Lewis Acid-Base Interaction in Ph3P-BH3
Przegląd
Source: Tamara M. Powers, Department of Chemistry, Texas A&M University
One of the goals of chemistry is to use models that account for trends and provide insights into the properties of reactants that contribute to reactivity. Substances have been classified as acids and bases since the time of the ancient Greeks, but the definition of acids and bases has been modified and expanded over the years.1
The ancient Greeks would characterize substances by taste, and defined acids as those that were sour-tasting, such as lemon juice and vinegar. The term "acid" is derived from the Latin term for "sour-tasting." Bases were characterized by their ability to counteract or neutralize acids. The first bases characterized were those of ashes from a fire, which were mixed with fats to make soap. In fact, the term "alkaline" is derived from the Arabic word for "roasting." Indeed, it has been known since ancient times that acids and bases can be combined to give a salt and water.
The first widely-used description of an acid is that of the Swedish chemist, Svante Arrhenius, who in 1894 defined acids as substances which dissociate in water to give hydronium ions, and bases as substances which dissociate in water to give hydroxide ions. This definition is thus limited to aqueous acids and necessitates that an acid contribute a proton.2 For example, in water, HCl is an acid, as it dissociates to give the hydronium ion (H3O)+ and the chloride ion. Boron trichloride would not be considered an acid, as in water it hydrolyzes to give B(OH)3 and 3 HCl; the product HCl though is an Arrhenius acid.
In 1923, Johannes Nicolaus Brønsted and Martin Lowry independently defined acids and bases on their ability to donate and accept hydrogen ions, or protons. Thus came the concept of acid-base conjugate pairs, and the expansion of the definition of acids and bases in solvents other than water. For example, ammonium is an acid, as it can donate a proton and generate ammonia. Ammonia can accept a proton, to give ammonium. Thus, ammonia is the conjugate base of ammonium. This acid-base reaction can occur in water, ammonia, or other solvents.
This video deals with the acid-base definition of the American chemist, Gilbert N. Lewis, who also defined acids and bases in 1923. Indeed, this is the same Lewis from Lewis-dot structures in General Chemistry. His approach focuses not on the ability of acids and bases to donate and accept protons, but rather on their ability to accept and donate electron pairs, respectively. This encompasses the Brønsted-Lowry definition, as H+ accepts an electron pair from a Brønsted base during protonation. However, it greatly expands the definition of an acid, now encompassing metal ions and main-group compounds. Here, we compare the 31P NMR of the Lewis acid-base adduct Ph3P-BH3 to free triphenylphosphine.
Zasady
Consider the bonding between triphenylphosphine and borane. We will first consider what both molecules look like before they form a Lewis adduct.
Recall Lewis dot structures, and valence shell electron-pair repulsion (VSEPR) theory, from general chemistry. The Lewis dot structure of triphenylphosphine is shown in Figure 1. There are three covalent bonds between the phosphorous atom and one of the carbon atoms in each of the three phenyl rings. Two electrons (a lone-pair) reside on the phosphorous atom to complete the octet. The phosphorous center is sp3 hybridized and has a tetrahedral electronic geometry, with the lone-pair of electrons residing in an sp3 orbital. Triphenylphosphine has a lone-pair that can be donated to another molecule and is therefore classified as a Lewis base.
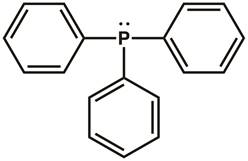
Figure 1. Lewis dot structure of triphenylphosphine.
The Lewis dot structure of borane is shown in Figure 2. There are three covalent bonds between the boron atom and the hydrogen atoms. In this case, the boron center only has six valence electrons and therefore does not follow the 8 e- rule. Borane is thus planar and sp2 hybridized, with the sp2 orbitals forming bonds to the hydrogen atoms and the lone p orbital being empty. Borane is thus a Lewis acid.

Figure 2. Lewis dot structure of borane.
Since the phosphorous in triphenylphosphine has a filled orbital and the boron of borane has an empty orbital, a Lewis acid-base adduct can form, with the triphenylphosphine donating its two electrons to boron. Upon adduct formation, the boron center becomes sp3 hybridized (Equation 1).
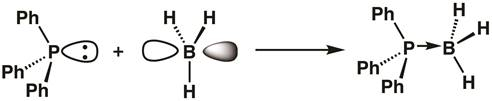 (1)
(1)
Equation 1 demonstrates the idea of Lewis acidity, with Lewis acids accepting lone-pairs of electrons, and Lewis bases donating pairs of electrons. Sometimes, Lewis acids are referred to as electrophiles, and Lewis bases as nucleophiles. Bonds between Lewis acids and bases are often called coordinate covalent or dative bonds, and sometimes are designated with arrows as opposed to lines.
Procedura
1. Setup of the Schlenk Line for the Synthesis of the Borane Triphenylphosphine Complex
NOTE: For a more detailed procedure, please review the "Schlenk Lines Transfer of Solvent" video in the Essentials of Organic Chemistry series). Schlenk line safety should be reviewed prior to conducting this experiment. Glassware should be inspected for star cracks before using. Care should be taken to ensure that O2 is not condensed in the Schlenk line trap if using liquid N2. At liquid N2 temperature, O2 condenses and is explosive in the presence of organic solvents. If it is suspected that O2 has been condensed or a blue liquid is observed in the cold trap, leave the trap cold under dynamic vacuum. Do NOT remove the liquid N2 trap or turn off the vacuum pump. Over time, the liquid O2 will evaporate into the pump; it will only be safe to remove the liquid N2 trap once all of the O2 has evaporated.
- Close the pressure release valve.
- Turn on the N2 gas and the vacuum pump.
- As the Schlenk line vacuum reaches its minimum pressure, prepare the cold trap with either liquid N2 or dry ice/acetone.
- Assemble the cold trap.
2. Synthesis of Borane Triphenylphosphine Complex3
- Add 5.3 g (20.3 mmol) of triphenylphosphine to a 250 mL Schlenk flask A and prepare the Schlenk flask for the cannula transfer of solvent.
- Add 20 mL of dry/degassed THF to the Schlenk flask A via cannula transfer. Stir the solution to dissolve the triphenylphosphine.
- Prepare a second Schlenk flask (B) containing 1.15 g (30.5 mmol) NaBH4 for cannula transfer.
- Cool both Schlenk flask A and B in an ice bath.
- Cannula transfer the contents of Schlenk flask A into Schlenk flask B.
- With positive N2 pressure, replace the rubber septum on Schlenk flask B with an addition funnel fitted with a rubber septum.
- To the addition funnel, add 8 mL of dry/degassed THF by cannula transfer.
- With positive N2 pressure, remove the septum from the top of the dropping funnel and add 2 mL of glacial acetic acid to the addition funnel.
- Keeping Schlenk flask B in an ice bath, add the THF/glacial acetic acid dropwise over 30 min. During the addition, frothing might occur. Make sure that the reaction is stirring vigorously to minimize this.
- After the addition, allow the reaction to warm to room temperature and stir for an additional hour.
- Remove the dropping funnel and slowly add 20 mL of water.
- Prepare a solution of 2 mL glacial acetic acid in 25 mL of water. Slowly add this mixture to the reaction.
- If crystals do not spontaneously form, cool the reaction in an ice bath to promote crystallization.
- Filter the product by suction through a fritted funnel. Wash the resulting solid with 20 mL of water 3 times.
- Allow the product to dry in the hood before preparing the sample for NMR analysis.
3. 31P NMR Analysis of Borane Triphenylphosphine Complex
- Prepare an NMR sample of triphenylphosphine and borane triphenylphosphine complex in CDCl3.
- Collect a 31P NMR of each sample (referenced to phosphoric acid) and observe how the phosphorous signal of triphenylphosphine shifts upon coordination to borane.
Wyniki
Borane triphenylphosphine complex:
31P NMR (chloroform-d, 500 MHz, δ, ppm): 20.7 (broad doublet)
Triphenylphosphine:
31P NMR (chloroform-d, 500 MHz, δ, ppm): -5.43
The 31P NMR signal of the borane triphenylphosphine complex is downfield relative to free triphenylphosphine. This is consistent with removal of electron density from the phosphorous center, which is deshielded upon adduct formation.
Wniosek i Podsumowanie
The borane triphenylphosphine complex is an example of a Lewis-adduct, whereby a Lewis base donates electrons to a Lewis acid. Though BH3 and PPh3 would not necessarily be considered an acid and base, respectively, using other acid-base theories, Lewis acid-base theory predicts correctly that the molecules form a stable adduct.
Small Molecule Activation:
While transition metal ions have historically been regarded as Lewis acids, the notion that they can serve as Lewis bases is being advanced. For example, Jonas Peters and co-workers at Caltech have shown that metal-borane complexes, which can donate electrons to the Lewis acid borane (a Z-type ligand), can give rise to novel reactivity. A nickel borane species was shown to reversibly add H2, heterolytically cleaving the H-H bond.4 The H2-added species is a catalyst for hydrogenations of olefins. The group also reported that iron-borane complexes can catalytically reduce nitrogen to ammonia.5 This was the first example of an iron-based homogeneous catalyst for this challenging yet critical reaction.
Frustrated Lewis Pairs:
Another current area of research is that of "Frustrated Lewis Pairs," or FLPs. These are Lewis acid-base "adducts" that due to steric reasons, cannot form a dative bond.6 Douglas Stephan and co-workers from the University of Toronto pondered what reactivity such adducts would have, particularly with the idea of using them for small molecule activation and catalysis. Thinking about transition metal complexes, which can both accept and donate electron density to and from substrates, they hypothesized donor/acceptor properties of what they termed "Frustrated Lewis Pairs" might have with regards to reactivity.
In 2006, Stephan and co-workers reported in Science that the zwitterionic (C6H2Me3)2PH(C6F4)BH(C6F5)2 reversibly loses H2 to give (C6H2Me3)2P(C6F4)B(C6F5)2.7 This was the first example of reversible H2 activation with main group elements, and other examples followed (Figure 3). This study paved the way for the development of FLP research. Since then, FLPs have been developed that are competent hydrogenation catalysts, and can activate a variety of small molecules including CO2. This is an active and exciting new area of research.
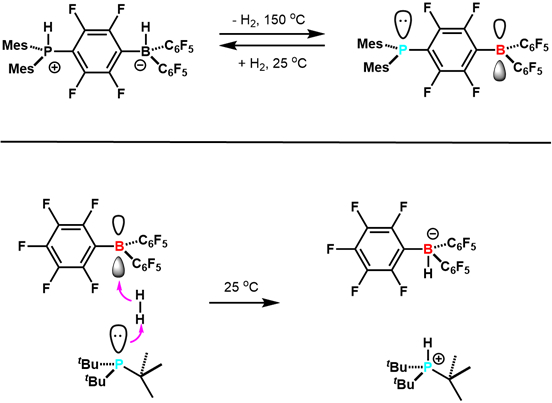
Figure 3. Early examples of reactivity of FLPs with H2. Adapted from reference 5.
Odniesienia
- Lesney, Today's Chemist at Work, 2003, 47-48.
- Miessler, P. J. Fischer and D. A. Tarr, Inorganic Chemistry, Pearson, 2014.
- McNulty, J.; Zhou, Y. Tetrahedron Letters, 2004, 45, 407-409.
- Harman and J. C. Peters, J. Am. Chem. Soc., 2012, 134, 5080-5082.
- Anderson, J. Rittle and J. C. Peters, Nature, 2013, 501, 84-87.
- Stephan, J. Am. Chem. Soc., 2015, 137, 10018-10032.
- Welch, R. R. S. Juan, J. D. Masuda and D. W. Stephan, Science, 2006, 314, 1124-1126.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Lewis Acid-Base Interaction in Ph3P-BH3
Inorganic Chemistry
38.7K Wyświetleń

Synthesis Of A Ti(III) Metallocene Using Schlenk Line Technique
Inorganic Chemistry
31.5K Wyświetleń

Glovebox and Impurity Sensors
Inorganic Chemistry
18.6K Wyświetleń

Purification of Ferrocene by Sublimation
Inorganic Chemistry
54.3K Wyświetleń

The Evans Method
Inorganic Chemistry
68.0K Wyświetleń

Single Crystal and Powder X-ray Diffraction
Inorganic Chemistry
103.9K Wyświetleń

Electron Paramagnetic Resonance (EPR) Spectroscopy
Inorganic Chemistry
25.3K Wyświetleń

Mössbauer Spectroscopy
Inorganic Chemistry
21.9K Wyświetleń

Structure Of Ferrocene
Inorganic Chemistry
79.1K Wyświetleń

Application of Group Theory to IR Spectroscopy
Inorganic Chemistry
44.9K Wyświetleń

Molecular Orbital (MO) Theory
Inorganic Chemistry
35.1K Wyświetleń

Quadruply Metal-Metal Bonded Paddlewheels
Inorganic Chemistry
15.3K Wyświetleń

Dye-sensitized Solar Cells
Inorganic Chemistry
15.7K Wyświetleń

Synthesis of an Oxygen-Carrying Cobalt(II) Complex
Inorganic Chemistry
51.5K Wyświetleń

Photochemical Initiation Of Radical Polymerization Reactions
Inorganic Chemistry
16.7K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone