Method Article
Extraction and Tissue Clearing Preparation of Mouse Brain-Spinal Cord Samples
* These authors contributed equally
In This Article
Summary
This study presents a protocol for extracting and preparing cleared whole-brain and spinal cord samples with preserved fluorescent signals, enhancing experimental efficiency and data integrity to advance neuroscience research.
Abstract
Neuroscience research focused on the central nervous system necessitates a thorough understanding of various factors, including cell distribution, neuronal connectivity, and molecular dynamics. Traditional methodologies for investigating the entire brain and spinal cord often involve isolating, sectioning, and scanning tissue slices, followed by the labor-intensive process of three-dimensional image reconstruction. This conventional approach can be both time-consuming and cumbersome. Advancements in tissue clearing and whole-organ imaging techniques have revolutionized the analysis of the entire brain and spinal cord. To maximize the potential of these innovative methods, it is essential to extract and clear brain samples while maintaining their connection to the spinal cord. This protocol provides a detailed and systematic guide for preparing samples of the brain connected to the spinal cord, outlining the extraction and clearing procedures. By streamlining these processes, this approach significantly enhances experimental efficiency and data integrity, thereby fostering advancements in neuroscience research and enabling more comprehensive investigations into the complexities of the central nervous system.
Introduction
Accurate mapping and analysis of nerve distribution provide valuable insights into the structural and functional organization of the central nervous system, paving the way for innovative therapeutic strategies and enhancing our overall comprehension of neural mechanisms. Currently, comprehensive video tutorials are lacking to guide researchers in preparing and extracting brain samples connected to the spinal cord and achieving successful tissue clearing.
There are several tissue-clearing approaches available: hydrophobic, hydrophilic, hydrogel-based, and tissue-expansion clearing methods1,2,3. The technique of tissue clearing is widely applied in the study of organs such as spleen4, lungs5, gastrocnemius muscle6, brain7, spinal cord8, and kidneys9. Our protocol will provide detailed instructions on how to prepare whole-brain samples connected to the spinal cord which have been labeled with fluorescent marker using STOCK Tg (Thy1-EGFP) MJrs/J strain mice. It will offer step-by-step guidance for sample extraction and describe the clearing protocol using tissue-clearing hydrophilic kits. This protocol helps researchers to master the entire process from brain-spinal cord sample extraction to preparation, and subsequent scanning. This will not only enhance experimental efficiency but also ensure the integrity and quality of the samples, providing more accurate and reliable data to support neuroscience research.
Compared to conventional sectioning, imaging, and three-dimensional reconstruction methods10,11, the approach presented here offers several key advantages, such as (1) enhanced structural integrity: by maintaining the whole-organ structure, this method reduces the risk of losing critical information that can occur with sectioning12; (2) comprehensive data acquisition: the use of a tissue clearing kit allows for detailed mapping of cells that are difficult to achieve with traditional techniques; (3) efficiency and accuracy: the protocol streamlines the entire process from sample extraction to imaging, reducing the time required by immunohistochemistry or staining procedures and minimizing errors associated with sectioning and mounting13.
The protocol overcomes the limitations of traditional sectioning methods, which often result in incomplete and fragmented data. By preserving the intact brain-spinal cord connection and utilizing modern tissue-clearing methods, this protocol provides a more holistic view of the central nervous system, which is crucial for understanding complex neural mechanisms and functions. The primary objective of this method is to enable comprehensive imaging of the central nervous system by preparing whole-brain samples connected to the spinal cord, which have been labeled with fluorescent neuronal markers. This protocol aims to facilitate detailed visualization of neural structures and cell distributions using advanced anatomical methods and tissue-clearing techniques.
Protocol
All animal experiments were conducted in compliance with the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The present study was approved by the Animal Care and Use Committee of Renji Hospital, Shanghai Jiaotong University School of Medicine. Here, 7-8 week-old STOCK Tg (Thy1-EGFP) MJrs/J strain male mice (C57BL/6J x CBA origin) were used for the present study. The animals were commercially obtained (see Table of Materials) and housed in standard cages (22 °C ± 2 °C, 12 h/12 h light/dark cycle, food, and water ad libitum).
1. Perfusion
- Deeply anesthetize adult mice using 1.25% tribromoethanol (0.02 mL/kg, see Table of Materials) administered by intraperitoneal injection using a 1 mL syringe. Check the depth of anesthesia by toe pinch response.
- Fix the mouse's limbs to the white acrylic board using steel clamps. Cut the skin between the two femurs of the mouse and continue cutting upwards until reaching the diaphragm. Open both sides of the thorax through the diaphragm to expose the heart using scissors and straight forceps.
- Insert a 0.7 mm infusion needle into the left ventricle. Make a 1-2 mm incision in the right atrial appendage.
- Perfuse with 1x PBS containing 10 U/mL sodium heparin at a rate of 10 mL/min with a peristaltic pump (see Table of Materials) to completely clear the blood. The liver turning completely pale indicates successful clearance.
- Perfuse with 50 mL of pre-cooled 4% PFA with a peristaltic pump at a rate of 10 mL/min. Monitor for signs such as mouse tail curling and muscle twitching.
NOTE: Unsuccessful perfusion can affect subsequent clearing processes.
2. Sample extraction
- Carefully dissect the entire brain and spinal cord using scissors and forceps to avoid damaging the sample.
- Separate the skin on the back and head of the mouse using ophthalmic scissors. Sever the spine at the point where the line connecting the upper rear edges of both limbs intersects with the vertebral column.
- Remove muscle and fat tissue from the neck and back of the mouse to expose the spine and skull. Cut along the midline gap between the left and right sides of the spine and spinal cord from the lower end of the vertebral canal using venus scissors.
- Remove the upper portion and bilateral sides of the severed spine to expose the spinal cord. Repeat the steps until reaching the brain connection.
- Place the tip of one blade of the venus scissors between the foramen magnum and the brain. Slide and cut along the mid-sagittal suture to open the skull. Use forceps to remove the skull and expose the brain.
- Remove the mouse brain-spinal cord tissue from the cranial end using forceps and venus scissors.
NOTE: Cuts or surface damage can be exacerbated during subsequent clearing, potentially causing unpredictable sample damage. Handle samples with care. - Use dissecting forceps to remove the spinal cord membrane under the stereomicroscope.
- Secure the brain-spinal cord sample to the fixing plate (see Table of Materials) using sutures. Add at least 20 times the sample volume of 4% PFA and place it on a shaker at 4 °C for slow agitation. Fix overnight for 16-24 h.
NOTE: Over-fixation leads to excessive protein cross-linking, reducing clearing efficiency14. Insufficient or delayed fixation can cause antigen degradation and tissue morphology destruction. - Discard the PFA and wash the sample 3x with 1x PBS. Ensure the volume of PBS is at least 10x the sample volume, fully submerging the sample. Each wash lasts 2 h with agitation on a shaker at a speed of no less than 60 rpm to thoroughly remove residual PFA.
3. Tissue clearing
NOTE: Here, a tissue clearing kit is used (see Table of Materials).
- Prepare the lipid-removal solution by mixing Solution A and Solution B at a mass ratio of 9:1.
NOTE: It is recommended that the volume of the lipid-removal solution should be more than 20x the volume of the sample. - Place the fixed tissue into a 50 mL centrifuge tube and add 50 mL of the lipid-removal solution. Place the centrifuge tube in a shaker at 37 °C, shaking gently at 60 rpm for lipid removal. Change the lipid-removal solution daily for 3-5 days. To determine completion, place the centrifuge tube on a light box with a scale line. If the black scale line is clearly visible and undistorted through the sample, the process is complete.
- Place the sample in 20 mL of Solution C on a shaker at 25 °C, shaking at less than 60 rpm for refractive index matching. Change Solution C after 24 h. Place the culture dish with the sample fully immersed in Solution C on a light box with a scale line. If the black scale line is clearly visible and undistorted through the sample, the matching process is complete.
NOTE: For adult mouse tissues, refractive index matching typically completes within 2 days.
4. Tissue embedding
- Prepare Gel solution with 147 g of Solution C in a 200 mL glass bottle, add 3.0 g of agarose, mix well by vortexing, then microwave until boiling and immediately turn off the microwave. Transfer the centrifuge tube to an incubator at 37 °C until the agarose dissolves.
NOTE: It is recommended to prepare the gel solution fresh as needed. - Embed the cleared sample with a 2 mm deep layer of 37 °C gel solution in the mold and cool in a 4 °C refrigerator for 30 min until semi-solidified. Place the refractive index-matched sample into the mold and add gel solution to be level or slightly below the top of the sample.
- Place the mold back in the 4 °C refrigerator for 2 h. Add gel solution until level with the mold surface. Cover with a coverslip. Accelerate solidification in the 4 °C refrigerator for 2 h. Store the embedded sample overnight in the 4 °C refrigerator. Proceed with imaging as soon as possible.
5. Scanning
- Remove the coverslip with tweezers and take out the embedded sample. Place the sample in a 50 mL tube and add 30 mL of imaging solution to fully submerge the sample.
- Apply 502 adhesive to firmly affix the sample onto the sample holder. Fix the holder onto the imaging device.
- Run the scanning software as described. Select the magnification level of 4x at the magnification section. Click the Calibration button to activate the calibration mode.
- Click the Preview button to enter preview mode. Click the Z-direction movement button to slowly move the sample upward from the bottom of the sample chamber until the generated light sheet passes through the sample.
- Select the appropriate laser channel and illumination mode option. Select the scanning path direction for the sample. Click the Auto Scan button to start capturing the sample.
Results
This protocol successfully isolates the entire brain connected with the spinal cord in STOCK Tg (Thy1-EGFP) MJrs/J strain male mice. It also renders the samples transparent, ensuring that the fluorescent signals are fully preserved and captured, providing clear and detailed images that maintain the integrity of the original fluorescence.
Figure 1 displays an intact brain-spinal cord, demonstrating that the dissection steps outlined in this protocol are precise. Additionally, the tissue clearing was performed with high accuracy, producing transparent specimens that enable the visualization of intricate neural structures. Furthermore, Figure 2 shows that the brain-spinal cord sample with the fluorescent signal imaging is remarkably well-preserved, providing clear and detailed images that retain the integrity of the original fluorescence. Figure 3 presents a higher magnification image of the individual cells depicted in Figure 2.
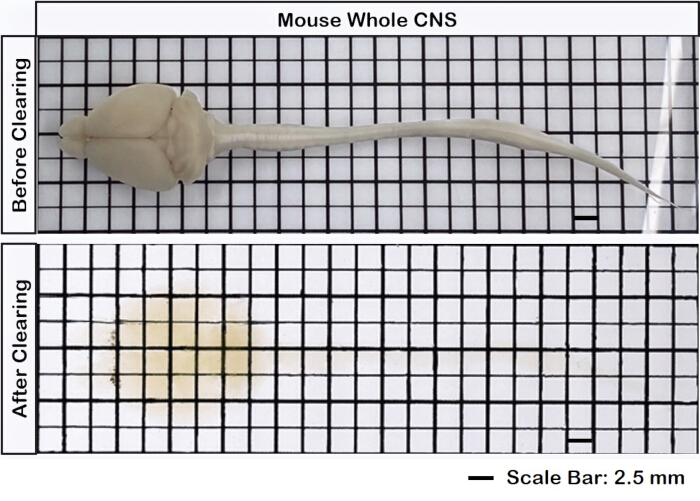
Figure 1: Intact brain spinal cord. The brain tissue is connected to the spinal cord, both before and after clearing. Scale bar = 2 mm. Please click here to view a larger version of this figure.
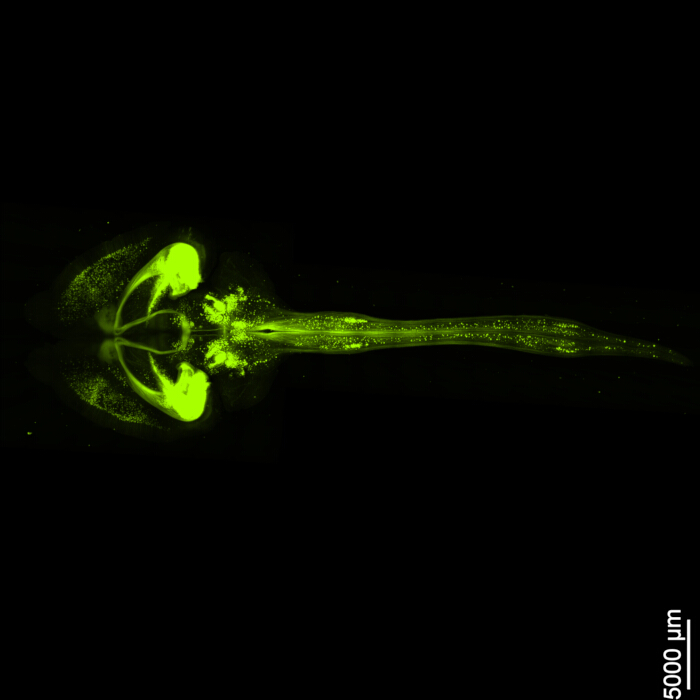
Figure 2: Three-dimensional fluorescent EGFP. The image shows the entire brain along with its connection to the spinal cord. Scale bar = 5000 µm. Please click here to view a larger version of this figure.
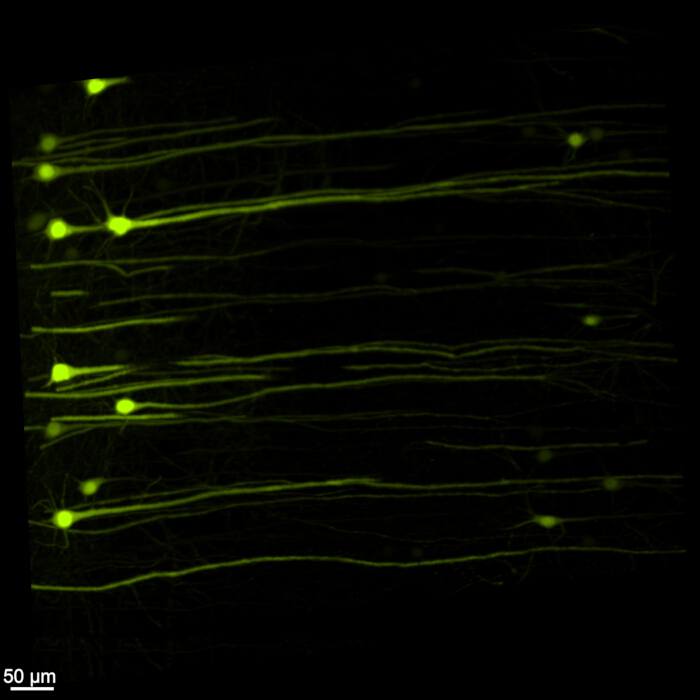
Figure 3: High magnification three-dimensional fluorescent EGFP. A higher magnification image highlighting individual EGFP-expressing cells within the brain-spinal cord sample. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Discussion
The proposed experimental protocol involves using mice whose brains are connected to spinal cords labeled with fluorescent neural tracing viruses. This protocol provides comprehensive and detailed instructions on preparing whole-brain samples that remain connected to the spinal cord. The protocol meticulously outlines each step, ensuring that researchers can replicate the process with precision.
Several critical steps in this protocol contribute to improving the quality of clearing and imaging. Completely extracting whole-brain samples that are connected to the spinal cords is an important task. Maintaining the integrity of the brain and the spinal cord is crucial for accurate tracing and subsequent analysis. When collecting samples, incisions or surface damage may exacerbate during the subsequent clearing process, potentially leading to unpredictable damage. To ensure the integrity of the samples, it is crucial to use the venus scissors and dissection tweezers specified in the materials for meticulous sampling. Securing the brain-spinal cord sample to the fixing plate with sutures is a crucial step to prevent deformation caused by gravity, pressure, or other factors while submerged in liquid.
Previous research has often concentrated exclusively on either the brain or spinal cord8 structure and function. However, the central nervous system functions as a cohesive unit. The transmission of signals from the brain downwards and the upward transmission of peripheral signals through the spinal cord to higher centers is a complex process. Access to comprehensive brain-spinal cord samples is advantageous for investigating the integration of these signals.
There are several primary tissue-clearing approaches available: hydrophobic, hydrophilic, hydrogel-based, and tissue-expansion clearing methods1,2,3. The underlying principles and methods are the same: substituting molecules that diffract light (such as lipids, pigments, and calcium phosphate) with other molecules that match the refractive index of the imaging medium2,15. Hydrophilic clearing methods typically pose fewer toxicity issues and are more effective at preserving the signals of fluorescent proteins and dyes1,2,3; however, they have several drawbacks. They can cause tissue swelling and volume fluctuations, potentially affecting the structural integrity of the samples. The use of hyperhydrating compounds like urea and formamide may lead to protein denaturation, impacting the biological relevance of the tissues. Additionally, while these methods are generally simple and compatible with fluorescent labeling, achieving optimal clearing for larger tissues often requires complex protocols involving various chemicals, which can complicate the process. Moreover, the need for precise chemical mixtures to adjust specific tissue compartments adds to the complexity, requiring careful optimization for specific applications.
Advancements in tissue clearing and imaging techniques have enabled us to map the distribution of nerves and volume of different cells that either intrinsically express fluorescent proteins or are labeled with fluorescently tagged antibodies, using light-sheet fluorescence microscopy16. The development of multiplexed molecular detection techniques in cleared tissues is advancing rapidly. These methods allow simultaneous profiling of various biological analytes from single samples, which is crucial for understanding complex biological systems like tumor microenvironments and dense neuronal networks. Additionally, methods like FISH and DNA Exchange Imaging show promise in enhancing multiplexing through DNA barcoding and rapid imaging. The future of highly multiplexed protein imaging may lie in using nucleic acid-conjugated antibodies for specific, sensitive, and multiplexed detection, enabling comprehensive analysis of complex tissues17.
In conclusion, we describe a promising way to extract and prepare cleared brain-spinal cord samples. Our study will help researchers to carry out relevant research. Our protocol achieved transparency tissue in PFA-perfused mice. However, a recent publication reports that scientists have discovered a non-toxic dye that can be applied to mouse skin to temporarily render it transparent18. This allows researchers to observe the physiological structures beneath the skin, including blood vessels and internal organs. This technology may become more advanced in the near future, with improvements in the thickness and degree of transparency, enabling in vivo observation of various mouse organs, including the central and peripheral nervous systems, respiratory system, and circulatory system19.
Disclosures
The authors declare no competing interests.
Acknowledgements
National Natural Science Foundation of China (grant NO. 82101249 and NO. 82471204 to XY Sun). Postdoctoral Research Foundation of China (grant NO. 2022M722125 to XY Sun).
Materials
| Name | Company | Catalog Number | Comments |
| 1 ml syringe | Shandong Weigao Group Medical Polymer | ||
| 502 glue | Deli Group | ||
| BD insulin syringe | Becton,Dickinson and Company | 328421 | |
| Bend toothed dissecting forceps | Jinzhong | JD1050 | |
| Circular steel clamp | Weili | ||
| Fine scissors | Jinzhong | y00030 | |
| Hemostatic forceps bent with tooth | Jinzhong | J31020 | |
| Hemostatic forceps straight with tooth | Jinzhong | J31010 | |
| Infusion needle 0.7mm | Kindly Group | ||
| Light box scale line | Nuohai Life Science | NH210901 | |
| Microdissection straight forceps | Jinzhong | WA3020 | |
| NobeliumSoftware | Nuohai Life Science | Scanning software | |
| paraformaldehyde | Biosharp | BL539A | |
| Peristaltic pumps | Nuohai Life Science | NH1000 | |
| Peristaltic pumps head | Nuohai Life Science | NH-15 | |
| Phosphate buffered saline | Servicebio | G4202 | |
| Sodium heparin | Shanghai Pharma | H31022051 | |
| STOCK Tg (Thy1-EGFP) MJrs/J strain mice | Jackson Laboratory | 007788 | |
| Straight toothed dissecting forceps | Jinzhong | JD1060 | |
| Tissue clearing Kit(hyrophilic) | Nuohai Life Science | NH-CR-210701 | |
| Tissue culture treater 100mm x 20mm | NEST | 704001 | |
| Tissue scissors | Jinzhong | J21040 | |
| Tribromoethanol | Aibei Biotechnology | M2910 | |
| Venus scissors | Jinzhong | YBC010 |
References
- Ueda, H. R., et al. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci. 21 (2), 61-79 (2020).
- Tainaka, K., Kuno, A., Kubota, S. I., Murakami, T., Ueda, H. R. Chemical principles in tissue clearing and staining protocols for whole-body cell profiling. Annu Rev Cell Dev Biol. 32, 713-741 (2016).
- Susaki, E. A., Ueda, H. R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: Toward organism-level systems biology in mammals. Cell Chem Biol. 23 (1), 137-157 (2016).
- Wu, M., et al. Innervation of nociceptor neurons in the spleen promotes germinal center responses and humoral immunity. Cell. 187 (12), 2935-2951.e19 (2024).
- Liu, T., et al. Local sympathetic innervations modulate the lung innate immune responses. Sci Adv. 6 (20), eaay1497 (2020).
- Qi, Y., et al. FDISCO: Advanced solvent-based clearing method for imaging whole organs. Sci Adv. 5 (1), eaau8355 (2019).
- Bagnoli, S., Terzibasi Tozzini, E., Cellerino, A. Whole-Brain clearing and immunofluorescence in Nothobranchius furzeri. Cold Spring Harb Protoc. 2023 (9), 698-704 (2023).
- Lu, T., Shinozaki, M., Nagoshi, N., Nakamura, M., Okano, H. 3D imaging of supraspinal inputs to the thoracic and lumbar spinal cord mapped by retrograde tracing and light-sheet microscopy. J Neurochem. 162 (4), 352-370 (2022).
- Saritas, T., Puelles, V. G., Su, X. T., Ellison, D. H., Kramann, R. Optical clearing and imaging of immunolabeled kidney tissue. J Vis Exp. (149), e60002 (2019).
- Song, J. H., et al. Precise mapping of single neurons by calibrated 3D reconstruction of brain slices reveals topographic projection in mouse visual cortex. Cell Rep. 31 (8), 107682 (2020).
- Fournel, R., Veruki, M. L., Hartveit, E. Digital reconstruction and quantitative morphometric analysis of bipolar cells in live rat retinal slices. J Comp Neurol. 530 (10), 1700-1728 (2022).
- Lin, R., et al. Cell-type-specific and projection-specific brain-wide reconstruction of single neurons. Nat Methods. 15 (12), 1033-1036 (2018).
- Peng, Y. C., et al. Rapid histological assessment of prostate specimens in the three-dimensional space by hydrophilic tissue clearing and confocal microscopy. J Histochem Cytochem. 70 (8), 597-608 (2022).
- Singhal, P., et al. Evaluation of histomorphometric changes in tissue architecture in relation to alteration in fixation protocol - An in vitro study. J Clin Diagn Res. 10 (8), ZC28-ZC32 (2016).
- Tainaka, K., et al. Chemical landscape for tissue clearing based on hydrophilic reagents. Cell Rep. 24 (8), 2196-2210.e9 (2018).
- Lee, S. H., Son, H. J. Second wave, late-stage neuroinflammation in cleared brains of aged 5xFAD Alzheimer's mice detected by macrolaser light sheet microscopy imaging. Int J Mol Sci. 24 (23), 242317058 (2023).
- Zhao, J., Lai, H. M., Qi, Y., He, D., Sun, H. Current status of tissue clearing and the path forward in neuroscience. ACS Chem Neurosci. 12 (1), 5-29 (2021).
- Ou, Z., et al. Achieving optical transparency in live animals with absorbing molecules. Science. 385 (6713), eadm6869 (2024).
- Ou, Z., et al. Achieving optical transparency in live animals with absorbing molecules. Science. 385, eadm6869 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved