1. Intradermal administration
- Most intradermal injections are aqueous-based compounds. The solutions must be physiologically buffered to have a neutral pH, in order to avoid tissue necrosis at the injection site.
- The needle size range is 25-30 gauge, the smallest possible.
- The dose range per injection site is 50-100 µL. Injecting excessive volumes can result in necrosis at the injection site or leakage of the compound out of the site due to pressure.
- For accurate placement of the needle into the intradermal space, it is necessary to anesthetize both mice and rats. Inhalation anesthesia allows for a rapid induction and recovery; however, injectable anesthesia has the advantage of providing ample time for preparation of the area and performing the injection.1
- Remove the hair at the injection site through the use of a depilatory cream or by shaving the area.
- Thoroughly remove any residual depilatory cream or hair debris.
- Apply a topical antiseptic, such as an iodine solution, chlorhexidine solution, or alcohol.
- Administration procedures
- Stretch the skin taut between the thumb and index finger. This provides stability to the skin when positioning the needle.
- Place the needle bevel up on the skin.
- Gently insert the needle into the skin between the epidermis and the dermis. Advance the needle just beyond the bevel.
- Inject the substance slowly. Injection of the compound will create a bleb, or small blister, in the skin.
- Pause after the injection to allow the skin to stretch and adjust before withdrawing the needle.
- Precautions
- Pulling back on the plunger is not necessary.
- If the needle is inserted into the subcutaneous space, no bleb is formed. Injecting too deeply results in a subcutaneous injection.
- Avoid blotting or wiping the area, as that can cause the compound to leak from the injection site.
- When performing multiple injections, space them so that the blebs do not overlap.
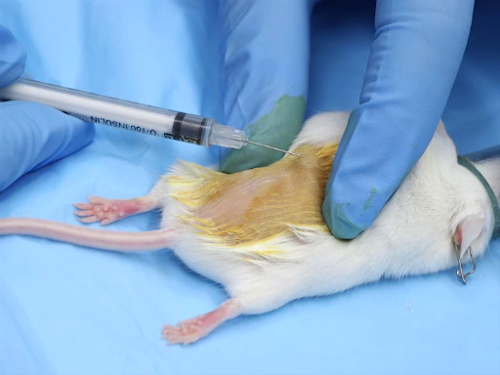
Figure 1. Intradermal injection in mice.
2. Intranasal administration
- Equipment
- Use a micropipetting unit pipette that can be calibrated to deliver an accurate volume.
- Disposable pipette tips should be used to avoid cross-contamination. TB syringes, blunt needles, and flexible tubing can also be used for dosing.
- Total injection volumes for rats should not exceed 40-100 µL, and should be given in 6-10 µL drops. For mice, the maximum total volume is 24 µL, given in 3-4 µL drops.
- Administration in conscious animals
- Manual restraint in conscious animals requires that the head be relatively immobile so that the pipette tip or blunt needle can be placed close enough to the nares to deliver the compound, but not so close as to poke or lacerate the nasal tissue.
- Restrain the animal and hold it in a vertical position.
- Place a small drop of a liquid compound at the nasal opening. The animal should inhale the droplet.
- Administer an additional volume, alternating nares until the entire volume has been given.
- When administering larger volumes, it is important to not constrict the chest during restraint. Chest compression impedes the ability of the animal to take sufficiently deep breaths to draw the liquid into the bronchi and lungs.
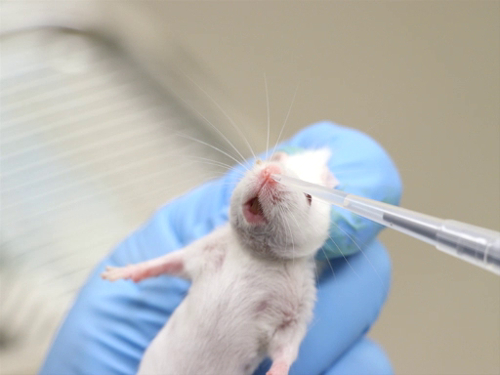
Figure 2. Intranasal administration in conscious mice.
- Administration in unconscious animals
- Using inhalation anesthetics allows for an immobilized animal during the approach and delivery of the compound. This eliminates the possibility of the animal biting the dosing equipment, the loss of the compound due to jerking of the head, and injury to the animal's nasal tissue, eyes, or facial skin. The animal is also less likely to snort and spray the compound from the nares upon administration.
- Place the animal in the supine position. The position of the head influences the placement of the solution in the nares. It has been demonstrated that the ideal position for CNS delivery is with the animal supine; this allows for better absorption.2
- Administer half of the compound directly into one side of the nasal opening, timing it with inhalation. Then, turn the animal over.
- As above, administer the other half of the volume into the other nasal opening.

Figure 3. Intranasal administration in unconscious mice.
3. Intracranial administration in neonatal mice and rats
- Mice or rats must be anesthetized for intracranial injections.
| Mouse |
Rat |
| Age (days) |
Needle gauge (g) |
Age (days) |
Needle gauge (g) |
| 0-7 |
29-30 |
0-5 |
27-29 |
| 7-14 |
27 |
5-10 |
25-27 |
| 14-28 |
25 |
10-14 |
25 |
| Age (days) |
Needle length (mm) |
Age (days) |
Needle length (mm) |
| 0-7 |
2 |
0-4 |
2-3 |
| 7-14 |
3 |
4-7 |
3 |
| 14-21 |
4 |
7-10 |
4 |
| 21-28 |
5 |
10-14 |
5 |
| Age (days) |
Volume (µL) |
Age (days) |
Volume (µL) |
| 0-5 |
<20 |
1-3 |
<20 |
| 6-20 |
<60 |
4-10 |
<60 |
| 20-28 |
<100 |
11-14 |
<100 |
Table 1. Needle gauge, needle length and maximum volume of intracranial administration as per the age of mice and rats.4
- Equipment
- Determine the correct needle gauge and maximum volume of administration as per Table 1.
- Prepare a needle guard prior to anesthetizing the animal.
NOTE: The injection depth of the needle is controlled through the use of a guard that is created with the needle cap.
- To create the guard, a needle is measured against the needle cap, and a mark is placed on the cap to indicate where to cut. The cut should be made so that 2 to 5 mm of the needle are exposed when the cap is replaced on the needle.
- The length of the exposed needle must be long enough to penetrate the skin and skull, and reach the desired depth in the brain.
- The needle lengths needed for mice and rats are listed in Table 1.
- A heating source is required to prevent hypothermia in the pups. There are several types: an electric heating pad set on low, a circulating water blanket, or a reusable chemical reaction heat pouch.
- Restraint
- Mouse and rat pups 10 days of age or younger do not require anesthesia for this procedure. Restrain them manually by holding them just behind the head and pulling the skin caudally to put slight pressure over the shoulders.
- Anesthetize pups over 10 days of age with isoflurane inhalation. Attach an induction chamber to a precision vaporizer or a bell jar with a cotton ball soaked with isoflurane. Once the pup is immobilized, the anesthesia is effective for about 40 seconds, which provides ample time for the injection.
- Injection technique
- Draw the substance into the syringe and place the cap/needle guide over the needle.
- The injection volume recommended as the maximum volume per neonatal mouse or rat is 100 µL, and that for weanling or older mice is up to 300 µL.
- To inject into the cerebral cortex of neonates, insert the needle 5 mm behind the eye, approximately 3 mm off the midline of the skull.
- The weanling mouse injection site is approximately half way between the eye and ear, and just off the midline.

Figure 4. Intracranial administration in a mouse pup.


















