by scatter characteristics (side scatter) and staining with SYTOX Green.
- Gate, sort, and collect single, live, retinoid-containing cells using emission at 455 nm upon excitation at 350 nm.
NOTE: Using the described procedure, about 5·105 retinoid-containing lung cells can be collected from one mouse lung. - Perform FACS data analysis using flow cytometry software.
Results
Isolation of lung retinoid-containing cells
Mouse lungs (from Lrat+/+ and Lrat-/-) mice were enzymatically digested, and single-cell suspensions were prepared and subjected to FACS according to the procedure outlined above. Cell sorting and data acquisition were performed on a Cytek Aurora™ Cell Sorter System operated by SpectroFlo CS software version 1.3.0 using a 100 µm nozzle and 15 psi pressure. First, plots for forward scatter (forward scatter height/FSC-H versus forward scatter area/FSC-A) were applied to select and gate single cells (singlets), followed by dead cell discrimination using side scatter and SYTOX Green staining (Figure 2). Finally, to sort the target population of retinoid-containing cells, a distinct population of live single cells with high autofluorescence at λ = 455 nm was selected (Figure 2A). To confirm the specificity of the applied gaiting and overall approach, lung cell suspension from the Lrat-/- mice was used as a negative control for adjusting the gating of retinoid autofluorescence. Lrat-/- mice are unable to synthesize REs and cannot accumulate retinoids in their lungs1. Notably, using the identical FACS gating strategy, a distinct cell population with high autofluorescence at λ = 455 nm cannot be detected in the single cell suspension from the Lrat-/- lungs (Figure 2B).
Once collected, primary lung retinoid-containing cells can be directly analyzed or cultured for further analyses of retinoid concentrations, cell morphology, gene expression, and responsiveness to pharmacological manipulations (Figure 1).
Using this procedure, we were able to show earlier that different cell populations are present among lung retinoid-containing cells1. Specifically, by undertaking single-cell RNA sequencing of FACS-collected retinoid-containing cells, we showed that among these cells the stromal cell group (83% of total collected cells) was the most abundant, followed by endothelial (7% of total collected cells), epithelial (5% of total collected cells), and myeloid cells (about 5% of total collected cells)1. The data on the heterogeneity of isolated retinoid-containing lung cells do not diminish the specificity of the described procedure; rather, these data highlight previously unsuspected complexity of retinoid metabolism in the adult lung that involves phenotypically diverse cells and extensive retinoid-mediated cellular communication.
Isolation of lung Col1a2+ retinoid-containing cells
Given the presence of heterogeneous cell populations among isolated retinoid-containing cells, the investigators may wish to focus on studying a specific lung cell type involved in retinoid metabolism. For this purpose, additional in vivo cell-specific labeling of the target cell population can be performed.
Among highly heterogeneous lung cell populations, a subpopulation of lung mesenchymal stromal cells with fibroblastic characteristics, referred to as pulmonary lipid interstitial cells or lung lipofibroblasts, is the predominant cell type capable of accumulating retinoids 1,57,58. To label a subpopulation of lung fibroblasts in vivo, mice expressing the tdTomato gene in fibroblasts (F-tdT mice) were generated by crossing mice harboring a tdTomato reporter cassette with Col1a2-CreER mice. Cre expression in F-tdT mice was induced through ip injection of 2 mg of tamoxifen once every 24 h for a total of 5 consecutive days; one month after the final tamoxifen injection, the mice were used for experiments. This approach allowed for labeling Col1a2+ fibroblasts with tdTomato protein and their isolation from single cell suspensions of the digested lung using a procedure described above. The approach allows to capture live (SYTOX Green negative), single tdTomato+ cells (Figure 3). Next, a subpopulation of retinoid-containing cells can be segregated from all of the captured tdTomato+ cells using a gating strategy based on retinoid autofluorescence (Figure 3) as described above. In addition, this procedure allows the subsequent sorting and separate collection of different populations of tdTomato+ cells based on the intensity of retinoid fluorescence signal into high, intermediate, and low retinoid-containing cell subpopulations.
Effective Cre recombination and tdTomato expression were confirmed by isolating the target cell population of lung fibroblasts from the tamoxifen-treated F-tdT mice (Figure 4A) using anti-Pdgfrα magnetic beads and magnetic-activated cell sorting (MACS). In addition, HPLC analysis was undertaken to confirm the presence of retinoids in the sorted retinoid-containing cells (Figure 4B).
Once collected, primary lung retinoid-containing fibroblasts can be directly analyzed or cultured for further analyses of retinoid concentration, cell morphology, gene expression, and responsiveness to pharmacological manipulations. For example, one of the characteristic features of lung retinoid-containing fibroblasts isolated from a wild-type (Lrat+/+) animal is the presence of lipid droplets that can be visualized in cultured cells using standard immunocytochemistry techniques (Figure 4C).
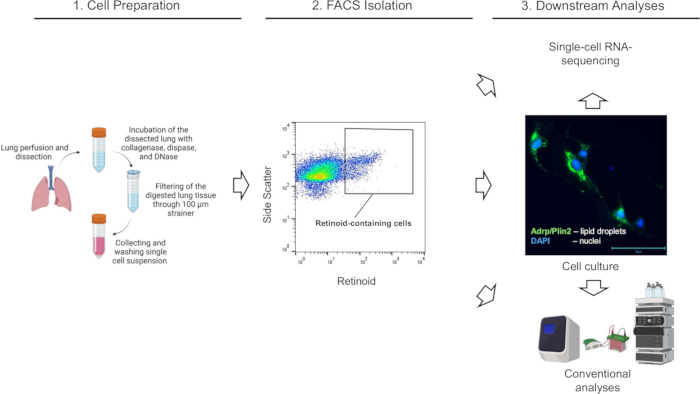
Figure 1: A schematic representation of the described experimental workflow. Please click here to view a larger version of this figure.
(Created with Biorender.com)

Figure 2: FACS isolation of lung retinoid-containing cells. (A) A gating strategy for sorting single, live, retinoid-containing cells (defined by the enclosed area) using emission at λ = 455 nm upon excitation at λ = 350 nm from lung cell suspensions isolated from a wild-type C57Bl6/J (Lrat+/+) mouse. (B) A gating strategy for sorting single, live, retinoid-containing cells using emission at λ = 455 nm upon excitation at λ = 350 nm applied to lung cell suspensions isolated from a Lrat-/- mouse; the enclosed area depicts no detected retinoid-containing cells in the Lrat-/- lungs; numbers indicate the percentage of gated cells. Please click here to view a larger version of this figure.
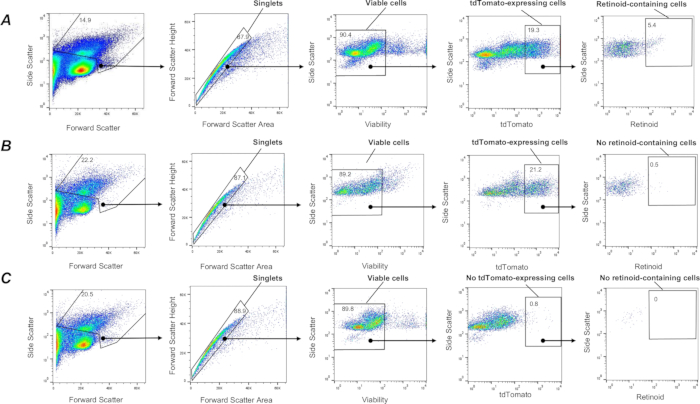
Figure 3: FACS isolation of lung retinoid-containing Col1a2+ fibroblasts. (A) A gating strategy for sorting single, live, tdTomato+ retinoid-containing cells (defined by the enclosed area) using emission at λ = 455 nm upon excitation at λ = 350 nm from lung cell suspensions isolated from a F-TdTom mouse on a wild type (C57Bl6/J) genetic background. Panel (B) A gating strategy for sorting single, live, tdTomato+ retinoid-containing cells (defined by the enclosed area) using emission at λ = 455 nm upon excitation at λ = 350 nm applied to lung cell suspensions isolated from a F-TdTom mouse on a Lrat-/- (C57Bl6/J) background; enclosed area depicts no detected retinoid-containing cells in the lungs from F-TdTom mice on a Lrat-/- background. (C) A gating strategy for sorting single, live, tdTomato+ retinoid-containing cells (defined by the enclosed area) using emission at λ = 455 nm upon excitation at λ = 350 nm applied to lung cell suspensions isolated from a tdTomato- mouse; enclosed area depicts no detected tdTomato+; numbers indicate the percentage of gated cells. Please click here to view a larger version of this figure.
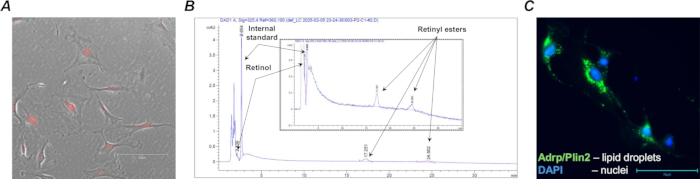
Figure 4: Retinoid-containing Col1a2+ / tdTomato+ fibroblasts. (A) A representative microphotograph of MACS isolated wild type (Lrat+/+) Col1a2+ / tdTomato+ lung cells in culture; the cultured cells with characteristic tdTomato fluorescence were captured using TX Red filter and 20x magnification; the scale bar is 150 µm. (B) An HPLC profile (with inserted magnification) showing characteristic peaks and retention times of retinoid species (retinol and retinyl esters) extracted from 1·105 sorted retinoid-containing cells. (C) A representative microphotograph of FACS isolated wild type (Lrat+/+) Col1a2+ / tdTomato+ lung cells in culture stained for lipid droplet-associated adipose differentiation-related protein (Adrp/Perilipin 2); the stained cells were captured using GFP filter and 40x magnification; the scale bar is 75 µm. Please click here to view a larger version of this figure.
Discussion
The ability of vitamin A detection in human and animal tissues and its histologic visualization using fluorescent microscopy was first reported as early as the 1940s59,60. The phenomenon of retinoid autofluorescence was then successfully applied to studies aimed at locating high concentrations of vitamin A in tissues in vitro61 and characterizing the morphogenesis of animal embryonic tissues62 using fluorescent microscopy. It was later observed and confirmed experimentally that the distinctive autofluorescence of cellular retinoids (excitation at λ = 350 nm with the characteristic blue-green emission at λ = 455 nm) is associated with specific cell types and, therefore, can be applied to isolate and characterize these cells63,64,65.
Retinoid autofluorescence has been used in published studies to establish the identities of retinoid-containing cells and/or to quantify the abundance of these cells in the liver41,42,43,44, pancreas45,46, kidneys41,47, and lung41. Moreover, several research groups reported the use of retinoid fluorescence to isolate by FACS and study primary retinoid-containing cells from living tissues, including liver44,48,49,50,51,52,53 and lung1.
Here, we describe the protocol where retinoid autofluorescence can be used as the primary feature to identify and sort lung retinoid-containing cells using FACS. The fluorescent retinoids detected include retinol and retinyl esters only. Thus, only cells containing these vitamin A derivatives (primarily retinyl esters) can be sorted. In addition, we provide additional advancement of the protocol where the investigators may utilize additional cell-specific labeling approaches to narrow down the cell specificity. This can be achieved by labeling lung retinoid-containing cells with fluorochrome-conjugated antibodies and additional sorting1. Alternatively, additional cell-specific labeling of lung cells can be achieved in vivo by expressing reporter fluorescent proteins using cell-specific Cre-recombinase expressing animal models following validation of Cre expression efficiency and cell specificity. The protocol described here highlights the use of Co1a2-driven expression of tdTomato protein in lung fibroblasts as one of the many options of this approach. However, this approach can be applied to other lung cell types, including endothelial, epithelial, and myeloid cells, when an appropriate Cre expression model is used.
The described protocol can also be applied to cell isolations from the injured (inflamed, fibrotic, etc.) lungs; however, in this case, several limitations should be taken into consideration. Lung injuries are associated with a progressive decline in retinoid concentrations1, which, therefore, can limit the application of this protocol and reduce cell yield. On the other hand, lung injuries are associated with fibroblast activation and elevated expression of extracellular matrix proteins, including Col1a2. Given that Cre expression is driven by the Col1a2 promoter, tdTomato expression can be enhanced, thus affecting the number of cells expressing tdTomato as well as the intensity of tdTomato signal in the lungs of the F-tdT mice that were used in our study.
Taken together, the protocol described here provides a specific and powerful tool to gain deeper insight into cellular aspects of retinoid metabolism in the lungs and lipid-mediated cellular communications in vivo.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was funded by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) R01 HL171112 (to I.S.), a career development award (to I.S) from Rutgers Center for Environmental Exposures & Disease funded by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) P30 ES005022, and start-up funds from Rutgers, The State University of New Jersey (to I.S.). The authors would like to acknowledge the staff of the Immune Monitoring and Flow Cytometry Shared Resource at the Rutgers Cancer Institute (supported, in part, with funding from the NCI-CCSG P30CA072770-5920) for their contributions to the work presented in this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 10 mL serological pipette | Avantor/VWR | 76452-284 | |
| 100 µm strainer | Greiner Bio-One | 542000 | |
| 15 mL falcon tube | Corning | 352099 | |
| 40 µm strainer | Greiner Bio-One | 542040 | |
| 50 mL falcon tube | Corning | 352070 | |
| Cell culture dish, 35 mm ´ 10 mm | Corning | 430165 | |
| Cell sorting media | Gibco | A59688DJ | |
| Collagenase type IV | Worthington Biochemical Corporation | LS004188 | |
| Cytek Aurora Cell Sorter System | Cytek Biosciences | ||
| Dispase II | Sigma-Aldrich | D4693 | |
| DNase I | Sigma-Aldrich | DN25 | |
| Falcon brand 5-ml polypropylene round bottom tube, 12 mm ´ 75 mm | Corning | 352063 | |
| Falcon brand 5-ml polystyrene round-bottom tube with cell-strainer cap, 12 mm ´ 75 mm | Corning | 352235 | |
| FlowJo software | Becton Dickinson | flow cytometry software | |
| HBSS with Ca2+/Mg2+ | Gibco | 14925-092 | |
| HBSS without Ca2+/Mg2+ | Gibco | 14175-095 | |
| Nalgene Rapid-Flow Sterile Disposable Bottle Top Filters | ThermoFisher | 595-3320 | |
| Red Cell lysis buffer | Sigma-Aldrich | R7757 | |
| SpectroFlo CS software | Cytek Biosciences | Version 1.3.0 | |
| Surgical Design Royaltek Stainless Steel Surgical Scalpel Blades | Fisher Scientific | 22-079-683 | |
| SYTOX Green dead cell stain | Invitrogen | S34860 | |
| Tamoxifen | Sigma-Aldrich | T2859 |
References
- Shmarakov, I. O., et al. Retinoids stored locally in the lung are required to attenuate the severity of acute lung injury in male mice. Nat Commun. 14 (1), 851 (2023).
- Bogue, C. W., Jacobs, H. C., Dynia, D. W., Wilson, C. M., Gross, I. Retinoic acid increases surfactant protein mRNA in fetal rat lung in culture. Am J Physiol Lung Cell Mol Physiol. 271 (5), L862-L868 (1996).
- Ng-Blichfeldt, J. -. P., et al. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine. 36, 461-474 (2018).
- Chen, F., et al. Prenatal retinoid deficiency leads to airway hyperresponsiveness in adult mice. J Clin Invest. 124 (2), 801-811 (2014).
- Esteban-Pretel, G., Marin, M. P., Renau-Piqueras, J., Barber, T., Timoneda, J. Vitamin A deficiency alters rat lung alveolar basement membrane: reversibility by retinoic acid. J Nutr Biochem. 21 (3), 227-236 (2010).
- Massaro, G. D., Massaro, D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 270 (2 Pt 1), L305-L310 (1996).
- Hind, M., Maden, M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J. 23 (1), 20-27 (2004).
- Belloni, P. N., Garvin, L., Mao, C. P., Bailey-Healy, I., Leaffer, D. Effects of all-trans-retinoic acid in promoting alveolar repair. Chest. 117 (5 Suppl 1), 235S-241S (2000).
- Veness-Meehan, K. A., Bottone, F. G., Stiles, A. D. Effects of retinoic acid on airspace development and lung collagen in hyperoxia-exposed newborn rats. Pediatr Res. 48 (4), 434-444 (2000).
- Cho, S. J., George, C. L., Snyder, J. M., Acarregui, M. J. Retinoic acid and erythropoietin maintain alveolar development in mice treated with an angiogenesis inhibitor. Am J Respir Cell Mol Biol. 33 (6), 622-628 (2005).
- Massaro, G. D., Massaro, D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 3 (6), 675-677 (1997).
- Stinchcombe, S. V., Maden, M. Retinoic acid induced alveolar regeneration: critical differences in strain sensitivity. Am J Respir Cell Mol Biol. 38 (2), 185-191 (2008).
- Fraslon, C., Bourbon, J. R. Retinoids control surfactant phospholipid biosynthesis in fetal rat lung. Am J Physiol. 266 (6 Pt 1), L705-L712 (1994).
- Zachman, R. D. Role of vitamin A in lung development. J Nutr. 125 (6 Suppl), 1634s-1638s (1995).
- van Bennekum, A. M., et al. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J Lipid Res. 40 (3), 565-574 (1999).
- Blaner, W. S., et al. Lipoprotein lipase hydrolysis of retinyl ester: possible implications for retinoid uptake by cells. J Biol Chem. 269 (24), 16559-16565 (1994).
- Trites, M. J., Febbraio, M., Clugston, R. D. Absence of CD36 alters systemic vitamin A homeostasis. Sci Rep. 10 (1), 20386 (2020).
- Wassef, L., Quadro, L. Uptake of dietary retinoids at the maternal-fetal barrier: in vivo evidence for the role of lipoprotein lipase and alternative pathways. J Biol Chem. 286 (37), 32198-32207 (2011).
- Spiegler, E., Kim, Y. -. K., Wassef, L., Shete, V., Quadro, L. Maternal-fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta Mol Cell Biol Lipids. 1821 (1), 88-98 (2012).
- Kedishvili, N. Y. Enzymology of retinoic acid biosynthesis and degradation. J Lipid Res. 54 (7), 1744-1760 (2013).
- Channabasappa, S., Caldwell, S., Kanthan, R., Singh, B. Retinoid receptors are expressed in mouse and human lungs. Anat Rec. 305 (9), 2281-2289 (2022).
- Kimura, Y., et al. Retinoid receptors in the developing human lung. Clin Sci (Lond). 103 (6), 613-621 (2002).
- Yang, L., Naltner, A., Yan, C. Overexpression of dominant negative retinoic acid receptor alpha causes alveolar abnormality in transgenic neonatal lungs. Endocrinology. 144 (7), 3004-3011 (2003).
- Snyder, J. M., et al. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res. 57 (3), 384-391 (2005).
- Gao, R. -. w., et al. Retinoic acid promotes primary fetal alveolar epithelial type II cell proliferation and differentiation to alveolar epithelial type I cells. In Vitro Cell Dev Biol Anim. 51 (5), 479-487 (2015).
- Dirami, G., et al. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol. 286 (2), L249-L256 (2004).
- Massaro, G. D., Massaro, D., Chambon, P. Retinoic acid receptor-alpha regulates pulmonary alveolus formation in mice after, but not during, perinatal period. Am J Physiol Lung Cell Mol Physiol. 284 (2), L431-L433 (2003).
- Massaro, G. D., et al. Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics. 4 (1), 51-57 (2000).
- McGowan, S., et al. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 23 (2), 162-167 (2000).
- Yan, C., et al. Retinoic acid-receptor activation of SP-B gene transcription in respiratory epithelial cells. Am J Physiol Lung Cell Mol Physiol. 275 (2), L239-L246 (1998).
- Ghaffari, M., Whitsett, J. A., Yan, C. Inhibition of hSP-B promoter in respiratory epithelial cells by a dominant negative retinoic acid receptor. Am J Physiol Lung Cell Mol Physiol. 276 (3), L398-L404 (1999).
- Yang, L., et al. Synergy between signal transducer and activator of transcription 3 and retinoic acid receptor-α in regulation of the surfactant protein b gene in the lung. Mol Endocrinol. 18 (6), 1520-1532 (2004).
- Metzler, M. D., Snyder, J. M. Retinoic acid differentially regulates expression of surfactant-associated proteins in human fetal lung. Endocrinology. 133 (5), 1990-1998 (1993).
- George, T. N., Snyder, J. M. Regulation of surfactant protein gene expression by retinoic acid metabolites. Pediatr Res. 41 (5), 692-701 (1997).
- Naltner, A., Ghaffari, M., Whitsett, J. A., Yan, C. Retinoic acid stimulation of the human surfactant protein B promoter is thyroid transcription factor 1 site-dependent. J Biol Chem. 275 (1), 56-62 (2000).
- Ng-Blichfeldt, J. P., et al. Deficient retinoid-driven angiogenesis may contribute to failure of adult human lung regeneration in emphysema. Thorax. 72 (6), 510-521 (2017).
- Lochbaum, R., et al. Retinoic acid signalling adjusts tight junction permeability in response to air-liquid interface conditions. Cell Signal. 65, 109421 (2020).
- Mamidi, S., Hofer, T. P., Hoffmann, R., Ziegler-Heitbrock, L., Frankenberger, M. All-trans retinoic acid up-regulates prostaglandin-e synthase expression in human macrophages. Immunobiology. 217 (6), 593-600 (2012).
- Hashimoto, S., et al. Retinoic acid differentially regulates interleukin-1beta and interleukin-1 receptor antagonist production by human alveolar macrophages. Leuk Res. 22 (11), 1057-1061 (1998).
- Li, S., Lei, Y., Lei, J., Li, H. Alltrans retinoic acid promotes macrophage phagocytosis and decreases inflammation via inhibiting CD14/TLR4 in acute lung injury. Mol Med Rep. 24 (6), (2021).
- Nagy, N. E., et al. Storage of vitamin A in extrahepatic stellate cells in normal rats. J Lipid Res. 38 (4), 645-658 (1997).
- Thompson, K. C., et al. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation in vitro. Hepatology. 28 (6), 1518-1524 (1998).
- Zhang, X. Y., Sun, C. K., Wheatley, A. M. A novel approach to the quantification of hepatic stellate cells in intravital fluorescence microscopy of the liver using a computerized image analysis system. Microvasc Res. 60 (3), 232-240 (2000).
- D'Ambrosio, D. N., et al. Distinct populations of hepatic stellate cells in the mouse liver have different capacities for retinoid and lipid storage. PLoS One. 6 (9), e24993 (2011).
- Apte, M. V., et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 43 (1), 128-133 (1998).
- Kim, N., et al. Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut. 58 (10), 1382-1390 (2009).
- Kida, Y., et al. Characterization of vitamin A-storing cells in mouse fibrous kidneys using Cygb/STAP as a marker of activated stellate cells. Arch Histol Cytol. 70 (2), 95-106 (2007).
- Ogawa, T., et al. Identification of vitamin A-free cells in a stellate cell-enriched fraction of normal rat liver as myofibroblasts. Histochem Cell Biol. 127 (2), 161-174 (2007).
- Tacke, F., Weiskirchen, R. Update on hepatic stellate cells: pathogenic role in liver fibrosis and novel isolation techniques. Expert Rev Gastroenterol Hepatol. 6 (1), 67-80 (2012).
- Bartneck, M., et al. Isolation and time lapse microscopy of highly pure hepatic stellate cells. Anal Cell Pathol (Amst). , (2015).
- Balaphas, A., et al. Optimized isolation and characterization of C57BL/6 mouse hepatic stellate cells. Cells. 11 (9), (2022).
- Kubota, H., Yao, H. L., Reid, L. M. Identification and characterization of vitamin A-storing cells in fetal liver: implications for functional importance of hepatic stellate cells in liver development and hematopoiesis. Stem Cells. 25 (9), 2339-2349 (2007).
- Larsen, A. K., et al. Autofluorescence in freshly isolated adult human liver sinusoidal cells. Eur J Histochem. 65 (4), (2021).
- Shaner, N. C., Patterson, G. H., Davidson, M. W. Advances in fluorescent protein technology. J Cell Sci. 120 (24), 4247-4260 (2007).
- National Research Council. . Guide for the Care and Use of Laboratory Animals. , (2011).
- O'Byrne, S. M., et al. Retinoid absorption and storage is impaired in mice lacking lecithin: retinol acyltransferase (LRAT). J Biol Chem. 280 (42), 35647-35657 (2005).
- McGowan, S. E., Torday, J. S. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 59, 43-62 (1997).
- McGowan, S. E. The lipofibroblast: more than a lipid-storage depot. Am J Physiol Lung Cell Mol Physiol. 316 (5), L869-L871 (2019).
- Meyer, K. A., Popper, H., Ragins, A. B. Histologic distribution of vitamin A in biopsy specimens of the liver. Arch Surg. 43 (3), 376-385 (1941).
- Popper, H. L. Histological demonstration of vitamin A in rats by means of fluorescence microscopy. Proc Soc Exp Biol Med. 43, 133-136 (1940).
- Van Exan, R. J., Hardy, M. H. Localization of vitamin A by autofluorescence during induced metaplastic changes in cultures of skin. In Vitro. 15 (8), 631-640 (1979).
- Schweigert, F. J., Siegling, C., Tzimas, G., Seeger, J., Nau, H. Distribution of endogenous retinoids, retinoid binding proteins (RBP, CRABPI) and nuclear retinoid X receptor beta (RXRbeta) in the porcine embryo. Reprod Nutr Dev. 42 (4), 285-294 (2002).
- Wake, K. Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structure in and around the liver sinusoids, and vitamin A-storing cells in extrahepatic organs. Int Rev Cytol. 66, 303-353 (1980).
- Senoo, H., Kojima, N., Sato, M. Vitamin A-storing cells (stellate cells). Vitam Horm. 75, 131-159 (2007).
- Senoo, H., et al. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 34 (12), 1247-1272 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved