Method Article
A Simple and Efficient Method for Testing Immunomodulatory Agents for Generation of Tolerogenic Dendritic Cells from Human CD14+ Monocytes
In This Article
Summary
We describe a procedure to assess the capacity for pharmacologic agents to generate tolerogenic dendritic cells from naïve monocyte-derived dendritic cells in vitro and validate their potency by autologous regulatory T cell generation.
Abstract
Tolerogenic dendritic cells (tolDCs) are a subset of dendritic cells (DCs) that are known to influence naïve T cells toward a regulatory T cell (Treg) phenotype. TolDCs are currently under investigation as therapies for autoimmunity and transplantation, both as a cell therapy and method to induce tolDCs from endogenous DCs. To date, however, the number of known agents to induce tolDCs from naïve DCs is relatively small and their potency to generate Tregs in vivo has been inconsistent, particularly therapies that induce tolDCs from endogenous DCs. This provides an opportunity to explore novel compounds to generate tolerance.
Here we describe a method to test novel immunomodulatory compounds on monocyte-derived DCs (moDCs) in vitro and validate their functionality to generate autologous Tregs. First, we obtain PBMCs and isolate CD14+ monocytes and CD3+ T cells using commercially available magnetic separation kits. Next, we differentiate monocytes into moDCs, treat them with an established immunomodulator, such as rapamycin, dexamethasone, IL-10, or vitamin D3, for 24 h and test their change in tolerogenic markers as a validation of the protocol. Finally, we co-culture the induced tolDCs with autologous T cells in the presence of anti-CD3/CD28 stimulation and observe changes in Treg populations and T cell proliferation. We envision this protocol being used to evaluate the efficacy of novel immunomodulatory agents to reprogram already differentiated DCs towards tolDC.
Introduction
Dendritic cells (DCs) are critical mediators between innate and adaptive immunity. DCs, which mainly reside in mucosal membranes, skin and lymphoid tissue, are the primary antigen presenting cells (APCs)1. DCs uptake foreign proteins and process and present them on major histocompatibility (MHC) proteins to naïve T cells. DCs specifically express MHC class II proteins, such as human leukocyte antigen-DR (HLA-DR) in humans. The activation state of the DCs upon antigen exposure is critical for the downstream T cell response2. Immature DCs express various pattern recognition receptors (PRRs) that recognize classes of molecules call pathogen associated molecular patterns (PAMPs), such as the bacterial wall component, lipopolysaccharide (LPS)3. Upon PRR stimulation, DCs become matured DCs and upregulate important T cell co-stimulatory proteins, such as CD80, CD86, and CD40, and secrete pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNFα), facilitating the differentiation of naïve T cells into conventional effector or helper T cells2. On the contrary, if DC maturation is interrupted or if DCs develop in a tolerogenic environment, DCs can generate a tolerogenic DC state (tolDCs)4. TolDCs downregulate classical T cell co-stimulatory receptors and instead upregulate tolerance receptors such as programmed cell death ligand 1 (PD-L1) and B- and T-lymphocyte attenuator (BTLA) and generate suppressive cytokines such as interleukin 10 (IL-10) and transforming growth factor beta (TGF-β)4. This is not a comprehensive list of tolerance markers and, in fact, there is limited consensus as to which tolDC markers are appropriate to define the tolDC state5. Considering this, we propose regulatory T cell (Treg) generation as a functional marker that ought to be used to compare the effectiveness of various tolDC induction agents.
In addition to tolDC/matured DC activation states, DCs can also be categorized based on their lineage or tissue location, with each subset displaying slightly different functionality. While the tolDC/matured DC division is less definitive and exists more as a continuum, lineage divisions have well-defined markers in both human and mice. DC precursors are formed in the bone marrow, but there are two main subtypes of DCs based on their lineage: 1) plasmacytoid dendritic cell (pDCs), which derive from lymphoid lineages and 2) conventional dendritic cells (cDCs), which derive from myeloid lineages. In humans, pDCs are matured in lymphoid organs, express CD303, and are highly responsive in viral infections6. CD11c expressing cDCs, meanwhile, are matured in peripheral tissues and exist in two distinct subtypes, CD1c+ cDC1s and CD141+ cDC2, which each generate distinct T cell responses7. Furthermore, all cDCs can exist in either tissue-resident (CD103-) or migratory (CD103+) substates8. Finally, under certain conditions, cells from monocyte lineages (CD14+) can be induced toward a dendritic cell phenotype and are identified as CD14-, CD141+, CD1c+ 9. These cells, known as monocyte-derived DCs (moDCs), are the most commonly used for ex vivo analysis in humans as monocytes constitute approximately 10-30% of human peripheral blood mononuclear cells (PBMCs), whereas pDCs constitute only 1-3%10. This makes moDCs an attractive choice, but it is also known that moDCs are more inflammatory that typical cDCs isolated from primary tissue9.
There are currently two broad categories of efforts to employ tolDCs to generate clinical tolerance. First, tolDCs are generated from monocytes for use as a cell therapy. In this paradigm, moDCs are typically differentiated using IL-4/GM-CSF concurrently with immunomodulators such as vitamin D3, rapamycin (rapa), IL-10, dexamethasone, or combinations of these11,12. These tolDCs have been explored as autologous cell therapies for autoimmunity and transplants13. The other use of tolDCs is to reprogram endogenous DCs towards tolDCs using free drugs or nanocarriers to deliver both immunomodulator and antigen of interest14,15,16. Induction of already differentiated DCs is more challenging, however, due to the development of robust metabolic phenotypes of DCs that are typically in contrast with tolDC metabolism17,18. This is a high bar for most pharmacological immunomodulators; for this reason, most endogenous DC reprogramming studies report effective DC suppression and often some Treg induction, but lack clinical success, often due to lack of T cell persistence15,19,20. This highlights the need for strategies to identify potential tolDC induction agents from existing DCs.
Here, we present a method for in vitro evaluation of immunomodulatory agents against differentiated moDCs with the end metric of autologous Treg induction. This protocol is designed to assess the effectiveness of immunomodulatory agents to reprogram already-differentiated human moDCs towards tolerance. Furthermore, this protocol validates the functionality of reprogrammed tolDCs to generate Tregs against autologous T cells isolated from the same PBMC sample. This is in contrast to other protocols that induce tolerance during differentiation and/or challenge tolDCs with T cells from allogenic donors21. In this protocol, we use the common tolerizing agent rapa as an example but also demonstrate the limited effectiveness of rapa-treated moDCs to generate Tregs. In our representative results, we also show the efficacy of other common immunomodulatory treatments such as IL-10, dexamethasone, and vitamin D3. We envision this protocol being used to screen potentially more effective tolDC-inducting agents against already established moDCs22.
Protocol
All human peripheral blood mononuclear cell (PBMC) samples were obtained from the University of Pennsylvania's Human Immunology Core from deidentified donors with prior approval from the University of Pennsylvania's Institutional Review Board (IRB) with patient consent.
Optional: While in this method, we used freshly isolated PBMCs obtained from an academic laboratory, PBMCs can be isolated from either whole blood or leukapheresis-enriched blood products. We recommend using the density gradient centrifugation method, as this is a well-established and reliable method that is described elsewhere23.
1. Isolation of monocytes/T cells and moDC differentiation
- Isolation of monocytes and T cells from PBMCs-Day 1
- Obtain 200 million human PBMCs from a healthy donor. Keep the cells in a 15 mL tube and transfer them on ice.
- Aliquot 50 mL of Separation Buffer (provided in commercially available separation kits) in a conical tube.
NOTE: Separation buffer can also be replaced with DPBS with 2% fetal bovine serum (FBS) and 1 mM Ethylenediaminetetraacetic acid (EDTA). - Remove the PBMC tube from ice and fill it with Separation Buffer. Centrifuge at 300 x g for 5 minutes.
- Aspirate the supernatant using a 10 mL serological pipette. Resuspend the cell pellet in 4 mL of Recommended Medium using a serological pipette.
- Divide the suspension into two different 15 mL tubes, adding 2 mL per tube (5 × 107 cells/mL per tube). Label one tube "T" and the other "Monocytes."
- Retrieve the Human Monocyte Isolation Kit and follow the manufacturer's protocol to isolate monocytes from the monocyte-labeled tube.
- Retrieve the Human T Cell Isolation Kit and follow the manufacturer's protocol for the second tube to isolate T cells.
- Plating of monocytes for differentiation and freezing of T cells-Day 1
- Preheat 50 mL of moDC Culture Medium (Table 1) at 37 °C for at least 10 min.
- Centrifuge the 15 mL tubes with enriched monocytes and the 15 mL tube with T cells from step 1.1 at 250 × g for 5 min.
- Aspirate the supernatant. Resuspend monocytes in 1 mL of moDC Culture Medium and T cells in T Cell Culture Medium (Table 1) by pipetting up and down. Ensure the pellet is properly dispersed.
- Count the cells using an automated cell counter or manual hemacytometer using Trypan Blue staining.
NOTE: For the representative data, 10 million T cells and 3 million monocytes were isolated. - Take the tube with monocytes and add 9 mL of warm moDC Culture Medium to the suspension for a final volume of 10 mL. Then, add 100 µL each of 10 µg/mL GM-CSF and 10 µg/mL IL-4 stock for a final concentration of 100 ng/mL of both GM-CSF and IL-4 in the media. Transfer the suspension to a Petri dish and label as "moDC" (Day 1) and incubate at 37 °C with 5% CO2.
- Take the T cell tube and mix 1 mL of T Cell Freeze Medium (Table 1) with 1 mL of T cell suspension. Divide into two 2 mL cryovials (1 mL/vial); seal tightly (final concentration of 5 million cells per vial). Place the cryovials in a freezing chamber with balancing tubes in unused wells. Store the chamber at −80 °C overnight, then transfer the cryovials to a cryotank.
- On Day 4, refresh the medium with the addition of 5 mL of fresh moDC Culture Medium and add 100 µL each of GM-CSF and IL-4 stock. Differentiated moDCs will be ready on Day 7.
2. Adding immunomodulatory drugs for generating tolerogenic moDCs
- Set up the moDC plate.
- On Day 7, retrieve the differentiated moDCs from the incubator and transfer the moDC cell suspension into a 50 mL tube.
- Centrifuge the suspension at 250 × g for 5 min. Aspirate the supernatant and resuspend the cell pellet in 1 mL of warm moDC culture media by pipetting up and down. Count the cells with hemacytometer and dilute the cells to a concentration of 3 × 105 cells/mL in warm moDC culture medium containing 100 ng/mL GM-CSF and IL-4.
- Add 3 × 104 cells/well in 100 µL to the desired number of wells in a flat bottom 96-well tissue culture plate.
NOTE: For representative results, we used a total of 48 wells (four conditions done in triplicate prepared for four different analyses in sections 3 and 4), but this can be adjusted for up to 60 wells. Fill all remaining wells with 100 µL/well PBS to prevent drying, especially outer wells. We recommend preparing different plates for each method of analysis. - Add 1 µL/well of immunomodulatory drugs to the desired wells (here 10 ng/mL rapa). Incubate the plate overnight at 37 °C with 5% CO2.
NOTE: If including immunostimulation on day 8, prepare wells for the immunomodulatory drug both with and without immunostimulation. Use of immunostimulation to mature moDCs is optional.
- Rethaw T cells.
- On Day 8, retrieve two cryovials from the cryotank and thaw the cryovials in a hot bead bath or water bath at 37 °C until the contents start melting (in <3 min).
- As the contents start to melt, transfer the vials to a cell culture hood and pipet 1 mL of warm T cell culture media into the vial to rapidly thaw. Transfer the entire contents of the vial into a 50 mL tube. Rinse the cryovials with 1 mL of T cell culture medium to ensure all cells are transferred into the tube.
- Top up the suspension with Flow Staining Solution (Table 1) to 15 mL. Centrifuge at 200 × g for 10 min and aspirate the supernatant. Then, resuspend the pellet in 5 mL of Flow Staining Solution and centrifuge again.
- Aspirate the supernatant and resuspend the cells in 1 mL of warm T cell culture medium. Count the cells using a hemacytometer.
NOTE: Viability should be >70% at this stage; if large amounts of dead cells remain, transfer to a fresh tube, add 10 mL of cell stain solution, centrifuge at 200 × g for 5 min, resuspend in 1 mL of T cell media, and recount. - Add 9 mL of warm T cell culture medium to make a final volume of 10 mL. Transfer the suspension into a Petri dish. Label it as "Rethawed T Cells" and incubate overnight at 37 °C with 5% CO2 to let the cells rest.
- Wash the moDC plate and add the immunostimulator if desired.
- On Day 8, centrifuge the moDC plate (from Day 7) at 300 × g for 5 min.
- Aspirate the supernatant, wash with warm HBSS, repeat centrifugation, aspirate again, and resuspend the cells in warm moDC culture medium containing GM-CSF and IL-4 (100 µL/well).
NOTE: Be careful not to disturb the cells. Tilt the plate during aspiration. Approximately 10 µL can remain in the well. - If using immunostimulation, add 1 µL/well of 100x stock LPS (or other immunostimulatory agent) to the desired wells. Incubate the plate overnight at 37 °C with 5% CO2.
3. Flow analysis for moDC (Validation + Tolerance)
- Flow analysis for validation of moDC
- On day 8, prepare antibody cocktails for the Validation Panel, targeting the following markers: HLA-DR, CD14, dendritic cell-specific C-type lectin (DC-SIGN), CD1c, and CD40 as described in Table 2. Dilute all antibodies with flow stain solution.
NOTE: The panel was designed for a 7 channel, Red/Blue laser flow cytometer. - Retrieve the moDC plate from the incubator. Centrifuge the plate at 300 × g for 5 min. Remove the supernatants. Optional: Retain the supernatants for ELISA analysis for cytokines IL-10 and TNFα using commercially available kits according to the manufacturers' instructions.
- Add 200 µL/well of Flow Staining Solution. Centrifuge the plate at 300 × g for 5 min.
- Aspirate the supernatant and add 50 µL/well of Fc receptor binding inhibitor antibody diluted 1:200 in flow staining solution to block unspecific binding. Incubate at room temperature (RT) for 30 min.
- Add 50 µL/well of the prepared Validation Panel antibody cocktail.
- Prepare compensation controls in the plate or in 1.5 mL tubes by mixing 50 µL of compensation beads with 50 µL of each diluted antibody.Incubate the plate at 4 °C for 1 h.
NOTE: Compensation beads are used here rather than cells for compensation due to potentially low expression of markers on moDCs. - Wash by centrifuging at 300 × g for 5 min, removing the supernatant, and resuspending in 200 µL of Flow Staining Solution. Perform two washes.
- After the final wash, centrifuge again and resuspend the cells in 110 µL/well of Flow Staining Solution. The samples are now ready for flow cytometry analysis.
- On day 8, prepare antibody cocktails for the Validation Panel, targeting the following markers: HLA-DR, CD14, dendritic cell-specific C-type lectin (DC-SIGN), CD1c, and CD40 as described in Table 2. Dilute all antibodies with flow stain solution.
- Flow analysis for tolerogenic moDC (Tolerance Panel)
- Similar to step 3.1 on day 8, prepare antibody cocktails for the Tolerance Panel, targeting the following markers: CD86, PD-L1, DC-SIGN, CD1c, BTLA, and CD40 (Table 2).
- Repeat steps 3.1.2-3.1.8, substituting the antibody cocktail with the Tolerance Panel cocktail.
4. Flow analysis of T cells
- Combine T cells with treated Tolerogenic moDCs.
- On day 9, retrieve the rethawed T cell Petri dish from the incubator and transfer all T cells into a 50 mL tube. Top up with Flow Staining Solution.
- Optional: If performing T cell proliferation analysis, divide T cells into two tubes.
- With the first tube, spin down at 250 × g for 5 min and remove the supernatant. Stain this tube with cell proliferation dye according to the manufacturer's instructions.
NOTE: We used Fixable Viability Dye eFluor 670, which requires a 30 min incubation step, followed by two washing steps. We suggest using 50 mL conical tubes to prevent cell loss and using HBSS with 10% HIFBS to wash after staining. - Use the second tube containingunstained T cells for Treg analysis. Similarly spin down at 250 × g for 5 min, remove the supernatant, resuspend in warm cell culture media, and keep in the incubator during the staining process. If no T cell proliferation is required, spin down all T cells and replace them with warm T cell culture media.
- With the first tube, spin down at 250 × g for 5 min and remove the supernatant. Stain this tube with cell proliferation dye according to the manufacturer's instructions.
- Count the cells and obtain at least 4 million (for just Treg analysis) or 8 million (for Treg and T proliferation analysis).
- Retrieve the moDC plate from incubator with the remaining wells, centrifuge at 300 × g for 5 min, and apirate the supernatants. Add 200 µL/well T cells at 1.6 × 105 cells/well. Add unstained T cells for Treg analysis plates and stained T cells for T cell proliferation plates.
NOTE: This will maintain a T cell to moDC ratio of 5:1, as the initial 3 ×104 moDCs typically expand slightly. - Add 25 µL/mL of anti-CD3/CD28 antibody cocktail to all groups except negative control wells to stimulate T cells. Incubate the plate for 72 h at 37 °C with 5% CO2 until day 12.
NOTE: Be sure to use stained T cells for proliferation analysis and unstained for Treg analysis.
- Treg flow analysis
- Surface marker staining
- On day 12, prepare antibody cocktails for the Treg Panel (Table 2).
- Transfer all cell suspensions from the 96-well culture plate into a V-bottom plate with Flow Staining Solution. Retain the supernatants for cytokine analysis.
- Wash the cells 2x by centrifugation (200 × g for 5 min followed by 200 µL of Flow stain solution). Set aside some cells for compensation controls. Set one aside for FOXP3 staining and for unstained controls.
NOTE: We suggest combing 10 µL of all samples prior to the final spin in step 4.2.1.3 to obtain necessary compensation controls. This provides a representative sample of all surface level expression of each marker on the cells. - After the second wash, centrifuge at 200 x g for 5 min at room temperature and aspirate, then add 50 µL/well Fc receptor binding inhibitor antibody (diluted 1:200 in Flow Staining Solution). Incubate at room temperature for 10 min.
- Add 50 µL/well of the prepared Treg Panel antibody cocktail.
- For compensation controls, mix 50 µL of unstained compensation cells with 50 µL of each single stained antibody.
- Incubate the plate and compensation controls at 4 °C for 1 h. Wash the plate once with Flow Stain Solution and centrifuge at 300 × g for 5 min.
- Stain with Live/Dead Near-IR dye diluted in HBSS (1:1,000) at 200 µL/well. Incubate at 4 °C for 30 min.
- Wash the plate once with Flow Staining Solution (200 µL/well) at 300 × g for 5 min.
- Stain with FoxP3 according to the manufacturer's instructions, which will require an overnight incubation. A brief overview of the FoxP3 staining protocol is described below .
- Prepare the FoxP3 Fixation/Permeabilization Working Solution according to the manufacturer's instructions. Mix 1 part of FoxP3 Fixation/Permeabilization Concentrate with 3 parts of FoxP3 Fixation/Permeabilization Diluent. Prepare 200 µL of the working solution per sample.
- Incubate all samples in 200 µL/well of the working Fixation/Permeabilization solution. Leave at 4 °C for 1 h.
- Prepare 1x Permeabilization Buffer: Mix 1 part of 10x Permeabilization Buffer (obtained from commercial kit) with 9 parts of distilled water.
- Wash the plate 2x with 1x Permeabilization Buffer (200 µL/well) at 300 × g for 5 min.
- Stain with FoxP3 antibody (PE-Cy5.5, 1:300 dilution in 1x Permeabilization Buffer): Add 100 µL/well of the diluted FoxP3 antibody and incubate at 4 °C overnight.
- Wash the plate 2x with 1x Permeabilization Buffer (200 µL/well) at 300 × g for 5 min.
- Resuspend the cells in 110 µL/well of Flow Staining Solution.
- Perform flow cytometry analysis.
- Surface marker staining
- T cell proliferation analysis
- Prepare antibody cocktails for the T Proliferation Panel (Table 2).
- Transfer all cell suspensions from the 96-well culture plate into a V-bottom plate with Flow Staining Solution. Retain supernatants for cytokine analysis.
- Wash the cells 2x by centrifugation (200 × g for 5 min followed by 200 µL of Flow stain solution). Set aside some cells for compensation controls. Set one aside for FOXP3 staining and for unstained controls.
NOTE: We suggest combing 10 µL of all samples prior to the final spin in step 4.3.3 to obtain the necessary compensation controls. This provides a representative sample of all surface level expression of each marker on the cells. - After the second wash, centrifuge (200 × g for 5 min at room temperature) and aspirate, then add 50 µL/well Fc receptor binding inhibitor antibody (diluted 1:200 in Flow Staining Solution). Incubate at room temperature for 10 min.
- Add 50 µL/well of the prepared T Proliferation Panel antibody cocktail.
- For compensation controls, mix 50 µL of unstained compensation cells with 50 µL of each single stained antibody.
- Incubate the plate and compensation controls at 4 °C for 1 h. Wash the plate once with Flow Stain Solution and centrifuge at 300 × g for 5 min.
- Stain with Live/Dead Near-IR dye diluted in HBSS (1:1,000) at 200 µL/well. Incubate at 4 °C for 30 min.
- Wash the plate once with Flow Staining Solution (200 µL/well) at 300 × g for 5 min.
- Perform flow cytometry analysis.
Results
We have described a protocol for human PBMCs, isolate both CD3+ T cells and CD14+ monocytes using commercially available magnetic separation kits, differentiate monocytes into CD14-, HLA-DR+, CD141+, CD1c+ moDCs using GM-CSF and IL-4, treat them for 24 h, and co-culture with autologous T cells with anti-CD3/CD28 stimulation for 72 h. An experimental schematic is shown in Figure 1.
Isolation of monocytes/T cells and moDC differentiation
For our initial representative results, 200 million human PBMCs were purchased from the University of Pennsylvania Human Immunology Core (HIC) from one healthy donor and processed as described in protocol sections 1 and 2. Blood was drawn on day 1 and isolated using standard Ficoll fractionation at the HIC facility. We used 100 million PBMCs for T cell and monocyte isolation, each with yields of 10 million T cells and 3 million monocytes, respectively. Monocytes and moDCs after GM-CSF/IL-4 differentiation on day 7 were analyzed via flow cytometry. We observed two distinct groups in the undifferentiated monocytes, classical CD14+, HLA-DR- monocytes (>45%), and a small population of CD14-, HLA-DR+ cells that expressed minimal amounts of CD1c and no CD141. In contrast, on day 7 of differentiation, we observed most cells that were CD14-, HLA-DR+, CD141+, CD1c+, indicating moDCs (Figure 2). We obtained approximately 4 million moDCs from the initial 3 million monocytes.
Flow analysis for moDC (Validation + Tolerance)
On day 7, moDCs were incubated with rapa for 24 h, washed, incubated with LPS for 24 h, and then analyzed by flow cytometry on day 9 via protocol step 3. Treatment groups included: 1) no treatment + no treatment (UT), 2) no treatment+ 0.1 µg/mL LPS (LPS), 3) 10 ng/mL rapa + no treatment (rapa), or 4) 10 ng/mL rapa+ 0.1 µg/mL LPS (LPS + rapa). Treatment with rapa, both with and without LPS, decreased CD1c+, DC-SIGN+ populations from UT, although percentages remained >50% (Figure 3A). MoDCs on day 9 were also analyzed for the expression of DC maturation markers, CD86 and CD40. We observed a marked increase in CD86 when moDCs were treated with LPS, which was prevented when moDCs were pretreated with rapa (Figure 3B). When the signal was normalized to UT controls, we observed a significant decrease in both CD40 and CD86 in rapa treatment groups and no increase in signal with LPS + rapa groups (Figure 3C,D). This same pattern was observed for tolerogenic markers, PD-L1 and BTLA, with rapa treatment decreasing the signal and preventing an increase in response to LPS stimulation (Figure 3E,F). This indicates that rapa treatment prevented LPS maturation but simultaneously limited the expression of tolerogenic markers.
Flow analysis for Treg
We next analyzed autologous T cells after co-culture with moDCs and stimulation with CD3/CD28 stimulation on day 12 at a ratio of 1:5 moDC:T cells using protocol step 4.2. We identified Treg populations by CD4+, CD25+, FoxP3+ (Figure 4)24. Samples included T cells without stimulation (negative control), T cells with CD3/CD28 stimulation but no moDCs, and T cells with CD3/CD28 stimulation and co-cultured with the four moDC groups from the previous paragraph. We observed a clear Treg population and a marked increase in Treg when moDCs were added to the co-culture. There was, however, no significant difference in Treg frequency between the four moDC, contrary to some literature reports that demonstrate increased Treg generation from rapa-treated tolDCs (Figure 5A)25.
Flow analysis of T cell proliferation
We also analyzed our representative sample for T cell proliferation using protocol step 4.3. T cell-moDC co-cultures were similarly stimulated as in Treg analysis and then analyzed by flow for cell proliferation dye of either CD4+ or CD8+ T cells (Figure 5B). We observed that rapa-treated moDCs have a moderate decrease in T cell proliferation in both CD4+ and CD8+ T cell compartments compared to LPS-treated moDCs and untreated moDCs upon CD3/CD28 stimulation.
Analysis of additional patient samples and immunomodulators:
Our representative results indicate that rapa-treated moDCs reduce markers of DC maturity such as CD86 and CD40 and reduce T cell proliferation to non-specific CD3/CD28 stimulation. Rapa-treated moDCs, however, have little effect on positive markers of tolerance, such as PD-L1 or BTLA and do not increase Treg populations upon CD3/CD28 stimulation. To explore if similar T cell results would be observed with other patients and immunomodulators, we repeated the autologous co-culture experiment from additional human samples (for a total of 4 or 5 depending on the analysis). We further tested 1 µM Dexamethasone (Dex), 10 ng/mL IL-10 (IL-10), 1 µg/mL Lipopolysaccharide (LPS), or 1 nM vitamin D3 (VD3) in addition to 10 ng/mL Rapa as the immunomodulators given in step 2.1.4 but omitting immunostimulation. We also included a sample that was given Dex in step 2.1.4 and treated with 0.1 µg/mL LPS in step 2.3.3 on day 8 (Dex + LPS). We first tested supernatants from moDC (without T cell addition) either 24 h or 72 h after step 2.3.3 on day 8 for the common tolerance cytokine IL-10 and the inflammatory cytokine TNFα (Figure 6A-D). We observed that rapa-treated moDCs did not increase IL-10 in any samples at 24 h or 72 h, but IL-10-treated samples did significantly increase IL-10 production at 24 h, even after two washing steps to remove exogenously added IL-10. (Figure 6A). We also observed that Dex-treated moDCs did significantly increase IL-10 production but only after resting for 72 h (Figure 6B). We further observed that no immunomodulator on its own generated TNFα, but the pretreatment of Dex reduced the average TNFα generation upon LPS treatment at 24 h, although this result was not significant (Figure 6C). The data further show that while moDC expression of PD-L1 is not increased by any immunomodulator at 24 h, significant increases in PD-L1+ moDCs were observed in the Dex + LPS- and Rapa-treated groups at 72 h (Figure 6E,F). Like our single representative sample, we also observe a suppression of CD86 expression from all immunomodulator treated groups at both 24 h and 72 h (Figure 6G,H). These confirm that immunomodulators can increase tolerogenic markers for moDCs, although the effect be delayed.
We further analyzed T cell responses after co-culture on day 12. We observed that immunomodulator treated moDCs did not increase CD4+ T cell proliferation relative to untreated moDC controls similar to other published reports26 (Figure 6I). Interestingly, we did not observe significant increases in Treg populations for any immunomodulator treated moDCs in the larger sample (Figure 6J). We believe this lack of significance is due to high variability between patient samples; two of the patient samples (1 and 2) did not seem to increase Tregs to any treatment, while the other two (3 and 4) showed increases with nearly all immunomodulatory treatments (Figure 6J). This highlights the need to perform analysis of immunomodulators with multiple patient samples and suggests that Treg proliferation via immunomodulator treated moDCs is highly variable between patients.

Figure 1: Experimental overview schematic. Monocytes and T cells are isolated from PBMCs, monocytes differentiated into moDCs, challenged with immunomodulatory agents to generate tolDCs, and co-cultured with T cells. Abbreviations: PBMCs = peripheral blood mononuclear cells; moDCs = monocyte-derived dendritic cells; tolDCs = tolerogenic dendritic cells. Please click here to view a larger version of this figure.
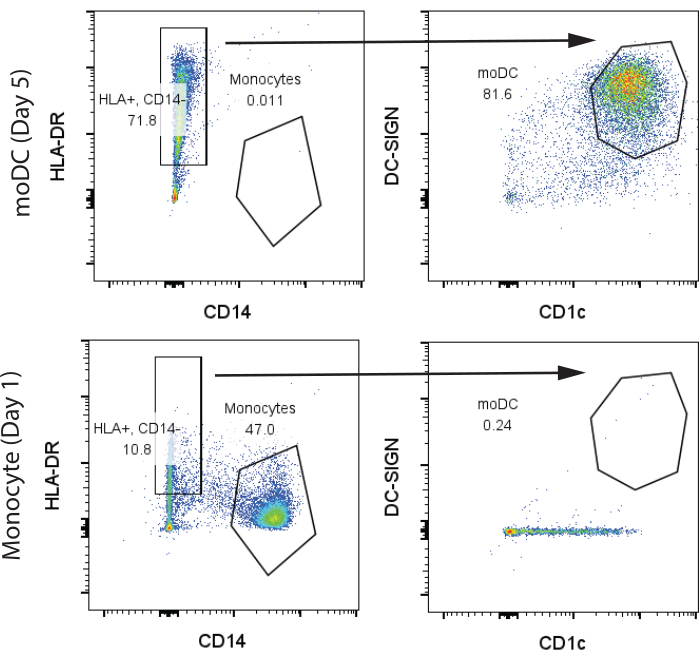
Figure 2: Representative gating strategy for identification of CD14+, HLA-DR- monocytes and CD14-, HLA-DR+, CD1c+, DC-SIGN+ moDCs. Samples were analyzed on day 7. Abbreviations: moDC = monocyte-derived dendritic cell; HLA-DR = human leukocyte antigen-DR; DC-SIGN = dendritic cell-specific C-type lectin. Please click here to view a larger version of this figure.

Figure 3: Flow cytometry analysis of moDCs post rapa and/or LPS treatment. On day 6, 105 moDCs were incubated with immunomodulators for 24 h, then washed and incubated with or without 0.1 µg/mL LPS for 24 h. On day 9, moDCs were washed and analyzed by flow cytometry. (A) Identification of CD14+, HLA-DR- monocytes, and CD14-, HLA-DR+, CD1c+, DC-SIGN+ moDCs. (B) Representative histograms of moDC CD86 signal after treatment; mean fluorescence intensity for (C) CD86, (D) CD40, (E) PD-L1, and (F) BTLA. Samples were in technical triplicates (N = 3) from the same patient PBMCs. Significance was determined by one-way ANOVA compared to UT group. *p < 0.05, **p < 0.01, ***p < 1 × 10-3, ****p < 1 × 10-4. Abbreviations: PBMCs = peripheral blood mononuclear cells; moDCs = monocyte-derived dendritic cells; HLA-DR = human leukocyte antigen-DR; rapa = rapamycin; LPS = lipopolysaccharide; MFI = mean fluorescence intensity; BTLA = B and T lymphocyte attenuator; PD-L1 = programmed cell death ligand 1; UT = Untreated; LPS moDC= moDCs treated with 0.1 µg/mL LPS on day 8; Rapa moDC = moDCs treated with 10 ng/mL rapa on day 7 then washed on day 8; Rapa + LPS moDCs = moDCs treated with 10 ng/mL rapa on day 7 then washed and treated 0.1 µg/mL LPS on day 8. Please click here to view a larger version of this figure.
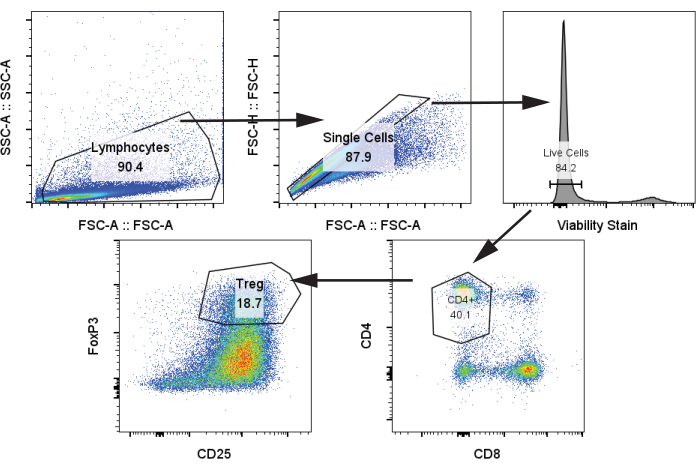
Figure 4: Representative gating strategy for identification of CD4+, CD25+, FoxP3+ Tregs. Sample was analyzed from thawed T cells on day 12. Abbreviations: SSC-A = side scatter-peak area; FSC-A = forward scatter-peak area; Tregs = regulatory T cells. Please click here to view a larger version of this figure.

Figure 5: Representative T cell analysis of moDC-T cell co-culture on day 12. (A) Representative CD25 versus FoxP3 flow plots of Live, single, CD4+ T cells to identify Tregs. Left column contains controls: T cells only (top) and CD3/CD28-stimulated T cells (bottom). Middle column: moDCs without rapa treatment, right column: moDcs with rapa treatment. For middle and right columns, top row is samples without LPS and bottom is with LPS treatment. (B) Representative gating for T cell proliferation. Either CD4+ (left) or CD8+ (right) T cells were plotted against proliferation stain. Peaks are indicated for 0, 1, 2, or 3 cell divisions. Histograms are labeled with the type of moDC treatment. Abbreviations: rapa = rapamycin; LPS = lipopolysaccharide; moDCs = monocyte-derived dendritic cells; T cells only = T cells with no moDCs without CD3/CD28 treatment; UT = Untreated; LPS moDC = moDCs treated 0.1 µg/mL LPS on day 8; Rapa moDC = moDCs treated with 10 ng/mL rapa on day 7 then washed on day 8. Note that rapa treatment increases relative amounts of cells with 0 divisions. Please click here to view a larger version of this figure.

Figure 6: Analysis of multiple moDC/T cell samples with additional immunmodulators. MoDCs were treated with 1 µM Dexamethasone, 10 ng/mL IL-10, 1 µg/mL Lipopolysaccharide, or 1 nM vitamin D3 in addition to 10 ng/mL Rapa as the immunomodulators given in step 2.1.4 but omitting immunostimulation. We also included a sample that was given Dex in step 2.1.4 and treated with 0.1 µg/mL LPS in step 2.3.3 on day 8 (Dex + LPS). (A,-D) Cytokine analysis of moDCs. Supernatants were tested for IL-10 either (A) 24 h or (B) 72 h after step 2.3.3 or tested for TNFα for C) 24 h or (D) 72 h after step 2.3.3 using commercial ELISA kits. N = 5. (E-H) Analysis of moDC surface markers. Similarly, moDCs were analyzed via flow cytometry for PD-L1 at (E) 24 h or (F) 72 h or for CD86 at (G) 24 h or (H) 72 h. N = 5. (I) Analysis of T cell proliferation. CD4+ T cells on day 12 were analyzed for T cell proliferation and the number of cell divisions per 100 T cells were calculated. Samples were incubated at a 1:5 moDC:T cell ratio, N = 4. (E) Treg analysis. MoDCs differentiated from five different patients were analyzed in a similar fashion as in Figure 5. Left, untreated moDCs; right, moDCs treated with 10 ng/mL rapa. Each colored line represents a different patient sample. Significance was determined by one way ANOVA. *p < 0.05, **p < 0.01.Abbreviations: rapa = rapamycin; LPS = lipopolysaccharide; TNFα = tumor necrosis factor-alfa; moDCs = monocyte-derived dendritic cells; UT = Untreated; LPS moDC = moDCs treated 0.1 µg/mL LPS; Rapa moDC = moDCs treated with 10 ng/mL rapa, Dex = moDCs treated with 1 µM Dexamethasone, IL-10= moDCs treated with 10 ng/mL IL-10, VD3= moDCs treated with 1 nM vitamin D3, Dex + LPS= moDCs treated with 1 µM Dexamethasone on day 7 and then 0.1 µg/mL LPS on day 8. Please click here to view a larger version of this figure.
| moDC Culture Medium | Flow Staining Solution | T Cell Culture Medium | T Cell Freeze Medium | |
| Media Base | RPMI 1640 | HBSS | RPMI 1640 | T cell Culture Media |
| Component 1 | 10% heat-inactivated FBS (HI-FBS) | 1% BSA w/v | 10% heat-inactivated FBS (HI-FBS) | 10% DMSO |
| Component 2 | 1x Penicillin-Streptomycin | 0.1 mM EDTA | 1x Penicillin-Streptomycin | N/A |
| Component 3 | N/A | N/A | 1 mM HEPES | N/A |
| Component 4 | N/A | N/A | 1 x Non-essential amino acids | N/A |
| Component 5 | N/A | N/A | 50 μM β-mercaptoethanol | N/A |
Table 1: Description of Cell Culture Reagents
| Panel | Antibody | Fluorophore | Dilution | Channel |
| Validation | HLA-DR | Alexa Fluor488 | 1:500 | Blue 1 |
| CD14 | PE | 1:500 | Blue 2 | |
| DC-SIGN | Percp-Cy5.5 | 1:500 | Blue 3 | |
| CD1c | PE-Cy7 | 1:500 | Blue 4 | |
| CD40 | APC | 1:500 | Red 1 | |
| Tolerance | CD86 | FITC | 1:500 | Blue 1 |
| PD-L1 | PE | 1:5000 | Blue 2 | |
| DC-SIGN | Percp-Cy5.5 | 1:500 | Blue 3 | |
| CD1c | PE-Cy7 | 1:500 | Blue 4 | |
| BTLA | APC | 1:250 | Red 1 | |
| CD40 | APC efluor 780 | 1:500 | Red 3 | |
| Treg | CD4 | Alexa Fluor488 | 1:500 | Blue 1 |
| CD8 | PE-Cy7 | 1:500 | Blue 4 | |
| CD25 | APC | 1:500 | Red 1 | |
| T Proliferation | CD4 | Alexa Fluor488 | 1:500 | Blue 1 |
| CD69 | PE | 1:500 | Blue 2 | |
| CD8 | PE-Cy7 | 1:500 | Blue 4 | |
| *All antibodies are diluted in Flow Stain Solution | ||||
Table 2: Description of Flow Cytometry Antibody Panels
Discussion
Here we describe a reliable and versatile method to assess the functionality of immunomodulatory agents to induce tolDCs from moDCs and validate their functionality to generate Tregs from autogenic T cells ex vivo. There are several critical steps in this protocol. First, monocytes are notoriously sensitive cells and must be obtained from fresh, not previously frozen PBMCs for the best results. Monocytes should be isolated as soon as possible and placed in the differentiation cocktail. Typically, poor monocyte yield or high rates of monocyte death in the first 24 h are due to freeze/thawing cycles or prolonged periods between isolation of PBMCs and monocytes. Second, cytokines used for differentiation of moDCs and T cell culture media must be from a high-quality source. Typically, poor differentiation of moDCs is due to poor quality or incorrect concentrations of cytokines. Third, we recommend that T cells be frozen between day 1 and day 7 and not kept in culture to preserve their naïve and unstimulated phenotype. Fourth, if may be helpful to allow treated moDCs to rest for up to 72 h to allow for increases in tolerogenic markers such as PD-L1 and IL-10 as seen in Figure 6. Finally, as seen in our data, there is wide patient variability in Treg generation capacity, so each control must be run with each patient sample to draw conclusions. We recommend testing multiple patient samples.
In our representative results, we chose a rather restrictive experimental design for demonstrating tolerance with a high bar. We chose a moderately low concentration of rapa (10 ng/mL), only incubated with already-differentiated moDCs for 24 h and then incubated in a 1:5 moDC:T cell ratio for 72 h with CD3/CD28 non-specific stimulation. This protocol is highly versatile as any of the following conditions can be easily altered: immunomodulator concentration, immunomodulator incubation time, whether incubation occurs concurrently with moDC differentiation or separately, moDC:T cell co-culture ratio, co-culture incubation time, and T cell co-stimulation. All these factors can alter how readily moDC treatment induces Treg generation. Of note is the use of non-specific CD3/CD28 stimulation to induce T cell proliferation. This could readily be replaced with an antigen of interest -- a control antigen such as CEFT peptide pools with healthy patient samples or autoantigens like insulin with type 1 diabetes patient samples27,28. This would provide antigen-specific tolerance data, which would likely be different from non-specific Treg mediated tolerance.
While versatile, this protocol does have some limitations. Namely, it is limited to only a few tolDC markers, CD86, CD40, PD-L1, and BTLA. These are commonly accepted markers and a limitation of the number of available channels in our laboratory flow cytometer. Other markers such as ILT-3 and ILT-4 or other could also be chosen25. This protocol also does not evaluate other types of suppressor T cell subsets, such as type 1 regulatory T cells (Tr1) cells, which can demonstrate potent protective phenotypes in vivo29. We contend, however, that this protocol evaluates the functionality of DCs to generate classical FoxP3+ CD25+ Tregs, which is sufficient for the initial screening of novel immunomodulatory compounds and the evaluation of DC tolerogenic capacity.
Another interesting aspect of human moDC evaluation is patient variability and the limited IL-10, PD-L1, BLTA and, most importantly, Treg response to immunomodulators shown in our representative results, particularly to rapa. The literature shows conflicting results. Similar results to this paper have been seen in both rapa-treated murine and human DCs, showing decreases in markers of DC maturity (CD80/CD86/CD40) and prevention of IL-2 release from T cells when non-specifically stimulated, but no T cell death or Treg generation26,30. However, other studies in both humans and mice show that ex vivo rapa-treated DCs show high levels of Treg generation25. Rapa is a mechanistic target of rapamycin (mTOR) inhibitor, a protein kinase in the PI3K/AKT/mTOR signaling pathway having several crucial cellular functions such as cell growth, proliferation, and survival31. Rapa is often used as an anti-rejection medication for transplantation and has a well-established clinical benefit in preventing immunological responses. Specifically for its use as a tolDC-induction agent, however, rapa can have varying effectiveness from patient to patient.
This highlights the need to both rigorously investigate tolDC induction agents against wider patient populations and identify novel immunomodulatory compounds. An overwhelming number of clinical studies on human tolDCs use either rapa, IL-10, dexamethasone, or vitamin D3 as immunomodulators32. This leaves a wide chemical space unexplored. We present this assay as an investigative tool to assess the tolDC induction potential of novel immunomodulators and to evaluate their potential to generate tolDCs from endogenous DCs. This protocol is simple, versatile, requires only a flow cytometer, and could be adapted for high-throughput functionality.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We would like to thank the University of Pennsylvania's Human Immunology Core (HIC) for providing fresh human PBMCs from donors. The HIC is supported in part by NIH P30 AI045008 and P30 CA016520.
Materials
| Name | Company | Catalog Number | Comments |
| 0.1-10 µL Filtered Pipet tips | VWR | 76322-158 | General Cell Culture |
| 1.5 mL Centrifuge Tube | VWR | 77508-358 | General Cell Culture |
| 10 mL Serological Pipets | VWR | 414004-267 | General Cell Culture |
| 100-1000 µL Filtered Pipet tips | VWR | 76322-164 | General Cell Culture |
| 15 mL Conical Tube | VWR | 77508-212 | General Cell Culture |
| 20-200 µL Filtered Pipet tips | VWR | 76322-160 | General Cell Culture |
| 2-Mercaptoethanol | MP Biomedical | 194834 | T Cell Culture |
| 50 mL Conical Tube | VWR | 21008-736 | General Cell Culture |
| 60 x 15 mm Dish, Nunclon Delta | Thermo Fischer | 150326 | General Cell Culture |
| 96 Well Conical (V) Bottom Plate, Non-Treated Surface | Thermo Fischer | 277143 | General Cell Culture |
| 96 well Flat Bottom Plate | Thermo Fischer | 161093 | General Cell Culture |
| APC/Cyanine7 anti-human CD272 (BTLA) Antibody | Biolegend | 344518 | Flow Cytometry |
| Attune NxT (Red/Blue Laser, 7 Channel) | Thermo Fischer | A24863 | Flow Cytometry |
| BSA | Thermo Fischer | 15260-037 | General Cell Culture |
| CD14 Monoclonal Antibody (61D3), PE | Thermo Fischer | 12-0149-42 | Flow Cytometry |
| CD1c Monoclonal Antibody (L161), PE-Cyanine7 | Thermo Fischer | 25-0015-42 | Flow Cytometry |
| CD209 (DC-SIGN) Monoclonal Antibody (eB-h209), PerCP-Cyanine5.5 | Thermo Fischer | 45-2099-42 | Flow Cytometry |
| CD25 Monoclonal Antibody (CD25-4E3), APC | Thermo Fischer | 17-0257-42 | Flow Cytometry |
| CD274 (PD-L1, B7-H1) Monoclonal Antibody (MIH1), PE | Thermo Fischer | 12-5983-42 | Flow Cytometry |
| CD4 Monoclonal Antibody (RPA-T4), Alexa Fluor 488 | Thermo Fischer | 53-0049-42 | Flow Cytometry |
| CD40 Monoclonal Antibody (5C3), APC | Thermo Fischer | 17-0409-42 | Flow Cytometry |
| CD40 Monoclonal Antibody (5C3), APC-eFluor 780 | Thermo Fischer | 47-0409-42 | Flow Cytometry |
| CD69 Monoclonal Antibody (FN50), PE | Thermo Fischer | MA1-10276 | Flow Cytometry |
| CD86 Monoclonal Antibody (BU63), FITC | Thermo Fischer | MHCD8601 | Flow Cytometry |
| CD8a Monoclonal Antibody (RPA-T8), PE-Cyanine7 | Thermo Fischer | 25-0088-42 | Flow Cytometry |
| Conical Bottom (V-well) 96 Well Plate | Thermo Fischer | 2605 | Flow Cytometry |
| Cryogenic Vials, 2 mL | Thermo Fischer | 430488 | T Cell Culture |
| Dimethylsulfoxide (DMSO), Sequencing Grade | Thermo Fischer | 20688 | General Cell Culture |
| DPBS | Thermo Fischer | 14200166 | General Cell Culture |
| EasySep Human Monocyte Isolation Kit | Stem Cell Technologies | 19359 | Cell Separation |
| EasySep Human T Cell Isolation Kit | Stem Cell Technologies | 17951 | Cell Separation |
| EasySep Magnet | Stem Cell Technologies | 18000 | Cell Separation |
| EDTA | Thermo Fischer | AIM9260G | General Cell Culture |
| Falcon Round-Bottom Polystyrene Tubes, 5 mL | Stem Cell Technologies | 38025 | Cell Separation |
| Fc Receptor Binding Inhibitor Polyclonal Antibody | Thermo Fischer | 14-9161-73 | Flow Cytometry |
| Fetal Bovine Serum | Thermo Fischer | A5670701 | General Cell Culture |
| Fixable Viability Dye eFluor 780 | Thermo Fischer | 65-0865-18 | Flow Cytometry |
| Foxp3 / Transcription Factor Staining Buffer Set | Thermo Fischer | 00-5523-00 | Flow Cytometry |
| FOXP3 Monoclonal Antibody (PCH101), PE-Cyanine5.5 | Thermo Fischer | 35-4776-42 | Flow Cytometry |
| HBSS | Thermo Fischer | 14170-112 | General Cell Culture |
| Heat Inactivated Fetal Bovine Serum | Thermo Fischer | A5670801 | General Cell Culture |
| HEPES (1 M) | Thermo Fischer | 15630106 | moDC Cell Culture |
| HLA-DR Monoclonal Antibody (L243), Alexa Fluor 488 | Thermo Fischer | A51009 | Flow Cytometry |
| Human CD3/CD28/CD2 T Cell Activator | StemCell Technologies | 10970 | T Cell Culture |
| Human GM-CSF Recombinant Protein | Thermo Fischer | 300-03 | moDC Cell Culture |
| Human IL-10 ELISA Kit, High Sensitivity | Thermo Fischer | BMS215-2HS | ELISA |
| Human IL-4, Animal-Free Recombinant Protein | Thermo Fischer | AF-200-04 | moDC Cell Culture |
| Human PBMC (Freshly Isolated) | UPenn HIC | N/A | Cells |
| Human TNF alpha ELISA Kit | Thermo Fischer | BMS223-4 | ELISA |
| Light Microscope (DMi1) | Lucia | 391240 | General Cell Culture |
| Lipopolysaccaride (LPS) | Invivogen | tlrl-eblps | moDC Cell Culture |
| LIVE/DEAD Fixable Near-IR Dead Cell Stain Kit | Thermo Fischer | L34975 | Flow Cytometry |
| MEM Non-Essential Amino Acids Solution (100x) | Thermo Fischer | 11140050 | T Cell Culture |
| Penicillin-Streptomycin (100x) | Thermo Fischer | 15140122 | General Cell Culture |
| Pipette Controller | VWR | 77575-370 | General Cell Culture |
| Rapamycin, 98+% | Thermo Fischer | J62473.MF | moDC Cell Culture |
| RPMI 1640 with Glutamax | Thermo Fischer | 61870-036 | General Cell Culture |
| Separation Buffer | Stem Cell Technologies | 20144 | Cell Separation |
| T Cell Stimulation Cocktail (500x) | Thermo Fischer | 00-4970-93 | T Cell Culture |
| UltraComp eBead Plus Compensation Beads | Thermo Fischer | 01-3333-41 | Flow Cytometry |
| Variable Pipette Set | Fischer Scientific | 05-403-152 | General Cell Culture |
References
- Banchereau, J., et al. Immunobiology of dendritic cells. Annu Rev Immunol. 18 (1), 767-811 (2000).
- Blander, J. M., Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 440 (7085), 808-812 (2006).
- Anderson, A. E., et al. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Bio. 85 (2), 243-250 (2009).
- Iberg, C. A., Hawiger, D. Natural and induced tolerogenic dendritic cells. J Immunol. 204 (4), 733-744 (2020).
- Ness, S., Lin, S., Gordon, J. R. Regulatory dendritic cells, T cell tolerance, and dendritic cell therapy for immunologic disease. Front Immunol. 12, 633436 (2021).
- Ali, S., et al. Sources of type I interferons in infectious immunity: Plasmacytoid dendritic cells not always in the driver's seat. Front Immunol. 10, 778 (2019).
- Fossum, E., et al. Targeting antigens to different receptors on conventional type 1 dendritic cells impacts the immune response. J Immunol. 205 (3), 661-673 (2020).
- Kedl, R. M., et al. Migratory dendritic cells acquire and present lymphatic endothelial cell-archived antigens during lymph node contraction. Nat Comm. 8 (1), 2034 (2017).
- Chow, K. V., Sutherland, R. M., Zhan, Y., Lew, A. M. Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immun Cell Biol. 95 (3), 244-251 (2017).
- Johnson, R. K., Overlee, B. L., Sagen, J. A., Howe, C. L. Peripheral blood mononuclear cell phenotype and function are maintained after overnight shipping of whole blood. Sci Rep. 12 (1), 19920 (2022).
- Boks, M. A., et al. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction - A comparative study of human clinical-applicable DC. Clinl Immunol. 142 (3), 332-342 (2012).
- Navarro-Barriuso, J., et al. Vitamin D3-induced tolerogenic dendritic cells modulate the transcriptomic profile of T CD4+ cells towards a functional hyporesponsiveness. Front Immunol. 11, 599623 (2021).
- Moorman, C. D., Sohn, S. J., Phee, H. Emerging therapeutics for immune tolerance: Tolerogenic vaccines T cell therapy, and IL-2 therapy. Front Immunol. 12, 657768 (2021).
- Kenison, J. E., et al. Tolerogenic nanoparticles suppress central nervous system inflammation. Proc Natl Acad Sci USA. 117 (50), 32017-32028 (2020).
- Neshat, S. Y., et al. Improvement of islet engrafts via Treg induction using immunomodulating polymeric tolerogenic microparticles. ACS Biomater Sci Eng. 9 (6), 3522-3534 (2023).
- Deak, P., Knight, R., Esser-Kahn, A. Robust tolerogenic dendritic cells via push/pull pairing of toll-like-receptor agonists and immunomodulators reduces EAE. Biomaterials. 286, 121571 (2022).
- Møller, S. H., Wang, L., Ho, P. -. C. Metabolic programming in dendritic cells tailors immune responses and homeostasis. Cell Mol Immunoly. 19 (3), 370-383 (2022).
- Adamik, J., et al. Distinct metabolic states guide maturation of inflammatory and tolerogenic dendritic cells. Nat Comm. 13 (1), 5184 (2022).
- Hals, I. K., et al. Investigating optimal β-cell-preserving treatment in latent autoimmune diabetes in adults: Results from a 21-month randomized trial. Diabetes Obes Metab. 21 (10), 2219-2227 (2019).
- Gammon, J. M., et al. Engineering the lymph node environment promotes antigen-specific efficacy in type 1 diabetes and islet transplantation. Nat Comm. 14 (1), 681 (2023).
- Sim, W. J., Malinarich, F., Fairhurst, A. -. M., Connolly, J. E. Generation of immature, mature and tolerogenic dendritic cells with differing metabolic phenotypes. J Vis Exp. (112), e54128 (2016).
- Jia, S., Kim, J., Esser-Kahn, A. P., Deak, P. High-throughput screening identification of novel immunomodulatory combinations for the generation of tolerogenic dendritic cells. Front Med. 10, 1298424 (2024).
- Dinh, B., et al. Isolation and cryopreservation of highly viable human peripheral blood mononuclear cells from whole blood: A guide for beginners. J Vis Exp. (212), e66794 (2024).
- Akkaya, B., et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol. 20 (2), 218-231 (2019).
- Stallone, G., et al. mTOR inhibitors effects on regulatory T cells and on dendritic cells. J Trans Med. 14 (1), 152 (2016).
- Dahlqvist, G., et al. Modulatory effect of rapamycin and tacrolimus on monocyte-derived dendritic cells phenotype and function. Immunobiology. 226 (1), 152031 (2021).
- Cimen Bozkus, C., Blazquez, A. B., Enokida, T., Bhardwaj, N. A T-cell-based immunogenicity protocol for evaluating human antigen-specific responses. STAR Protoc. 2 (3), 100758 (2021).
- Nakayama, M., Michels, A. W. Determining antigen specificity of human islet infiltrating T cells in type 1 diabetes. Front Immunol. 10, 365 (2019).
- Gregori, S., et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 116 (6), 935-944 (2010).
- Taner, T., Hackstein, H., Wang, Z., Morelli, A. E., Thomson, A. W. Rapamycin-treated, alloantigen-pulsed host dendritic cells induce Ag-specific T cell regulation and prolong graft survival. Am J Transpl. 5 (2), 228-236 (2005).
- Baroja-Mazo, A., Revilla-Nuin, B., Ramírez, P., Pons, J. A. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J Transplant. 6 (1), 183-192 (2016).
- Phillips, B. E., Garciafigueroa, Y., Trucco, M., Giannoukakis, N. Clinical tolerogenic dendritic cells: Exploring therapeutic impact on human autoimmune disease. Front Immunol. 8, 1279 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved