Method Article
Functional Analysis of Tumor-Infiltrating Myeloid Cells by Flow Cytometry and Adoptive Transfer
* These authors contributed equally
In This Article
Summary
This protocol provides reliable methods of solid tumor dissociation and myeloid cell isolation in murine intradermal or subcutaneous tumor models. Flow cytometry allows for phenotypic characterization of heterogeneous myeloid populations within the tumor microenvironment and sorting will demonstrate their functionality in the context of adoptive transfer.
Abstract
The tumor-infiltrating myeloid cell compartment represents a heterogeneous population of broadly immunosuppressive cells that have been exploited by the tumor to support its growth. Their accumulation in tumor and secondary lymphoid tissue leads to the suppression of antitumor immune responses and is thus a target for therapeutic intervention. As it is known that the local cytokine milieu can dictate the functional programming of tumor-infiltrating myeloid cells, strategies have been devised to manipulate the tumor microenvironment (TME) to express a cytokine landscape more conducive to antitumor myeloid cell activity. To evaluate therapy-induced changes in tumor-infiltrating myeloid cells, this paper will outline the procedure to dissociate intradermal/subcutaneous tumor tissue from solid tumor-bearing mice in preparation for leukocyte recovery. Strategies for flow cytometric analysis will be provided to enable the identification of heterogeneous myeloid populations within isolated leukocytes and the characterization of unique myeloid phenotypes. Lastly, this paper will describe a means of purifying viable myeloid cells for functional assays and determining their therapeutic value in the context of adoptive transfer.
Introduction
The tumor microenvironment (TME) is comprised of rapidly proliferating neoplastic cells and a surrounding heterogeneous stromal cell compartment. As growing tumors are often poorly vascularized, the TME is a peripheral site uniquely characterized by hypoxia, nutrient deprivation, and acidosis1. To survive in this landscape, tumor stress responses and metabolic reprogramming result in the secretion of soluble factors that promote tissue remodeling and angiogenesis as well as the selective recruitment of immune cells2. As myeloid cells are one of the most abundant type of hematopoietic cells in the TME, there is increasing interest in examining the role of tumor-infiltrating myeloid cells in the TME.
Myeloid cells are a heterogenous and plastic group of innate immune cells including monocytes, macrophages, dendritic cells, and granulocytes. Although they have critical roles in tissue homeostasis and adaptive immune response regulation, their function can be polarizing depending on the composition of activation signals within the local microenvironment3. Tumors take advantage of myeloid cell characteristics through the secretion of soluble factors within the TME. These alternative signals can divert myelopoiesis towards immature differentiation and skew the function of existing tumor-infiltrating myeloid cells3. Indeed, myeloid cells within the TME often promote cancer progression and can suppress antitumor immune responses, leading to adverse effects on cancer therapy.
Although therapeutic strategies promoting the depletion of immunosuppressive myeloid cells have been shown to delay tumor growth4, the lack of target specificity risks the removal of immunostimulatory myeloid cells, which by contrast, aid in the resolution of cancer. These inflammatory myeloid cells can exert profound antitumor effects including direct tumor cell killing and activation of cytotoxic CD8+ T cells5. Alternatively, strategies normalizing the composition and function of myeloid cells in the TME have shown therapeutic success6; however, the biological mechanisms underlying their re-education towards an antitumor phenotype have still not been fully understood. Ultimately, a comprehensive characterization of tumor myeloid cells is necessary for further improvement of cancer therapy.
Unfortunately, reproducible disaggregation of tumors for myeloid cell isolation is challenging. Tumor-derived myeloid cells are sensitive to ex vivo manipulation compared to other leukocyte subsets, and the aggressiveness of tumor processing can lead to enzymatic epitope cleavage and reduced viability of recovered cells7. The purpose of this method is to provide a reliable means of tumor dissociation to preserve surface marker integrity for analysis and cellular vitality for functional study. In comparison to tumor-infiltrating leukocyte (TIL) isolation protocols that favor harsher enzymatic mixes to enhance the reproducible release of various cellular subsets, this method favors more conservative enzymatic digestion to maximize myeloid cell recovery. High-level multi-color flow gating strategies are also provided to identify murine tumor myeloid cell subsets for further characterization and/or sorting.
Protocol
NOTE: All animal studies complied with the Canadian Council on Animal Care guidelines and were approved by McMaster University's Animal Research Ethics Board.
1. Tumor harvest and dissociation
- Inoculate 6-8-week-old, female, C57BL/6 mice intradermally/subcutaneously with 2 × 105 B16 melanoma cells as described by Nguyen et al.8 Allow tumors to grow for 7 days before harvesting.
- Euthanize the mouse by cervical dislocation while making sure to not disrupt the tumor when doing so. Spray the mouse down with 70% ethanol before harvesting.
- Using a scalpel and scissors, surgically remove the intradermal/subcutaneous tumor from surrounding tissue (including attached tumor-draining lymph nodes), and place the tumors into a preweighed microfuge tube. Keep on ice.
NOTE: Conduct tumor harvest in an animal-use biosafety cabinet. Use a 15 mL conical tube for larger tumors. - Weigh the tumors, and add 500 µL of RPMI-1640 medium with 10% fetal bovine serum (FBS) to each tube, using scissors to cut the tumors into small pieces within the tube or in a 6-well plate.
NOTE: The tumor pieces should be small enough to be mixed by an electric pipettor once the digestion medium has been added. - Prepare the dissociation mix by dissolving collagenase type IV at 0.5 mg/mL and DNase at 0.2 mg/mL in RPMI-1640 medium with 10% FBS and 5 mM calcium chloride.
NOTE: Dissociation mix must be prepared fresh to maximize collagenase activity. - Transfer the minced tumor suspension to a 15 mL conical tube, and add 10 mL of dissociation mix per 0.25 mg of tumor. Place the tube in a temperature-controlled orbital shaker for 30 min at 37 °C with 200 rpm agitation. Neutralize the collagenase activity by adding two volumes of cold RPMI-1640 medium with 10% FBS and 2 mM ethylenediamine tetraacetic acid (EDTA), and refrigerate for 10 min at 4 °C.
- Briefly vortex and pipette the suspension into a 40 µm strainer on a 50 mL conical tube. Use a syringe plunger and neutralizing media to disaggregate the residual tumor tissue, and wash it through the strainer. Centrifuge the suspension for 5 min (500 × g, 4 °C), discard the supernatant, and resuspend the pellet in phosphate-buffered saline (PBS) with 2% FBS and 1 mM EDTA.
2. TIL enrichment and flow cytometric staining (FACS)
- To enrich TILs for myeloid cell characterization, use a magnetic cell separation kit designed for biotin-positive selection with biotinylated CD45.2 antibodies according to the manufacturer's instructions (see the Table of Materials).
- Resuspend the cells in 200 µL of FACS buffer (PBS with 0.5% w/v bovine serum albumin (BSA)), and transfer them to a 96-well U-bottom plate.
NOTE: Do not exceed a staining concentration of 1 × 108 cells/mL. Adjust the volume, and split samples into multiple wells to compensate for high cell numbers. - Centrifuge the plate for 5 min (500 × g, 4 °C), and discard the supernatant. Add 50 µL of Fc block solution (1:200 dilution of purified rat anti-mouse CD16/CD32 [see the Table of Materials) in FACS buffer, final concentration of 2.5 µg/mL), and resuspend the cells by pipetting. Incubate for 10 min at 4 °C.
- Add 50 µL of FACS buffer containing 2x concentration of surface-staining antibody (1:50 dilution of CD45.2, NK1.1, CD11c, F4/80, CD8a, Ly6C, CD11b, CD4, Ly6G) and fixable viability stain (FVS, 1:500 dilution), and mix the cells by pipetting. Incubate for 20 min at 4 °C.
NOTE: Cover the plate with aluminum foil to minimize light exposure. Antibodies should be titrated prior to the experiment to empirically determine the optimal dilution. - Wash the cells twice by adding 200 µL of FACS buffer to each well, centrifuging the suspension (5 min, 500 × g, 4 °C), and discarding the supernatant.
- Add 100 µL of fixation/permeabilization solution (see the Table of Materials) to each well, mix the cells by pipetting, and incubate for 20 min at 4 °C.
- Add 100 µL of 1x permeabilization buffer (see the Table of Materials) to each well, centrifuge the plate for 5 min (500 × g, 4 °C), and discard the supernatant.
- Wash the cells by adding 200 µL of 1x permeabilization buffer to each well, centrifuging the suspension (5 min, 500 × g, 4 °C), and discarding the supernatant.
NOTE: The experiment can be paused overnight after resuspending the cells in permeabilization buffer. Store the sample at 4 °C and protected from light. Resume after briefly mixing the cells before centrifuging. - Add 100 µL of permeabilization buffer containing 1x concentration of intracellular staining antibody (1:100 dilution of nitric oxide synthase 2 (NOS2), arginase 1 (Arg1)), and mix the cells by pipetting. Incubate for 20 min at 4 °C.
NOTE: Cover the plate with aluminum foil to minimize light exposure. Antibodies should be titrated prior to the experiment to empirically determine the optimal dilution. - Add 100 µL of 1x permeabilization buffer to each well, centrifuge the plate for 5 min (500 × g, 4 °C), and discard the supernatant.
- Wash the cells by adding 200 µL of 1x permeabilization buffer to each well, centrifuging the suspension (5 min, 500 × g, 4 °C), and discarding the supernatant.
- Resuspend the cells in 300 µL of FACS buffer. Filter the sample through a 5 mL round-bottom polystyrene tube with 40 µm strainer cap before performing flow cytometry analysis.
3. Tumor myeloid cell sorting for functional studies
- After identifying the desired myeloid cell populations by flow cytometry analysis, pre-enrich bulk myeloid cells for sorting with a magnetic cell separation kit designed for CD11b- or CD11c-positive selection according to the manufacturer's instructions (see the Table of Materials).
- Using surface-staining antibodies specific for the desired myeloid cell subsets (1:100 dilution of CD11b, Ly6C, Ly6G), stain the pre-enriched cells as described in steps 2.2-2.5.
NOTE: Include fixable viability stain to ensure the sorting of live myeloid cells. Do not exceed a staining concentration of 1 × 108 cells/mL. Adjust the volume, and split the samples into multiple wells to compensate for high cell numbers. - Resuspend the cells in cold sorting buffer (PBS with 1% w/v BSA, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 1 mM EDTA). Filter the sample through a 5 mL round-bottom polypropylene tube with a 40 µm strainer cap. Keep the cells on ice, and cover the tube with aluminum foil.
NOTE: Adjust the concentration of cells/volume to the desired instrument specification for sorting. - Prepare a sample collection tube with capture medium (5 mL round-bottom polypropylene tube containing PBS with 50% FBS).
NOTE: Coat tubes with 5 mL capture medium overnight prior to sorting. Discard all but 1-2 mL of the capture medium on the next day before running the sample. - Modify the sorter instrument settings to decrease sample pressure and prevent perturbations in droplet formation. Equip the 130 µm nozzle tip, and utilize the 10 psi setting. Run the sample at a low flow rate with periodic sample agitation at 100 rpm, ensuring that the deposition of droplets is in the center of the tube. After the sort, keep the sample on ice.
- Incubate the sample for 10 min at 4 °C. Centrifuge the tube for 5 min (500 × g, 4 °C), discard the supernatant, and resuspend the pellet in the desired medium for functional assays or adoptive transfer.
4. Adoptive transfer of purified tumor myeloid cells
- Inoculate 6-8-week-old, female, C57BL/6 mice intradermally with 1 × 106 B16 melanoma tumor cells resuspended in 30 µL of PBS.
NOTE: Inoculate mice in advance such that tumor growth does not exceed 100 mm3 by the time of adoptive transfer. - Resuspend the sorted tumor myeloid cells in PBS with 25 mM HEPES and 1 mM EDTA at a concentration of 2 × 106 cells/mL. Filter the sample through a 5 mL round-bottom polystyrene tube with a 40 µm strainer cap. Keep on ice.
- Induce and maintain mice under anesthesia with 3% isoflurane. Apply ophthalmic ointment to prevent ocular dryness/injury.
- Load a 31 G syringe with 50 µL of cell suspension. Dislodge air bubbles by gently flicking the syringe. Clean the injection site using an alcohol swab.
- Using sterile forceps, lift the skin at the base of the tumor. Insert the needle into the subcutaneous space at a slight upward angle to enter the tumor from below the skin. Use the forceps to pinch the skin surrounding the needle, and slowly dispense the syringe volume. Continue to pinch the skin with the forceps while removing the needle slowly, and use a cotton swab to clean potential leakage.
NOTE: Proceed with any additional therapeutic treatments if desired. - Allow the mice to recover from anesthesia.
Results
The results demonstrate that this method produces a high yield of myeloid cells from solid murine tumors. The preservation of receptor integrity and cellular viability facilitates reliable functional analysis of the desired myeloid subsets. These improvements to myeloid cell isolation allowed the discernment of the changing function of intratumoral myeloid cells upon normalization of the TME with the class I histone deacetylase inhibitor (HDACi), MS-275, during adoptive T cell therapy. TIL isolation protocols typically do not take the steps to maximize myeloid cell yield9. As a result, enzymatic digestion is typically too harsh and leads to a loss of sample viability. When processed B16 melanoma tumors were treated with collagenase type I for 1 h (a commonly used condition) before positively enriching for CD45.2+ cells by magnetic selection, the morphology (forward Scatter-area (FSC-A) vs. side scatter-area (SSC-A)) and myeloid cell subgating (CD11b vs. CD11c) indicated that the yield of myeloid cells (CD11b+ or CD11c+) and non-myeloid cells (CD11b- CD11c-) was extremely low (Figure 1). To reduce the potentially excessive specific activity of collagenase, the duration of tumor digestion was decreased to 30 min. While there was a slight improvement in myeloid cell recovery, the overall yield was still low and there was no improvement in non-myeloid cell recovery.
Because lot variation may introduce proteases with specific activity high enough to cause excessive cell death, a separate lot of collagenase type I was requested from a different commercial supplier for comparison. Interestingly, the overall yield of myeloid and non-myeloid cells was much higher, with a slight enhancement in myeloid cell number upon the addition of FBS. Although FBS was added to stabilize the myeloid cells to collagenase-induced damage, this raised the question as to whether FBS was also neutralizing the tryptic activity of the collagenase type I preparation, which could be impairing cell recovery. As collagenase type I preparations have collagenase, caseinase, clostripain, and tryptic activities10, to reduce protease exposure, the digestion was attempted using collagenase type IV, which has higher collagenase-specific activity and lower tryptic activity. This condition resulted in a greater increase in myeloid cell yield, with the addition of FBS resulting in the highest yield. Interestingly, collagenase type I and type IV, with or without FBS, did not markedly change the overall yield of non-myeloid cells.
With these optimized enzymatic digestion conditions, leukocytes were isolated from murine B16 melanoma tumors, and flow cytometry was used to phenotype the different myeloid cell populations within the TME based on their expression of surface markers. Tumors were harvested and processed and the leukocytes isolated using a CD45.2 magnetic selection kit. The cells were then stained using a carefully designed panel of cell surface markers (Figure 2). The gating strategy described here starts with a morphological assessment of the cells using FSC-A vs. SSC-A. This allows the exclusion of cellular debris based on their small size. FSC-H vs. FSC-W was used to select single cells and exclude the doublets. Total live leukocytes were then gated based on CD45.2 and viability staining. Lymphocytes were excluded based on NK1.1, CD4, and CD8 staining; note that in BALB/c mice, Asialo-GM1 and/or DX5 can be used to exclude natural killer (NK) cells as NK1.1 is not expressed on BALB/c-derived NK cells. CD11b was then plotted against CD11c to identify tumor-associated dendritic cells (TADCs)/conventional dendritic cells (cDCs).
Cells that are negative for CD11c represent the bulk myeloid cells, which can be further separated based on Ly6C and Ly6G staining. Cells that express intermediate levels of Ly6C and high levels of Ly6G represent the neutrophils. This population shares the same phenotype as the granulocytic myeloid-derived suppressor cells (G-MDSCs). CD11b+ cells that stain negative for Ly6G, but positive for Ly6C can be divided into Ly6Chi, Ly6Cint, and Ly6Clo. Ly6Cint/hi cells express lower levels of F4/80 and represent inflammatory monocytes. However, Ly6Chi cells also share the same phenotype with monocytic myeloid-derived suppressor cells (M-MDSCs). Finally, Ly6Cint cells express high levels of F4/80 and are usually associated with tumor-associated macrophages (TAMs). While this characterization may not fully identify the myeloid cell subsets of interest, it provides a useful gating strategy to sort myeloid cell populations within the TME for further functional or genomic analyses. Within the context of immunotherapy, adoptive T-cell therapy incorporating epigenetic modifying drugs, such as the class I histone deacetylase inhibitor (HDACi) MS-275, can affect the TME to promote sustained tumor regression, while its absence results in tumor relapse8.
Although microarray analysis of bulk tumor RNA suggests a role for tumor-infiltrating myeloid cells, this phenotypic characterization did not indicate major surface marker changes during MS-275 treatment8. Interestingly, functional markers present in the flow cytometry staining panel identified a certain myeloid cell subset (CD11b+ Ly6Chi Ly6G-) differentially producing nitric oxide synthase 2 (NOS2) and arginase 1 (Arg1), which are implicit readouts of polarizing or divergent functional programming (Figure 3A). By sorting CD11b+ Ly6Chi Ly6G- cells from differentially treated, tumor-bearing mice, more extensive functional studies could be performed to understand their role. Using carboxyfluorescein succinimidyl ester (CFSE) labelling to monitor lymphocyte proliferation11, sorted myeloid cells derived from naïve and vaccinated mice were found to suppress T cell proliferation in vitro, while cells derived from vaccinated + MS-275-treated mice had reduced immunosuppressive function (Figure 3B). Adoptive transfer of these cells revealed that they instead possessed antitumor capability and promoted sustained regression of tumors during vaccination and prolonged mouse survival (Figure 3C).

Figure 1: Representative data showing the effectiveness of varying dissociation conditions. In C57BL/6 mice (n=3 per group), untreated intradermal B16F10-gp33 tumors were processed and dissociated under various enzymatic conditions before CD45.2 selection. Shown above are the flow cytometry gating strategies used to demonstrate cellular yield differences (FSC-A vs. SSC-A) illustrates tumor-infiltrating leukocyte yield implicitly by cell size/granularity discrimination. (CD11b vs. CD11c ) allows for the quantification of myeloid (CD11b+ or CD11c+) or non-myeloid (CD11b- CD11c-) cells. Error is defined by standard error of the mean. Abbreviations: CD = cluster of differentiation; SSC-A = side scatter-area; FSC-A = forward scatter area; FBS =fetal bovine serum. Please click here to view a larger version of this figure.
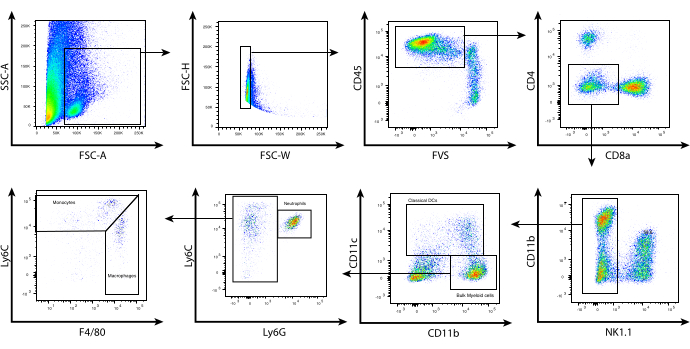
Figure 2: Representative flow cytometry analysis of tumor-infiltrating myeloid cells. Following tumor processing and CD45.2 selection, enriched cells were surface-stained as described in the protocol. Shown above is the gating strategy used to exclude the lymphocytes and identify the individual subsets of myeloid cells within the tumor microenvironment. Debris (SSC-A vs. FSC-A) and doublets (FSC-H vs. FSC-W) were excluded, and CD45.2+ live cells were determined using the fixable viability stain FVS510 (CD45.2 vs. FVS). CD4+, CD8+, NK1.1+ cells were gated out. CD11bhi/lo CD11c+ cells represent cDCs. CD11b+ CD11c- were then subgated based on Ly6C and Ly6G expression. Three populations were identified: (i) Ly6Cint Ly6G+ (neutrophils/G-MDSCs), (ii) Ly6Chi Ly6G- F4/80lo/int (monocytes/M-MDSCs), (iii) Ly6Clo/int Ly6G- F4/80hi (macrophages/TAMs). Abbreviations: CD = cluster of differentiation; SSC-A = side scatter-area; FSC-A = forward scatter area; FSC-H = forward scatter-height; FSC-W = forward scatter-width; FVS = fixable viability stain; cDCs = conventional dendritic cells; Ly = lymphocyte antigen; G-MDSCs = granulocytic myeloid-derived suppressor cells; M-MDSCs = monocytic myeloid-derived suppressor cells; TAMs= tumor-associated macrophages. Please click here to view a larger version of this figure.

Figure 3: Functional analyses of purified tumor-infiltrating myeloid cells. In C57BL/6 mice (n=3-5 per group), intradermal B16F10-gp33 tumors were either unvaccinated or administered adoptive T cell therapy in the presence or absence of the HDAC inhibitor, MS-275. Five days posttreatment, tumors were processed and positively enriched for CD11b+ cells. The cells were surface-stained to subgate on the desired tumor-infiltrating myeloid cell subset (CD11b+ Ly6Chi Ly6G-). (A) These cells were further stained intracellularly for markers that can delineate the polarity of functional activation, and the data are presented as the frequency of CD11b+ Ly6Chi Ly6G- cells that produce NOS2 or Arg1. Alternatively, the surface-stained CD11b+-enriched myeloid cells were sorted to obtain a pure Ly6Chi Ly6G- cell population. (B) These purified cells were peptide-pulsed and cocultured with CFSE-labeled, naïve TCR-transgenic T cells in varying ratios. CFSE dilution, as determined by flow cytometry, is shown as a representative histogram and quantified by cellular division index (1:1). (C) Separately purified CD11b+ Ly6Chi Ly6G- cells derived from vaccinated + MS-275-treated tumor-bearing mice were adoptively transferred into new tumor-bearing mice (n=5) in conjunction with vaccination and tumor regression, and survival curves were monitored. Error is defined by the standard error of the mean. *** p=0.0004, * p=0.0479, **** p<0.0001. This figure has been modified from Nguyen et al.8. Abbreviations: CD = cluster of differentiation; HDAC = histone deacetylase inhibitor; NOS2 = nitric oxide synthase 2; Arg1 = arginase 1 ; CFSE = carboxyfluorescein succinimidyl ester; TCR = T-cell receptor; Ly = lymphocyte antigen; NS= not significant. Please click here to view a larger version of this figure.
Discussion
Although tumor-infiltrating myeloid cells exist in varying activation and differentiation states within the tumor, several subsets have been identified including tumor-associated DCs (TADCs), tumor-associated neutrophils (TANs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs)12. Unfortunately, the overlapping expression of cell-surface markers used to identify these myeloid cell subsets makes it currently challenging to phenotypically differentiate tumor myeloid cells from other myeloid cells13. Similarly, therapy-induced phenotypic changes in tumor-infiltrating myeloid cells may not be easily observed with existing myeloid antibody staining panels. Taken together, the insufficiency of unique surface markers complicates the understanding of myeloid cell biology. To delineate natural, tumor-driven, and therapy-influenced myeloid cells, the evaluation of myeloid cells subsets must be done according to their function in addition to their phenotypic characteristics.
The methods described herein to harvest and dissociate tumor tissue allow for the isolation of myeloid cells with preserved viability and surface marker integrity. As tumor-infiltrating myeloid cells are sensitive to ex vivo manipulation7, the emphasis of the protocol is on less aggressive mechanical and enzymatic dissociation. However, depending on the tumor tissue type, different samples require variably aggressive treatment to generate a single-cell suspension. For more collagen-rich tumor models (i.e., CMS5 fibrosarcoma), a syringe plunger could be used in addition to scissors to more fully mechanically disaggregate tumor tissue prior to enzymatic treatment. Conversely, less collagen-rich tumor models (i.e., B16 melanoma) do not necessarily require enzymatic treatment. As demonstrated in these results, the type of collagenase preparation and the lot from which it was derived can significantly influence the variety and potency of proteases that cells are exposed to during enzymatic dissociation.
While non-myeloid leukocytes (i.e., lymphocytes) do not seem to be as sensitive, these data suggest that myeloid cells, in particular, may be very susceptible to excessive protease exposure (Figure 1), resulting in damage to membrane proteins and decreased viability. As a result, the use of collagenase type IV over collagenase type I is recommended for its increased collagenase-specific proteolytic activity and the incorporation of FBS into the digestion mix to neutralize residual tryptic activity. Furthermore, utilizing purified collagenase products, as opposed to crude preparations from commercial sources, can increase the reproducibility of cellular recovery. Once the digestion is complete, the addition of EDTA and incubation at 4 °C is mandatory to chelate the Ca2+ ions and reduce the temperature needed for collagenase activity14.
To characterize myeloid cell infiltrate within a heterogeneous TIL population, high-level gating strategies have been provided (Figure 2). The polychromatic flow cytometry panel should be designed in such a way that 1) dead and non-myeloid cells are excluded, 2) myeloid cell subsets can be identified, and 3) altered functionality can be implicitly observed across groups of samples (Figure 3). Depending on the number of myeloid cell subsets of interest and the depth of functional characterization, these criteria may not be fully accommodated within one staining panel. To free up channels for the panel, markers could be strategically pooled into a single dump channel. Although this method allows for high-dimensional analysis of tumor-infiltrating myeloid cells, it is still constrained by a relatively low parameter limit. More recent technologies using heavy metal reporter ions, such as mass cytometry, enable up to 40 independent parameters within a single panel, which will allow better study of the cellular and functional diversity of myeloid cells within the TME15.
However, the cost of instrumentation limits the ubiquity of its use. Flow cytometry is more accessible and produces reliable characterization data, although the mixing and matching of markers across multiple panels necessitates panel validation as an extremely important step prior to the experiment. Upon identification of tumor-infiltrating myeloid subsets of interest, several researchers15 describe techniques to sort these cells for functional assays and studies. Sorted cells can be reliably analyzed for their T cell-suppressive capacity in vitro as well as their antitumor capability in vivo after adoptively transferring them into mouse models of tumor relapse (Figure 3). Overall, the significance of the described methods is that, in comparison to other TIL isolation protocols, the conditions of tumor harvest, dissociation, cell enrichment, and cell sorting were tailored towards reproducibly acquiring a high yield of the myeloid cell compartment over all other leukocytes and without compromising their function.
Disclosures
No conflicts of interest declared.
Acknowledgements
This work was supported by the Ontario Institute for Cancer Research through funding provided by the Government of Ontario, as well as the Canadian Institutes of Health Research (FRN 123516 and FRN 152954), the Canadian Cancer Society (grant 705143), and the Terry Fox Research Institute (TFRI-1073).
Materials
| Name | Company | Catalog Number | Comments |
| Alexa Fluor 700 Mouse Anti-Mouse CD45.2 | BD Biosciences | 560693 | 1:100 |
| APC-Cy7 Mouse Anti-Mouse NK-1.1 | BD Biosciences | 560618 | 1:100 |
| Biotin Mouse Anti-Mouse CD45.2 | BD Biosciences | 553771 | |
| BV421 Hamster Anti-Mouse CD11c | BD Biosciences | 562782 | 1:100 |
| BV650 Rat Anti-Mouse F4/80 | BD Biosciences | 743282 | 1:100 |
| BV711 Rat Anti-Mouse CD8a | BD Biosciences | 563046 | 1:100 |
| Collagenase, Type IV, powder | Gibco | 17104019 | |
| DNase I | Roche | 10104159001 | |
| EasySep Mouse CD11b Positive Selection Kit II | Stemcell technologies | 18970 | |
| EasySep Mouse CD11c Positive Selection Kit II | Stemcell technologies | 18780 | |
| EasySep Release Mouse Biotin Positive Selection Kit | Stemcell technologies | 17655 | |
| FITC Rat Anti-Mouse Ly-6C | BD Biosciences | 553104 | 1:100 |
| Fixable Viability Stain 510 | BD Biosciences | 564406 | 1:1000 |
| Fixation/Permeabilization Solution Kit (BD Cytofix/Cytoperm) | BD Biosciences | 554714 | |
| PE Rat Anti-CD11b | BD Biosciences | 557397 | 1:100 |
| PE-Cy7 Rat Anti-Mouse CD4 | BD Biosciences | 552775 | 1:100 |
| PerCP-Cy5.5 Rat Anti-Mouse Ly-6G | BD Biosciences | 560602 | 1:100 |
| Perm/Wash (BD Perm/Wash) | BD Biosciences | 554723 | |
| Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block) | BD Biosciences | 553141 | |
| iNOS Monoclonal Antibody (CXNFT), APC | Thermo Fisher | 17-5920-82 | 1:100 |
| Human/Mouse Arginase 1/ARG1 Fluorescein-conjugated Antibody | R&D Systems | IC5868F | 1:100 |
References
- Paardekooper, L. M., Vos, W., vanden Bogaart, G. Oxygen in the tumor microenvironment: effects on dendritic cell function. Oncotarget. 10 (8), 883-896 (2019).
- Schouppe, E., De Baetselier, P., Van Ginderachter, J. A., Sarukhan, A. Instruction of myeloid cells by the tumor microenvironment: Open questions on the dynamics and plasticity of different tumor-associated myeloid cell populations. Oncoimmunology. 1 (7), 1135-1145 (2012).
- Jahchan, N. S., et al. Tuning the tumor myeloid microenvironment to fight cancer. Frontiers in Immunology. 10, 1611 (2019).
- Srivastava, M. K., et al. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 7 (7), 40677 (2012).
- Awad, R. M., De Vlaeminck, Y., Maebe, J., Goyvaerts, C., Breckpot, K. Turn back the TIMe: targeting tumor infiltrating myeloid cells to revert cancer progression. Frontiers in Immunology. 9, 1977 (2018).
- Strauss, L., et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Science Immunology. 5 (43), 1863 (2020).
- Cassetta, L., et al. Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice and non-human primates. Cancer Immunology, Immunotherapy. 68 (4), 687-697 (2019).
- Nguyen, A., et al. HDACi delivery reprograms tumor-infiltrating myeloid cells to eliminate antigen-loss variants. Cell Reports. 24 (3), 642-654 (2018).
- Newton, J. M., Hanoteau, A., Sikora, A. G. Enrichment and characterization of the tumor immune and non-immune microenvironments in established subcutaneous murine tumors. Journal of Visual Experiments: JoVE. (136), e57685 (2018).
- Engfeldt, P., Arner, P., Ostman, J. Nature of the inhibitory effect of collagenase on phosphodiesterase activity. Journal of Lipid Research. 26 (8), 977-981 (1985).
- Quah, B. J., Parish, C. R. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. Journal of Visual Experiments: JoVE. (44), e2259 (2010).
- Schupp, J., et al. Targeting myeloid cells in the tumor sustaining microenvironment. Cellular Immunology. 343, 103713 (2019).
- Gabrilovich, D. I., Ostrand-Rosenberg, S., Bronte, V. Coordinated regulation of myeloid cells by tumours. Nature Reviews Immunology. 12 (4), 253-268 (2012).
- Seglen, P. O. Preparation of isolated rat liver cells. Methods in Cell Biology. 13, 29-83 (1976).
- Roussel, M., et al. Mass cytometry deep phenotyping of human mononuclear phagocytes and myeloid-derived suppressor cells from human blood and bone marrow. Journal of Leukocyte Biology. 102 (2), 437-447 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved