Method Article
Rapid Generation of Primary Murine Melanocyte and Fibroblast Cultures
In This Article
Summary
This protocol outlines a rapid method to simultaneously generate melanocyte and fibroblast cultures from the skin of 0-4 day old mice. These primary cultures can be maintained and manipulated in vitro to study a variety of physiologically relevant processes, including skin cell biology, pigmentation, wound healing and melanoma.
Abstract
Defects in fibroblast or melanocyte function are associated with skin diseases, including poor barrier function, defective wound healing, pigmentation defects and cancer. Vital to the understanding and amelioration of these diseases are experiments in primary fibroblast and melanocyte cultures. Nevertheless, current protocols for melanocyte isolation require that the epidermal and dermal layers of the skin are trypsinized and manually disassociated. This process is time consuming, technically challenging and contributes to inconsistent yields. Furthermore, methods to simultaneously generate pure fibroblast cultures from the same tissue sample are not readily available. Here, we describe an improved protocol for isolating melanocytes and fibroblasts from the skin of mice on postnatal days 0-4. In this protocol, whole skin is mechanically homogenized using a tissue chopper and then briefly digested with collagenase and trypsin. Cell populations are then isolated through selective plating followed by G418 treatment. This procedure results in consistent melanocyte and fibroblast yields from a single mouse in less than 90 min. This protocol is also easily scalable, allowing researchers to process large cohorts of animals without a significant increase in hands-on time. We show through flow cytometric assessments that cultures established using this protocol are highly enriched for melanocytes or fibroblasts.
Introduction
Mammalian skin is a multilayered organ that protects the body from foreign pathogens and ultra violet irradiation (UVR). The skin also plays a critical role in homeostatic processes such as wound healing, temperature regulation and vitamin D production1,2,3. Mammalian skin consists of three major cell types: melanocytes, fibroblasts and keratinocytes. These cell types populate different layers of the skin, with keratinocytes making up the epidermis, fibroblasts residing in the dermis and melanocytes localizing to the epidermal-dermal junction and hair follicles4. Here, we detail a simple procedure that enables the concurrent generation of primary melanocyte and fibroblast cultures from murine skin.
Melanocytes are pigment-producing cells found in many locations throughout the human body, including the basal epidermis, iris, cochlea, brain and hair follicles5. The primary function of melanocytes is to generate and secrete melanin-containing vesicles called melanosomes5,6. Melanosomes contain two major classes of melanin: brown/black eumelanin and yellow/red pheomelanin6,7. Biochemical processes within the melanocyte regulate the relative abundance of each melanin species and help to determine hair, skin and eye color8,9. Melanin also serves to absorb UVR and protect sun-exposed tissues from mutagenesis10.
Melanocyte dysfunction can cause pigmentary defects and increase skin cancer susceptibility. For example, the hyper-pigmented skin patches characteristic of melasma are the result of focal melanin overproduction, whereas germline genetic mutations which compromise genes involved in melanin synthesis lead to albinism11,12. Intimate knowledge of melanocyte biology is required to develop strategies that will correct such pigmentary defects and ultimately improve the psychosocial well-being of individuals afflicted with these diseases. Deficits in melanin production and/or the preferential synthesis of pheomelanin are also associated with increased skin cancer risk10. This risk is believed to result from reduced UVR protection6,13. Thus, methods to enhance or restore eumelanin production in melanocytes may reduce the incidence of skin cancer in these populations.
Mesenchymal fibroblasts establish the connective tissue and structural support for all organs of the body, including the dermal layer of the skin14. Excretion of proteins such as collagen, elastin, laminin and fibronectin enable fibroblasts to form the extracellular matrix (ECM) that is essential for tissue integrity1,14. Fibroblasts also play essential roles in processes such as wound healing, inflammation, angiogenesis and cancer formation/progression1,15,16.
Similar to melanocytes, defects in fibroblast activation and function can promote tumorigenesis and disease. For example, inappropriate fibroblast activation commonly leads to the formation of fibrosis, resulting from the enhanced deposition of excess ECM components into the surrounding tissue. As fibroblasts maintain much of the body’s structural integrity, fibrosis promotes diseases that affect numerous tissues and organs, including idiopathic pulmonary fibrosis, systemic sclerosis, liver cirrhosis and cardiac fibrosis15. Fibroblasts also play a critical role in cancer16. Cancer associated fibroblasts (CAFs) are the most abundant non-malignant cells in the microenvironment of many tumors. CAFs have been shown to promote tumor proliferation, progression and therapeutic resistance by modulating tissue stiffness, local cytokine production and immune function16.
Primary cell cultures provide researchers with genetically tractable models to identify and mitigate melanocyte and fibroblast defects that lead to disease. However, current methods to establish melanocyte cultures are time consuming and technically challenging. The need for trypsinization and delicate separation of the epidermis and dermis contributes to variability in experimental yield and makes it difficult to perform large-scale experiments. Furthermore, protocols to simultaneously isolate melanocytes and fibroblasts from whole skin are currently lacking in the field.
We have developed a method to reduce the processing steps, variability and time required to establish melanocyte and fibroblast cultures from the same whole skin sample. Using a mechanical homogenization method followed by a brief digestion, our strategy significantly decreases the amount of hands-on time required to isolate primary melanocytes while enabling concurrent fibroblast isolation. The increased speed, efficiency and consistency of this protocol, in combination with the ability to isolate melanocytes from 0-4 day old mice, provides researchers the flexibility to perform a wider array of experiments than previously possible.
Protocol
Obtain approval from your institutional animal ethics committee before commencing this or any other study involving animals. Experiments performed in this protocol were approved by the Ohio State University’s Institutional Animal Care and Use Committee (IACUC, protocol #2012A00000134).
1. Protocol Preparation
NOTE: The following reagent preparation instructions are appropriate for the generation of 9 cm2 melanocyte and fibroblast cultures from a single mouse. Refer to the reagent preparation guide in Table 1 for larger scale isolations.
- Prepare 1.5 mL of Collagen Solution containing 50 µg/mL collagen in 0.1 M glacial acetic acid.
- In a laminar flow cabinet, coat one well of a 6-well cell culture dish with 8 µg/cm2 (1.44 mL) Collagen Solution. Ensure that the well is completely covered by the Collagen Solution. Incubate the dish at 37 °C for 3 h or at 4 °C overnight. Prior to use, wash the collagen-coated well twice with 1.35 mL of sterile phosphate buffered saline (PBS) (150 µL of PBS per 1 cm2).
- Assemble the tissue chopper in a laminar flow cabinet by carefully placing a chopping disk on the tissue chopper platform and securing a sterile blade to the movable arm.
- Prepare 3 mL of 1x Antibiotic/Antimycotic Solution by diluting 100x antibiotic/antimycotic stock solution in sterile PBS.
- Prepare 3 mL of fresh Skin Digestion Buffer containing 10% fetal bovine serum, 1% penicillin/streptomycin solution, 1% L-glutamine, 10 mg/mL collagenase type I, 0.25% porcine trypsin and 0.02 mg/mL deoxyribonuclease I in RPMI 1640.
- Prepare 6 mL of fresh Melanocyte Media containing 10% fetal bovine serum, 7% horse serum, 1% penicillin/streptomycin solution, 1% L-glutamine, 0.5 mM di-butyryl cyclic AMP (dbcAMP), 20 nM tetradecanoylphorbol 13-acetate (TPA) and 200 pM cholera toxin (CT) in Nutrient Mix F-12 Ham's media.
NOTE: Concentrated dbcAMP, TPA and CT stock solutions can be made, aliquoted and stored for >1 year at -80 °C. Base media lacking these components can be stored for up to 1 month at 4 °C. - Prepare 4 mL of Fibroblast Media containing 10% fetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine in Dulbecco’s Modified Eagle Medium. This media can be stored at 4 °C for up to 1 month.
- Prepare 40 mL of 4% Paraformaldehyde Solution by diluting 16% paraformaldehyde in PBS. Store any excess at 4 °C for 1 month or -20 °C for up to 1 year.
- Prepare a 1% saponin stock solution by mixing 0.5 g of saponin in 50 mL of PBS at 37 °C until the saponin has completely dissolved. Sterilize the solution for long-term storage using a 50 mL syringe fitted with a 0.2 µm PES filter. The resulting saponin stock solution can be kept at 4 °C for 1 month.
- Prepare 200 µL of 0.1% Saponin Solution per mouse by diluting 1% saponin stock solution in PBS containing 3% bovine serum albumin (BSA).
- Prepare 1 mL of Viability Solution by diluting 1 µL of fixable viability dye in 1 mL of PBS.
2. Melanocyte and Fibroblast Isolation
- Euthanize 0 to 4 day-old male and/or female C57Bl/6J pups by decapitation and remove extremities from the euthanized mice using surgical scissors.
- In a laminar flow cabinet, briefly roll the trunk of each mouse in a sterile Petri dish containing 70% ethanol. Remove the trunk from the ethanol and place it into an empty, sterile Petri dish.
- Using surgical scissors, sterilized in 70% ethanol, make an incision in the skin on the ventral side of the trunk starting from the neck to the tail. Peel the skin from the trunk of the mouse using sterile forceps.
- Place the skin dermis side down in a 6-well dish containing 3 mL of 1x Antibiotic/ Antimycotic Solution and incubate at room temperature for 2-3 min.
- Turn on the tissue chopper with the following settings in place: Slice thickness: 1 µm; Blade force: ~60% of the maximum; Speed: ~50% of the maximum.
- Transfer the skin, dermis side down, to a sterile tissue chopper disk and pass the skin completely through the activated tissue chopper 3 times.
- Transfer the homogenized skin to a sterile, 15 mL conical tube containing 3 mL of Skin Digestion Buffer. Mix the resulting suspension by pipetting up and down 10-15 times with a P1000 micropipette.
- Cap the conical tube and incubate the sample in a 37 °C water bath for 15 min, inverting the tube every 3-5 min.
- Pellet the cells in the skin homogenate by centrifuging the conical tube in a swinging bucket rotor at 750 x g for 5 min at room temperature.
- Using a P1000 micropipette, slowly and completely remove the Skin Digestion Buffer being careful not to disturb the pellet.
NOTE: A portion of whole skin can be saved at this stage and used as a control for Step 3: Confirmation of Cellular Purity. Strain these cells through a 70 μm cell strainer and then begin at step 3.5 for further processing. - Thoroughly resuspend the cell pellet in 1 mL of Melanocyte Media by pipetting up and down 15-20 times with a P1000 micropipette. Add the resulting cell solution dropwise to an uncoated well of a 6-well dish containing 1 mL of Melanocyte Media.
- Place the plated skin homogenate in a tissue culture incubator set at 37 °C and 5% CO2. Allow the cultures to incubate for 40 min.
NOTE: During this time, some fibroblasts in the skin homogenate will adhere to the uncoated dish while the melanocytes and keratinocytes remain in suspension. - Transfer the culture supernatant from the uncoated dish to one well of a pre-washed, collagen-coated 6-well dish. Add G418 to the media such that the final concentration is 100 ng/mL.
- Add 2 mL of Fibroblast Media to one well of the uncoated dish, now containing adherent fibroblasts.
- Incubate both cultures overnight in a tissue culture incubator set at 37 °C and 5% CO2.
- Separately aspirate the media and any debris from each culture, 16-24 h after plating. Wash each dish twice with 1 mL of sterile PBS, and then add 2 mL of fresh Melanocyte Media plus 100 ng/mL G418 to the melanocyte culture and 2 mL of fresh Fibroblast Media to the fibroblast culture.
- After the melanocyte cultures have been treated with G418 for 48 h, wash the cells twice with 1 mL of sterile PBS and add 2 mL of fresh Melanocyte Media without G418 to the culture.
NOTE: As fibroblasts in the melanocyte culture continue to die off post-G418 treatment, wash the dish with sterile PBS to remove dead cells and add fresh Melanocyte Media. Melanocyte and fibroblast cultures should be passaged when they reach 70-80% confluency.
3. Confirmation of Cellular Purity
- Aspirate the media from each culture and carefully wash each dish with 1 mL of sterile PBS.
- Add 0.7 mL of 0.25% trypsin to each culture and incubate the cultures in trypsin at 37 °C and 5% CO2 for 1 min.
- Dislodge the cells by pipetting the trypsin against the bottom of the dish using a P1000 micropipette.
- Add 0.7 mL of the appropriate media to each trypsinized culture and transfer the cell solution into a 1.5 mL microcentrifuge tube.
- Pellet the cells by centrifugation at 750 x g for 2 min at room temperature. Carefully remove and discard the supernatant using a P1000 micropipette.
NOTE: Steps 3.1-3.5 can be used to passage melanocyte and fibroblast cultures. The resulting pellet should be resuspended in the appropriate media and placed in a new, uncoated (fibroblasts) or pre-washed, collagen-coated (melanocytes) dish. - Resuspend the cell pellet in 0.5 mL of ice-cold PBS.
- Enumerate and transfer 0.5 million cells to a pre-chilled 1.5 mL microcentrifuge tube.
- Repeat step 3.5 and then resuspend the cells in 100 µL of Viability Solution. Incubate the cell suspension for 30 min at 4 °C in the dark.
- Repeat steps 3.5-3.6 twice.
- Repeat step 3.5, then fix the cells by resuspending the pellet in 100 µL of ice cold 4% Paraformaldehyde Solution. Incubate the suspension for 15 min at room temperature in the dark.
- Repeat step 3.5 twice, each time resuspending the pellet in 0.5 mL of 3% BSA in 1x PBS.
- Repeat step 3.5, then resuspend the cell pellet in 100 µL of 0.1% Saponin Solution. Incubate the suspension for 15 min at room temperature in the dark.
- Repeat step 3.5, then resuspend the cell pellet in 100 µL of 0.1% Saponin Solution containing 0.5 µg of rabbit anti-gp100 antibody, 0.5 µg of rabbit anti-Fibroblast-specific protein 1 (FSP1) antibody and 0.025 µg of mouse anti-Cytokeratin 14 (K14) antibody. Incubate the suspension for 1 h at room temperature in the dark.
NOTE: While the K14 antibody was purchased pre-conjugated to Alexa Fluor 647, the gp100 and FSP1 antibodies were conjugated to CF 555 and CF 488, respectively. The staining of control populations should also commence at this step. Control cell populations should be isolated from culture and processed as described in steps 3.5-3.12. - Repeat step 3.11 twice, to remove any unbound antibody.
- Repeat step 3.5, then resuspend the cell pellet in 200-400 µL of 3% BSA in 1x PBS.
- Pass the cell solution through a 40 µm cell strainer into a 5 mL polystyrene round-bottom tube and analyze the strained cells by flow cytometry.
Results
Male and female C57Bl/6J mice were euthanized on postnatal days 0-4 and the truncal skin was subjected to mechanical dissociation as described above. After chopping, the skin formed a viscous slurry lacking any sign of structural tissue. Centrifugation of this slurry resulted in the formation of a large cell pellet at the bottom of the conical tube and a layer of adipose floating on top of the supernatant. This adipose layer was discarded with the supernatant while the remaining cell pellet was resuspended and transferred to an uncoated well of a 6-well dish. The high density of cells caused the media to appear cloudy. However, after 40 min of incubation, larger cell and tissue conglomerates were observed in the media and adherent fibroblasts could be seen with an inverted light microscope (Figure 1).
The non-adherent cells from the fibroblast culture were transferred to one well of a collagen-coated 6-well dish in order to isolate melanocytes. Addition of G418 killed any remaining fibroblasts in the homogenate, leaving numerous dead cells floating in the media for the next 5 days (Figure 2A-C). After 4-5 days of growth, the plated melanocytes began to take on a stereotypical dendritic phenotype with melanocytic granules (Figure 2C). This phenotype persisted after passaging of the culture (Figure 2D). While clusters of contaminating keratinocytes were initially observed in the melanocyte cultures (Figure 2A), these populations were lost upon subsequent passaging.
Flow cytometric analyses were used to confirm the purity of the resulting primary melanocyte and fibroblast cultures. C5N mouse keratinocytes17, day 10 primary melanocyte cultures and day 6 primary fibroblast cultures were stained with: anti-gp100 (differentiated melanocytes), anti-Cytokeratin 14 (K14; keratinocytes) and anti-Fibroblast-specific protein 1 (FSP1; fibroblasts) (Figure 3). In these cells, gp100 staining was specific to the primary melanocytes, while FSP1 positivity was only observed in fibroblasts (Figure 3A,B). Expression of K14 was limited to C5N keratinocytes (Figure 3C). Additional analyses were performed to determine the purity of our melanocyte cultures 1, 2, 3, 4 and 10 days after isolation (Figure 4). Combined fibroblast and keratinocyte contamination never exceeded 25% of the total cells in culture (Figure 4B,C). Nevertheless, dramatic increases in gp100 were not observed until the fourth day in culture (Figure 4A). We attribute this observation to the reduced expression of gp100 in melanocyte precursors (i.e., melanoblasts), many of which appear fully differentiate by day 10 in culture (Figure 4A). In summary, these data demonstrate that our rapid isolation protocol simultaneously produces cultures enriched for melanocytes and fibroblasts.
| Step # | Reagent | 1 pup | 5 pups |
| 1.1 | Collagen Solution | 1.5 mL | 4.5 mL |
| 1.2 | Plate size | 6-well | 10 cm |
| 1.4 | Antibiotic / Antimycotic Solution | 3 mL | 15 mL |
| 1.5 | Skin Digestion Buffer | 3 mL | 15 mL |
| 1.6 | Melanocyte Media | 6 mL | 30 mL |
| 1.7 | Fibroblast Media | 4 mL | 20 mL |
Table 1: Reagent preparation guide for different cohort sizes.

Figure 1: Representative images of primary murine fibroblast cultures. Shown are 20x images of fibroblasts immediately following isolation (A), as well as 24 (B) and 48 (C) h post-isolation. Please click here to view a larger version of this figure.
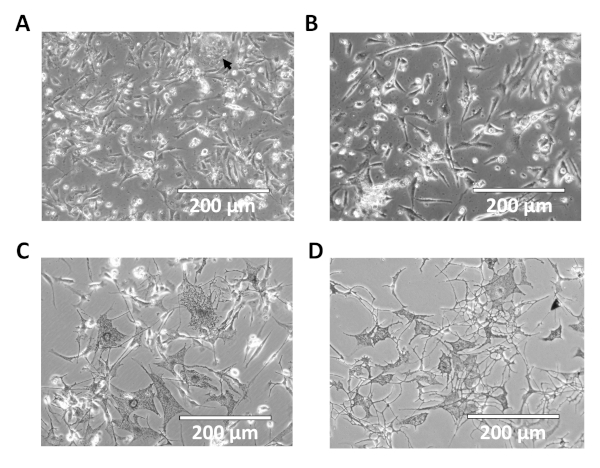
Figure 2: Representative images of primary melanocyte cultures. Shown are 20x images of melanocyte cultures 1 (A), 2 (B) and 4 days (C) post-isolation. Contaminating keratinocytes are indicated by the arrow in 'A'. (D) Representative image of primary melanocyte cultures after passaging. Please click here to view a larger version of this figure.

Figure 3: Flow cytometric assessments of culture purity. Representative histograms showing gp100 (A; differentiated melanocytes), FSP1 (B; fibroblasts) and K14 (C; keratinocytes) positivity in primary melanocytes (day 10), primary fibroblasts (day 6) and C5N keratinocytes. After gating on live cells, 10,000 events were analyzed from each population. Single color controls were used for compensation and the resulting data was visualized with FlowJo software. Please click here to view a larger version of this figure.
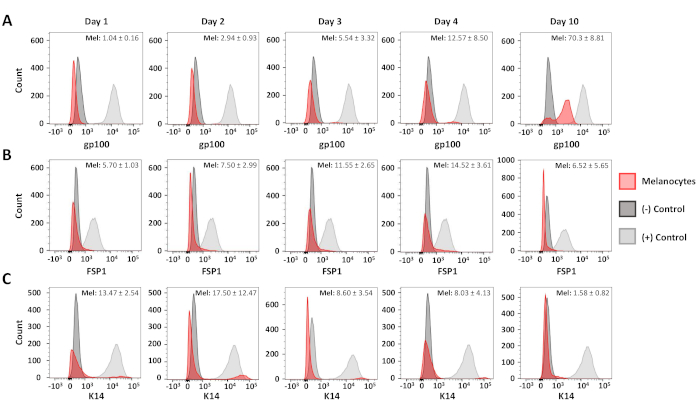
Figure 4: Time course analysis of melanocyte purity. Representative histograms showing melanocyte purity 1-10 days after initial isolation. Samples were stained, analyzed and graphed as described in Figure 3. Positive and negative control populations were as follows: gp100--human melanocytes (+), primary murine fibroblasts (-); FSP1--primary murine fibroblasts (+), human melanocytes (-); K14--C5N cells (+), human melanocytes (-). The average positivity and standard deviation for at least three distinct melanocyte cultures is shown in the upper right-hand corner of each graph. Please click here to view a larger version of this figure.
Discussion
The in vitro culture of primary melanocytes and fibroblasts has led to significant advancements in our understanding of skin biology and disease. This protocol improves upon prior melanocyte isolation methods by reducing the time and technical savvy needed to generate consistent melanocyte cultures while allowing for the simultaneous isolation of skin fibroblasts. A novel, time-saving element of this procedure is that the dermis and epidermis do not need to be separated. Instead, skin cells are isolated using the consistent chopping force and granularity of a mechanical tissue chopper along with selective media and plating techniques. By reducing processing complexity and hands-on time, this procedure also enables researchers to conduct large-scale experiments efficiently.
We recommend several steps to enhance the yield and consistency using this protocol. First, prior to step 2.4, we advise using curved forceps to scrape away any adipose tissue from the dermal side of the skin. This process will reduce the fat cake formed after centrifugation. Next, proper mechanical dissociation of the skin is critical to the success of this protocol and can be enhanced by rotating the homogenizer disk ~60° after each pass of the blade. Another key step is to ensure that all of the Skin Digest Buffer is removed in step 2.10. Failure to do so will hinder cell adhesion to the dish and significantly decrease yield. Because the pellet is not firmly affixed to the bottom of the 15 mL conical tube in step 2.10, the cells can be easily aspirated if the supernatant is not slowly removed. If thorough removal of the Skin Digestion Buffer is a challenge, we suggest washing the pellet in 2-3 mL of Melanocyte Media and then repeating steps 2.9-2.10 prior to plating. Finally, when resuspending the cell pellet in step 2.11, it is important to pipet vigorously in order to break up any clumps and ensure that the cells are evenly distributed on the surface of the cell culture dish. Debris and dead cells will be observed in the culture during the first few days after isolation. These dead cells may hinder the growth of the culture and should be removed by washing the dish with PBS and then adding fresh media.
This protocol outlines a method to generate primary melanocyte and fibroblast cultures from a single mouse. However, the procedure can be effectively adjusted to accommodate larger cohorts of mice (see Table 1). For larger cohorts, combining skin homogenate from 4-5 pups results in a 30-40% confluent 10 cm dish of melanocytes within 4-5 days of isolation. Several techniques can be used to improve the efficiency of this protocol when working with multiple animals. First, we typically euthanize the animals in sets of four, processing two animals at a time. We have found that homogenized skin from two pups can be combined at steps 2.7-2.8 by increasing the volume of Skin Digest Buffer to 6 mL. To save time, we begin isolating skin from the next pair of pups while the first set is undergoing homogenization and digestion. This approach ensures that the cell populations are isolated soon after euthanasia and reduces the processing time required for multiple animals. When working with older mice (i.e., postnatal days 2-4), we have found that passing the skin a fourth time through the active tissue chopper (step 2.6) enhances skin homogenization and cellular yield. Using this technique, we see no age-dependent difference in the melanocyte or fibroblast yield.
This protocol details how to simultaneously generate fibroblast and melanocyte cultures from the same skin sample. We have observed that keratinocytes in the initial melanocyte culture remain adhered to the dish when short-term trypsinization is performed along with the forceful dislodgement of melanocytes (see steps 3.2-3.3). While we have not optimized propagation methods for these remaining cells, we hypothesize that continued culture of such adherent populations in keratinocyte media supplemented with 100 ng/mL G418 could result in an enriched cell population.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors thank the Damon Runyon Foundation (Innovation Award #38-16 to C.E.B.) and Pelotonia (B.M.M.) for financial support. We are grateful to C. Haines and C. Wormsbaecher who provided comments to improve the manuscript text. This work benefitted from the Ohio State Comprehensive Cancer Center’s Analytical Cytometry Shared Resource which is supported by NIH P30 CA016058.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2 µm PES sterile syringe filter | VWR | 28145-501 | |
| 10 cm cell culture dish | Corning | 430167 | |
| 40 µm cell strainer | Fisher Scientific | 22363547 | |
| 5 mL polystyrene round-bottom tubes | Fisher Scientific | 352008 | |
| 6-well cell culture dish | Sigma-Aldrich | SIAL0516 | |
| 70 µm cell strainer | Fisher Scientific | 22363548 | |
| Antibiotic Antimycotic Solution (100x) | Sigma-Aldrich | A5955 | |
| Bovine Serum Albumin | Fisher Scientific | BP9706-100 | |
| CF 488A Mix-n-Stain Antibody Labeling Kit | Biotium | 92273 | |
| CF 555 Mix-n-Stain Antibody Labeling Kit | Biotium | 92274 | |
| Cholera Toxin | Sigma-Aldrich | C8052 | |
| Collagen from rat tail | Sigma-Aldrich | C7661 | |
| Collagenase Type I | Worthington Biochemicals | LS004156 | |
| Corning Penicillin/Streptomycin Solution | Fisher Scientific | 30-002-CL | |
| Cytokeratin 14 Antibody Alexa Fluor 647 | Novus Biologicals | NBP2-34403AF647 | |
| Deoxyribonuclease I | Worthington Biochemicals | LS002058 | |
| Di-butyryl cyclic AMP | Sigma-Aldrich | D0627 | |
| Dulbecco's Modified Eagle Medium | Gibco | 12800-082 | |
| Dulbecco's Phosphate Buffered Saline | Sigma-Aldrich | D8537 | |
| eBioscience Fixable Viability Dye eFluor 780 | Thermo Fisher | 65-0865-14 | |
| Ethanol, 200 proof | Fisher Scientific | 22032601 | |
| Fetal Bovine Serum | Sigma-Aldrich | 12306C | |
| FSP1/S100A4 antibody | Millipore Sigma | 07-2274 | |
| G418 Disulfide | P212121 | LGB-418-1 | |
| Glacial Acetic Acid | VWR | VWRV0714 | |
| Horse Serum | Fisher Scientific | 26050088 | |
| HyClone L-Glutamine | Fisher Scientific | SH3003402 | |
| McIlwain Tissue Chopper | Ted Pella | 10180 | |
| Melanoma gp100 antibody | Abcam | ab137078 | |
| Nutrient Mix F-12 Ham's Media | Sigma-Aldrich | N6760 | |
| Phorbol 12-Myristate 13-acetate | Sigma-Aldrich | P8139 | |
| Pierce 16% Formaldehyde | Thermo Fisher | 28908 | |
| Porcine Trypsin | Sigma-Aldrich | 85450C | |
| RPMI 1640 media | Sigma-Aldrich | R8758 | |
| Saponin | Sigma-Aldrich | S-7900 | |
| Tissue Chopper Blade | Ted Pella | 121-6 | |
| Tissue Chopper Plastic Disk | Ted Pella | 10180-01 | |
| Trypsin | VWR | VWRL0154-0100 |
References
- Rittie, L. Cellular mechanisms of skin repair in humans and other mammals. Journal of Cell Communucation and Signal. 10 (2), 103-120 (2016).
- Romanovsky, A. A. Skin temperature: its role in thermoregulation. Acta Physiologica (Oxf). 210 (3), 498-507 (2014).
- Holick, M. F., Smith, E., Pincus, S. Skin as the site of vitamin D synthesis and target tissue for 1,25-dihydroxyvitamin D3. Use of calcitriol (1,25-dihydroxyvitamin D3) for treatment of psoriasis. Archives of Dermatology. 123 (12), 1677-1683 (1987).
- Yamaguchi, Y., Hearing, V. J. Physiological factors that regulate skin pigmentation. Biofactors. 35 (2), 193-199 (2009).
- Yamaguchi, Y., Hearing, V. J. Melanocytes and their diseases. Cold Spring Harbor Perspectives in Medicine. 4 (5), (2014).
- Brenner, M., Hearing, V. J. The protective role of melanin against UV damage in human skin. Photochemistry and Photobiology. 84 (3), 539-549 (2008).
- Thody, A. J., et al. Pheomelanin as well as eumelanin is present in human epidermis. The Journal of Investigative Dermatology. 97 (2), 340-344 (1991).
- Swope, V. B., Abdel-Malek, Z. A. MC1R: Front and Center in the Bright Side of Dark Eumelanin and DNA Repair. International Journal of Molecular Science. 19 (9), (2018).
- Barsh, G. S. What controls variation in human skin color. PLoS biology. 1 (1), 27 (2003).
- Abdel-Malek, Z., et al. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigment Cell Research. 13, 156-162 (2000).
- Gronskov, K., Ek, J., Brondum-Nielsen, K. Oculocutaneous albinism. Orphanet Journal of Rare Diseases. 2, 43 (2007).
- Kwon, S. H., Hwang, Y. J., Lee, S. K., Park, K. C. Heterogeneous Pathology of Melasma and Its Clinical Implications. International Journal of Molecular Sciences. 17 (6), (2016).
- D'Orazio, J., Jarrett, S., Amaro-Ortiz, A., Scott, T. UV radiation and the skin. International Journal of Molecular Science. 14 (6), 12222-12248 (2013).
- Bonnans, C., Chou, J., Werb, Z. Remodelling the extracellular matrix in development and disease. Nature Reviews Molecular Cell Biology. 15 (12), 786-801 (2014).
- Kendall, R. T., Feghali-Bostwick, C. A. Fibroblasts in fibrosis: novel roles and mediators. Frontiers in Pharmacology. 5, 123 (2014).
- Kalluri, R. The biology and function of fibroblasts in cancer. Nature Reviews Cancer. 16 (9), 582-598 (2016).
- Dickson, M. A., et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Molecular and Cellular Biology. 20 (4), 1436-1447 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved