Method Article
Ex Vivo Imaging of Cell-specific Calcium Signaling at the Tripartite Synapse of the Mouse Diaphragm
In This Article
Summary
Here we present a protocol to image calcium signaling in populations of individual cell types at the murine neuromuscular junction.
Abstract
The electrical activity of cells in tissues can be monitored by electrophysiological techniques, but these are usually limited to the analysis of individual cells. Since an increase of intracellular calcium (Ca2+) in the cytosol often occurs because of the electrical activity, or in response to a myriad of other stimuli, this process can be monitored by the imaging of cells loaded with fluorescent calcium-sensitive dyes. However, it is difficult to image this response in an individual cell type within whole tissue because these dyes are taken up by all cell types within the tissue. In contrast, genetically encoded calcium indicators (GECIs) can be expressed by an individual cell type and fluoresce in response to an increase of intracellular Ca2+, thus permitting the imaging of Ca2+ signaling in entire populations of individual cell types. Here, we apply the use of the GECIs GCaMP3/6 to the mouse neuromuscular junction, a tripartite synapse between motor neurons, skeletal muscle, and terminal/perisynaptic Schwann cells. We demonstrate the utility of this technique in classic ex vivo tissue preparations. Using an optical splitter, we perform dual-wavelength imaging of dynamic Ca2+ signals and a static label of the neuromuscular junction (NMJ) in an approach that could be easily adapted to monitor two cell-specific GECI or genetically encoded voltage indicators (GEVI) simultaneously. Finally, we discuss the routines used to capture spatial maps of fluorescence intensity. Together, these optical, transgenic, and analytic techniques can be employed to study the biological activity of distinct cell subpopulations at the NMJ in a wide variety of contexts.
Introduction
The NMJ, like all synapses, is composed of three elements: a presynaptic terminal derived from a neuron, a postsynaptic neuron/effector cell, and a perisynaptic glial cell1,2. While the basic aspects of synaptic transmission were first demonstrated at this synapse3, many aspects of this process remain unknown, in part owing to the expression of the same molecules by the distinct cellular elements of this synapse. For example, receptors for both the purine adenine nucleotide ATP and acetylcholine (ACh), which are co-released by motor neurons at the vertebrate NMJ, are expressed by muscle, Schwann cells, and motor neurons, thus complicating the interpretation of any functional effect exerted by these substances (e.g., transmitter release or response, muscle force generation)4. Moreover, although the tripartite components of the NMJ are simple compared to, for example, neurons in the central nervous system which often exhibit multiple synaptic inputs, whether motor neurons, muscle cells, or Schwann cells vary in response to stimuli based on their intrinsic heterogeneity (e.g., embryonic derivation, fiber subtype, morphology) is unclear. In order to address each of these issues, it would be advantageous to simultaneously track the response of many cells within one synaptic element, as well as track, at the same time, such a response in either of the other separate elements. Conventional strategies using chemical dyes to measure calcium signaling cannot achieve these two goals, because bath-applied dye is taken up by multiple cell types after application to tissue, and intracellularly loaded dye can only be used to visualize individual or small cohorts of cells. Here, utilizing transgenic mice expressing GECIs designed to measure cell-specific calcium signaling, together with specific imaging and software tools5, we demonstrate the first of these two overall goals and discuss how the addition of new transgenic tools would help achieve the second. This technique will be useful for anyone interested in tracking calcium dynamics or other cellular signaling events observable through gene-encoded optical sensors in multiple cell populations at the same time.
Protocol
Animal husbandry and experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the IACUC at the University of Nevada.
1. Preparation of the Diaphragms and Phrenic Nerves from Transgenic Mice
- Purchase transgenic mice and oligonucleotide primers to genotype these mice.
Note: The primers are listed on the “Information” page for each of these mice.- Breed a 3- to 6-month-old mouse expressing one copy of the appropriate transgenic/knock-in Cre-driver allele and zero copies of the conditional GCaMP3/6 allele with a second mouse of the same age expressing one or two copies of the conditional GCaMP3/6 allele and zero copies of the Cre-driver allele.
- Genotype the pups and mark the ones that have both Cre and conditional GCaMP3/6 alleles—these will henceforth be called double-transgenic mice (e.g., Myf5-Cre, conditional GCaMP3)6.
Note: This way, all data will derive from mice expressing one copy of both Cre and conditional GCaMP3/6 alleles. This is particularly important when adding in other mutant mice (e.g., knockouts) to these crosses.
- When the double-transgenic mice are of the appropriate age (e.g., postnatal day 0 or 5 [P0 or P5] or adult), euthanize the mice by decapitating them with scissors (for mice younger than P10) or by placing them in an isoflurane inhalation chamber—when they are no longer responsive to pinching the tail with a pair of forceps, they are ready for sacrifice.
- Sacrifice the animal by decapitation with a pair of scissors.
- Transversely section across the entire animal just below the liver and just above the heart and lungs with iridectomy scissors.
- Dissect away the liver, the heart, and the lungs, being careful to maintain a length of the phrenic nerve that is sufficiently long to be drawn into a suction electrode (i.e., 1 - 2 cm).
Note: The left phrenic nerve can be identified as a white piece of tissue that enters the medial portion of the left diaphragm. It must not be cut when removing the lungs. The right phrenic nerve runs within a piece of fascia that also contains the superior vena cava and is thinner and whiter than the vena cava. Together, they both penetrate the right medial diaphragm. - Further remove the ribcage and the vertebral column, except for the thin ridge around the diaphragm.
- Place the diaphragm and the phrenic nerve sample in a microfuge tube with Krebs-Ringer solution with 1 µg/mL 594-αBTX for 10 min in the dark.
Note: This concentration of 594-αBTX labels ACh receptors (AChRs) without blocking their function (personal observation).
2. Stimulation and Recording of the Muscle Action Potentials
- Using minutien pins, immobilize the diaphragm by pinning it onto a 6-cm dish coated with silicone dielectric gel and filled with ~8 mL of oxygenated Krebs-Ringer solution and place it onto the microscope stage. Perfuse the diaphragm with more Krebs-Ringer solution (8 mL/min) for 30 min.
Note: This rinses the unbound 594-αBTX, as well as equilibrates the tissue after dissection. - Make a suction electrode according to the established methods7.
- At 4X magnification, using a micromanipulator, move the suction electrode over the left phrenic nerve and apply suction by pulling out the barrel of a 5-mL syringe connected to the tubing that is attached to the suction electrode.
Note: When successfully drawn into the suction electrode, the phrenic nerve is taut. Turn on the stimulator and stimulate the phrenic nerve by flipping the manual switch 1x. - Ensure that the diaphragm contracts in response to the 1-Hz stimulation by visually examining it with brightfield illumination. If not, adjust the voltage by turning the voltage knob incrementally to achieve a supramaximal pulse, which can be verified by a visual examination of muscle contraction. If still not visible, blow out the nerve with the syringe and attempt to draw it in again by applying suction.
- At 4X magnification, using a micromanipulator, move the suction electrode over the left phrenic nerve and apply suction by pulling out the barrel of a 5-mL syringe connected to the tubing that is attached to the suction electrode.
- Turn off the perfusion and add the muscle-specific myosin inhibitor BHC6 or the voltage-gated sodium channel antagonist µ-conotoxin8 to a final concentration of 100 µM.
- To make 100 µM BHC, pipette 4 µL of 200 mM stock in DMSO and predilute it in 1 mL of Krebs-Ringer solution.
- Remove 1 mL of Krebs-Ringer solution from the dish.
- Add the prediluted BHC, to the dish.
Note: This predilution helps prevent the induction by undiluted DMSO of a non-transient fluorescent response in GCaMP3-expressing cells. - Wait 30 min and then, turn on the perfusion of fresh Krebs-Ringer solution for another 20 - 30 min.
- Prepare the recording electrode.
- Wearing gloves, place a borosilicate filamented glass with an outer diameter (OD) of 1 mm and an inner diameter (ID) of 0.4 mm into a micropipette puller and tighten the dials to clamp it into position. Close the puller door.
- Using a P-97 puller, program the following setting: heat at 900, pull at 120, velocity at 75, time at 250, pressure at 500, and no additional loops.
Note: Resistance (R) is measured using software controls of the amplifier: the data acquisition software confirms resistance by solving the formula V = IR. The software controller passes a known current (I) (typically 1 nA) through the electrode and measures the change in voltage (V), thus enabling us to solve for R. - For embryonic diaphragms, ensure that the resistance is near 60 MΩ, and for older diaphragms, 10 - 20 MΩ. Load the recording electrode with 3 M KCl.
- At 10X magnification, lower the electrode into muscle, using a second micromanipulator on the opposite side of the stage as a stimulating electrode.
- Using electrophysiological data acquisition software, wait until the resting membrane potential changes from 0 to -65 mV or below.
- Stimulate at 1 Hz and verify the presence of a muscle action potential by checking for a large potential that exhibits a modest overshoot (potential that rises above 0 mV when it starts at -65 mV or below). Do not confuse stimulation artifact with an action potential.
Note: Potentials are significantly longer in duration (~5 ms) than stimulation artifacts.
3. Imaging of the Fluorescence of the Sample
- At 20X magnification, locate the endplate band at the center of the muscle by looking for 594-αBTX–labeled NMJs under green/yellow light excitation (550 nm). Switch to the blue light excitation (470 nm) to image Ca2+ responses in muscle, motor neuron, or Schwann cells.
- If desired, set up the image splitter with bandpass filters and a dichroic single-edge filter for the dual-wavelength imaging.
- In order to calculate the maximal fluorescence (Fmax) exhibited by GCaMP3/6-expressing tissue, add 12 µL of 3 M potassium chloride (KCl) to the diaphragm preparations6.
- Perform experiments with the brightness bar on the lookup table bar set to 110% of the level at which the GCaMP3/6-expressing tissue exhibits saturation at 20X magnification, without binning in response to KCl.
- Record at 20 frames per second to not miss any fast events.
- Stimulate with 1 - 45 s of 20 - 40 Hz of nerve stimulation by delivering a train of impulses using the suction electrode or add pharmacological agonists by bath application or by perfusion and collect dynamic fluorescent Ca2+ responses in one cell subtype together with the static 594-αBTX NMJ signal.
Note: If tissue-specific red or far-red GECI or GEVI mice become available for use at the NMJ, they can be used to collect two dynamic signals reflecting two distinct cellular elements at the NMJ. - When the imaging or electrophysiological experiments are finished because the desired results have been achieved, perfuse water through the perfusion lines and suck water 2x - 3x through the suction electrode to ensure that salts do not build up.
4. Export and Analysis of the Data by a Standard Deviation Map of Fluorescence Intensity (SDiu16)
- Record image sequences recorded as 16-bit TIFF stacks and load them into the desired imaging data analysis system for analysis.
- In the software’s 8d file menu, select Image stack of interest and click to load.
- Once the video loads, scan through the time to identify a section that has no cellular fluorescent activity.
Note: This region will be used to create a background sample. - Hold Shift and click to draw a region of interest (ROI) box in the area identified as the background sample area.
- After creating the box, press the space bar to generate a plot of background activity change.
- Right-click the trace and select the assorted option to present the option to Dump ROI as text to make the trace as an xy coordinate text file.
- Once the video loads, scan through the time to identify a section that has no cellular fluorescent activity.
- Moving back to the video of interest, scan again to identify the time region where the activity of interest is occurring.
- Using the middle mouse button, select this time region in the yellow time box.
- Right-click on the video and select Stack OPS and then Stat map option 5.
Note: This will generate a standard deviation map (SD map) in the left window. - Click on the SD map and then press the ] key 19x to apply the appropriate color heat map.
- Right-click the SD map and select STM load and save, which will present the option Save stm as tiff to save the SD map.
- Then, press the [ key 19x to return to a grayscale color map.
- Press C and then D to bring up density mapping tools. Using the left mouse button and the center mouse button, adjust the threshold to include all fluorescent activity shown in the SD map.
- Press C to close the density tools while maintaining the threshold settings.
- Right-click the SD map and select STM particles and then Find PTCLS.
Note: This will identify individual cells expressing fluorescent activity. - Right-click the SD map once more and select Create Particle ROIs.
Note: This will superimpose the selected cells on the original video of interest. - While holding Shift, right-click on any one of the now identified particle ROIs on the original video.
- Select ROI Marker and Measure Int in ROI.
Note: This will generate individual fluorescent activity plots for each identified ROI in the video of interest. These can be saved by right-clicking any one of these and selecting Assorted, followed by Dump ROI as text.
- For detailed logic underlying these operations, please see the source code file9.
Results
Several examples of fluorescence intensity changes, mediated by increases of intracellular Ca2+ within defined cell types of the NMJ, show the utility of this approach. These results are presented as spatial fluorescence intensity maps, which provide the location of responding cells, as well as the intensity of their responses, thus allowing for the evaluation of how many cells respond and how much each cell responds to a particular stimulus. For example, as shown in Figure 1, we took videos of the Ca2+ responses in a population of terminal/perisynaptic Schwann cells (TPSCs) at the NMJs of the diaphragm of a P7 Wnt1-Cre; conditional GCaMP3-expressing mouse in response to stimulation of the phrenic nerve and identified the subpopulations of the responding cells by spatial fluorescence intensity maps. These maps of fluorescence intensity are presented as heat maps and color-coded according to a Fire color lookup table (Fire CLUT). We recorded these videos with and without splitting the image to simultaneously view the clusters of α-BTX-labeled AChRs in the middle of the diaphragm (Videos 1 and 2), an approach that could easily be adapted to capture dynamic GECI or GEVI responses from two distinct cell types, provided that each of them exhibits non-overlapping excitation and emission spectra.
In Figure 2, we performed the same nerve stimulation experiment on the diaphragm of a P4 Myf5-Cre; conditional GCaMP3-expressing mouse and imaged the Ca2+ responses in muscle cells. Interestingly, when we used either the myosin blocker BHC or the skeletal muscle-specific voltage-gated sodium channel (Nav1.4) blocker µ-conotoxin (Figure 2A and Video 3 or Figure 2B and Video 4, respectively), we visualized Ca2+ transients that travel the full length of the muscle fiber, representing the action potential and mediated by the release of Ca2+ from the sarcoplasmic reticulum, or merely the length of the endplate band, representing the endplate potential and mediated by extracellular Ca2+ influx through the AChR.In addition to identifying subpopulations of responding cells with spatial fluorescence intensity maps (SD maps), as in Figure 1, we also measured the change in fluorescence over time in a population of these muscle cells with spatiotemporal (ST) maps. Each of these experiments represents a different cell type, a different age, a different treatment (nerve stimulation vs. nerve stimulation in the presence of different drugs) and different types of analysis (spatial vs. spatiotemporal fluorescence intensity maps). These figures also illustrate one of the most useful features of transgenic GCaMP-expressing mice, namely the ability to repeatedly stimulate and image the same sample and, therefore, test the effect of different treatment conditions.

Figure 1: Measurement of activity-induced Schwann cell Ca2+ responses in the diaphragm and phrenic nerve of P7 Wnt1-Cre; conditional GCaMP3 mice. (A) (Left) An average fluorescence intensity image, showing background levels of fluorescence in Schwann cells along the phrenic nerve branches and at the neuromuscular junction (NMJ), was captured before nerve stimulation (Prestim). The values of this background fluorescence were subtracted from fluorescence values obtained after nerve stimulation. (Right) A spatial map of the standard deviation of 16-bit fluorescence intensity units (SDiu16) of Ca2+ responses generated after 30 s of 40 Hz of phrenic nerve stimulation (Stim Map or SD Map) shows a robust response in the terminal/perisynaptic Schwann cells (TPSCs) at the NMJ. The fire CLUT heatmap is in SDiu16 and the scale bar is in microns. All images in panels B - E are the same magnification as those in panel A. (B) (Left) The same diaphragm was labeled with 594-conjugated α-bungarotoxin (α-BTX), which binds to and labels acetylcholine receptors (AChRs), and excited with green/yellow light to identify the NMJ. (Right) This panel shows a brightfield image of the same diaphragm, showing the tip of an intracellular recording electrode (arrow), which can be guided to an NMJ, based on α-BTX labeling. (C) The Ca2+ transient features (e.g., intensity, onset after stimulation, duration) of individual cells or groups of cells can be evaluated by demarcating individual regions in the spatial intensity map as particles (left), representing them as color-coded regions of interest (ROIs), and (D) plotting their intensities over time. (E) This panel shows dual-wavelength images of GCaMP3-mediated fluorescent Ca2+ responses and 594-α-labeled NMJs in the same diaphragm using the Gemini image splitter after nerve stimulation. Please click here to view a larger version of this figure.

Video 1: Movie without image splitting of activity-induced Schwann cell Ca2+ responses at P7, as described in detail in the Figure 1 legend. Please click here to view this video. (Right-click to download.)
Video 2: Movie with image splitting of activity-induced Schwann cell Ca2+ responses and 594-α-BTX-labeled AChRs at P7, as described in detail in the Figure 1 legend. Please click here to view this video. (Right-click to download.)
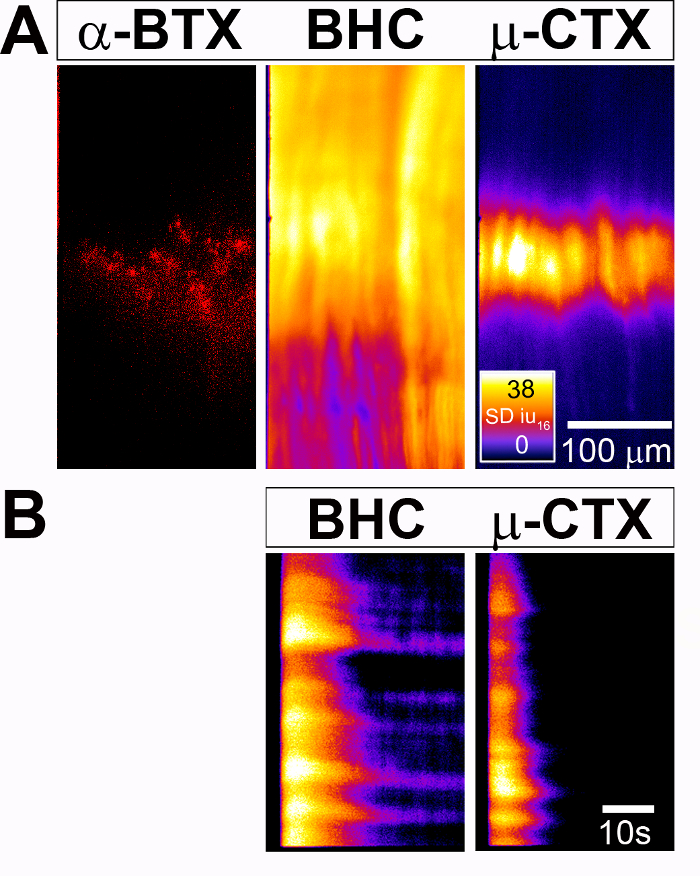
Figure 2: Measurement of activity-induced muscle cell Ca2+ responses in the diaphragm of P4 Myf5-Cre; conditional GCaMP3 mice. (A) (Left) Nicotinic AChR clusters of the centrally located endplate band of the diaphragm are labeled with 594-α-BTX. (Middle) A spatial map of Ca2+ transient intensities (SD map), generated after 30 s of 40 Hz of phrenic nerve stimulation in the presence of the myosin inhibitor BHC, shows a response throughout the entire region of all diaphragm muscle cells. (Right) In contrast, an SD map generated from the same diaphragm after the same stimulation, but in the presence of the Nav1.4 antagonist µ-conotoxin (µ-CTX), exhibits a spatially restricted response in the medial region of all diaphragm muscle cells that corresponds to the AChR cluster-enriched endplate band. The fire CLUT heatmap is in SDiu16 and the scale bar is in microns. (B) This panel shows spatiotemporal maps of Ca2+ transient intensities over time (ST maps) in a population of muscle cells (y-axis) followed over time (x-axis). The scale bar is in seconds. Please click here to view a larger version of this figure.

Video 3: Movie of activity-induced muscle cell Ca2+ responses in the presence of the myosin blocker BHC at P4, as described in detail in the Figure 2A legend. Please click here to view this video. (Right-click to download.)

Video 4: Movie of activity-induced muscle cell Ca2+ responses in the presence of the Nav1.4 antagonist µ-conotoxin at P4, as described in detail in the Figure 2B legend. Please click here to view this video. (Right-click to download.)
Discussion
Here we provide some examples of measuring Ca2+ responses in specific cells in intact neuromuscular tissue using GECI-expressing mice. In order to successfully perform these experiments, it is imperative not to injure the phrenic nerve during the dissection. To image Ca2+ responses in Schwann cells at either low or high power (i.e., 20X or 60X), it is necessary to use either BHC or µ-conotoxin to block movement. For low-power imaging of Ca2+ responses in muscle cells, it is possible to measure them in the absence of these drugs, thus permitting the simultaneous acquisition of muscle Ca2+ transient intensities and muscle length changes during high-frequency nerve stimulation6. When performing multiple experiments on the same sample, it is necessary to separate each one by at least 15 min, during which time the sample can be perfused. These steps allow for the repeated imaging of stimulation-induced Ca2+ responses from the same field of view in the same sample for at least 3 - 5 hours. It is also critical to predilute drugs dissolved in DMSO as described for BHC, as DMSO applied directly onto GCaMP-expressing tissue induces irreversible, stimulus-independent fluorescence responses.
We found that for reasons that are unclear, Wnt1-Cre; conditionalGCaMP3/6 mice fail to exhibit nerve stimulation or agonist-induced Ca2+ responses in Schwann cells after P15 - P20. However, Sox10-Cre; conditional GCaMP3/6 mice continue to exhibit these responses at least as late as P56, the oldest age that we have examined. In contrast, Myf5-conditional GCaMP3/6 mice exhibit responses as old as one year, the oldest age examined.
While GECI-expressing mice provide unique opportunities for imaging Ca2+ responses in whole populations of cells of a specific subtype, there are some limitations, such as the inability to perform ratiometric imaging and, thus, extract quantitative Ca2+ measurements. There are also limitations to the amount of depth of tissue from which these responses can be imaged using widefield fluorescence microscopy (i.e., as opposed to using confocal or multiphoton microscopy). Therefore, while the thinness of the diaphragm is amenable for the application of the techniques presented here, capturing cell-specific Ca2+ responses in cell types of the NMJ in other muscles that are thicker may require sub-dissection or other kinds of fluorescence microscopy.
These genetic and optical tools represent a significant advancement over previous Ca2+ imaging techniques, by which only multiple cell types or a few individual cells within one cell type could be imaged. An additional advantage is that Ca2+ responses can be repeatably imaged for long periods of time from the same cells using GECI mice, whereas this is not easily possible using traditional chemical Ca2+-binding fluorescent dyes. Finally, using an image splitter, we perform dual-wavelength imaging of a dynamic signal within one cell type (Schwann cells) and a fixed label within a second (muscle cells) and, thus, show how multiple cell-specific calcium or voltage responses can be evaluated (e.g., a Schwann cell Cre-driving mouse crossed to a conditional Cre-dependent GCaMP mouse as reported here, crossed to a transgenic Cre-independent mouse expressing a muscle cell-specific GECI or GEVI with non-overlapping fluorescence excitation/emission spectra10, would allow simultaneous tracking of dynamic Ca2+ and/or voltage changes in both Schwann and muscle cells). Such tools could help evaluate whether the response of one cell type to a specific stimulus, such as the purine ATP or its breakdown product adenosine, is direct or indirectly mediated by a direct effect on another cell type at the NMJ.
The main goal of these studies was to evaluate the spatiotemporal Ca2+ response pattern of cell subtypes to nerve stimulation, but the techniques employed to achieve this can be deployed toward other goals. For instance, they can be used to analyze Ca2+ responses in the presence of certain antagonists or in certain mutant backgrounds, such as in specific animal models of motor neuron disease, muscular dystrophy, or Charcot-Marie Tooth disease, to analyze the Ca2+ response to specific agonists to evaluate receptor expression, to assess the heterogeneity of Ca2+ response features within a cell subtype to a stimulus, or to compare Ca2+ responses in a cell subtype to other functional responses within that type (electrophysiologically recorded muscle endplate or action potentials, optically imaged muscle shortening, force-transducer-recorded muscle tension, etc.) or to other parameters (e.g., post hoc evaluation of nerve/muscle Schwann cell morphology or molecular expression via immunohistochemistry). Together, these studies show how cell-specific GECI or GEVI mice can be used to illuminate a wide spectrum of physiological processes at a synapse composed of genetically identifiable, cell-specific inputs.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported with funds from the National Institutes of Health (NIH) GM103554 and GM110767 to (T.W.G.) and from the National Center for Research Resources 5P20RR018751 and the National Institute of General Medical Sciences 8P20 GM103513 (to G.W.H.).
Materials
| Name | Company | Catalog Number | Comments |
| Myf5-Cre mice | Jax | #007893 | Drives muscle cell expression as early as E136 |
| Wnt1-Cre mice | Jax | #003829 | Drives expression into all Schwann cells at E13 but not P209 |
| Sox10-Cre mice | Jax | #025807 | Drives Schwann cell expression at older ages |
| Conditional GCaMP3 mice | Jax | #029043 | Expresses GCaMP3 in cell-specific fashion |
| Conditional GCaMP6f mice | Jax | #024105 | Expresses GCaMP6f in cell-specific fashion |
| BHC (3-(N-butylethanimidoyl)-4-hydroxy-2H-chromen-2-one) | Hit2Lead | #5102862 | Blocks skeletal muscle myosin but not neurotransmission6 |
| CF594-α-BTX | Biotium | #00007 | Labels acetylcholine receptor clusters at NMJ |
| µ-conotoxin GIIIb | Peptides Int'l | #CONO20-01000 | Blocks Nav1.4 voltage-dependent sodium channel8 |
| Silicone Dielectric Gel; aka Sylgard | Ellswoth Adhesives | # Sil Dielec Gel .9KG | Allows for the immobilization of the diaphragm by minutien pins |
| Minutien pins (0.1mm diameter) | Fine Science Tools | 26002-10 | Immobilizes diaphragm onto silicone dielectric gel |
| Eclipse FN1 upright microscope | Nikon | MBA74100 | Allows staging and observation of specimen |
| Basic Fixed Microscope Platform with Manual XY Microscope Translator | Autom8 | MXMScr | Allows movement of specimen |
| Manual micromanipulator | Narishige | M-152 | Holds recording and stimulating electrodes |
| Microelectrode amplifier | Molecular Devices | Axoclamp 900A | Allows sharp electrode intracellular electrophysiological recording |
| Microelectrode low-noise data acquisition system | Molecular Devices | Digidata 1550 | Allows electrophysiological data acquisition |
| Microelectrode data analysis system | Molecular Devices | PCLAMP 10 Standard | Performs electrophysiological data analysis |
| Square wave stimulator | Grass | S48 | Stimulates nerve to excite muscle |
| Stimulus Isolation Unit | Grass | PSIU6 | Reduces stimulation artifacts |
| Borosilicate filaments, 1.0 mm outer diameter, 0.5mm internal diameter | Sutter | FG-GBF100-50-15 | Impales and records nerve-evoked muscle potentials |
| Borosilicate filaments, 1.5 mm outer diameter, 1.17mm internal diameter | Sutter | BF150-117-15 | Lengthened and used for suction electrode |
| Micropipette Puller | Sutter | P-97 | Pulls and prepares recording electrodes |
| 1200x1200 pixel, back-illuminated cMOS camera | Photometrics | Prime 95b | Sensitive camera that allows high-resolution, high-speed imaging |
| Light Source | Lumencor | Spectra X | Provides illumination from LEDs for fluorescence obsevation |
| Infinity-corrected fluorescent water immersion objectives, W.D. 2mm | Nikon | CFI60 | Provide long working distances for visualization of specimen |
| Fiber Optic Illuminator with Halogen lamp | Sumita | LS-DWL-N | Provides illumination for brightfield observation |
| W-View Gemini Image Splitter | Hamamatsu | A12801-01 | Projects 1 pair of dual wavelength images separated by a dichroic to single camera |
| Single-band Bandpass Filters (512/25-25 and 630/92-25) | SemRock | FF01-512/25-25; FF01-630/92-25 | Permits dual band imaging |
| 560 nm Single-Edge Dichroic Beamsplitter | Sem Rock | FF560-FDi01-25x36 | Dichroic mirror which separates beams of light to allow dual-wavelength imaging |
| Imaging data acquisition system | Nikon | NIS Elements - MQS31000 | Allows imaging data acquisition |
| Wavelength control module | Nikon | MQS41220 | Module for imaging data acqusiition |
| Emission splitter hardware module | Nikon | MQS41410 | Module for imaging data acqusiition |
| Imaging data analysis system | NA | Volumetry 8D5, Fiji | Allows analysis of fluorescence intensity and other imaging data |
References
- Sanes, J. R., Lichtman, J. W. Development of the vertebrate neuromuscular junction. Annual Review of Neuroscience. 22, 389-442 (1999).
- Darabid, H., Perez-Gonzalez, A. P., Robitaille, R. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nature Reviews Neuroscience. 15 (11), 703-718 (2014).
- Fatt, P., Katz, B. An analysis of the end-plate potential recorded with an intracellular electrode. Journal of Physiology. 115 (3), 320-370 (1951).
- Todd, K. J., Robitaille, R. Purinergic modulation of synaptic signalling at the neuromuscular junction. Pflugers Archive. 452 (5), 608-614 (2006).
- Hennig, G. W., et al. Use of Genetically Encoded Calcium Indicators (GECIs) Combined with Advanced Motion Tracking Techniques to Examine the Behavior of Neurons and Glia in the Enteric Nervous System of the Intact Murine Colon. Frontiers of Cellular Neuroscience. 9, 436 (2015).
- Heredia, D. J., Schubert, D., Maligireddy, S., Hennig, G. W., Gould, T. W. A Novel Striated Muscle-Specific Myosin-Blocking Drug for the Study of Neuromuscular Physiology. Frontiers of Cellular Neuroscience. 10, 276 (2016).
- Johnson, B. R., Hauptman, S. A., Bonow, R. H. Construction of a simple suction electrode for extracellular recording and stimulation. Journal of Undergraduate Neuroscience Education. 6 (1), A21-A26 (2007).
- Hong, S. J., Chang, C. C. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. British Journal of Pharmacology. 97 (3), 934-940 (1989).
- Heredia, D. J., Feng, C. Y., Hennig, G. W., Renden, R. B., Gould, T. W. Activity-induced Ca2+ signaling in perisynaptic Schwann cells of the early postnatal mouse is mediated by P2Y1 receptors and regulates muscle fatigue. Elife. 7, e30839 (2018).
- Cho, J. H., et al. The GCaMP-R Family of Genetically Encoded Ratiometric Calcium Indicators. ACS Chemical Biology. 12 (4), 1066-1074 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved