Method Article
Advances in Human Induced Pluripotent Stem Cell-Derived Chimeric Antigen Receptor-Expressing Natural Killer Cells
W tym Artykule
Podsumowanie
Here, we present a method to differentiate and expand human iPSC-derived chimeric antigen receptor (CAR)-expressing natural killer cells with improved killing against various malignancies. This protocol demonstrates the differentiation and expansion of natural killer (NK) optimized iPSC-derived CAR-NK cells and the measurement of antitumor activity against various tumor cell lines.
Streszczenie
Natural killer (NK) cells are innate immune cells that play a crucial role in the body's defense against tumors and viral infections. The generation of human induced pluripotent stem cell (iPSC)-derived chimeric antigen receptor (CAR) expressing NK cells has emerged as a promising avenue for "off the shelf" cancer immunotherapy. Here, we utilized an NK cell-optimized CAR construct that includes the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain, and the CD3ζ signaling domain, which has been demonstrated to stimulate robust antigen-specific NK cell-mediated antitumor activity. The use of iPSCs for CAR NK cell generation offers several advantages, including homogenous CAR expression, scalability, reproducibility, and the potential for clinical application. This detailed step-by-step protocol from cell engineering to differentiation enables the generation of NK cell-optimized iPSC-derived CAR-expressing NK cells, providing a standardized and targeted cancer immunotherapy with improved antitumor activity and highlighting their potential as a promising treatment option for various malignancies.
Wprowadzenie
NK cells, a type of lymphocyte within the innate immune system, are pivotal in the early defense against tumors and virally infected cells1,2,3. Unlike T cells, NK cells do not require antigen presentation via major histocompatibility complex (MHC) molecules. Instead, NK cells have a repertoire of activating and inhibitory receptors that regulate their activity3. NK cell-mediated cytotoxic activity utilizes various mechanisms, including the release of perforin and granzymes, engagement of death receptors, and the production of pro-inflammatory cytokines such as IFN-γ and TNF-α. This unique mode of action positions NK cells as an attractive candidate for cancer immunotherapy, particularly in the treatment of solid cancers where immune evasion is a significant hurdle1,2,3,4.
Hepatocellular carcinoma (HCC) is one of the most common and deadly forms of liver cancer worldwide5. Traditional therapeutic approaches, including surgery, chemotherapy, and radiotherapy, typically provide limited clinical benefit and result in high recurrence rates6,7. Recent advances in immunotherapy and targeted therapy have significantly impacted the treatment of HCC8,9. Immune checkpoint inhibitors, like nivolumab and pembrolizumab, have shown promising results by enhancing the immune cell response against cancer cells10,11. These therapies have led to improved survival rates and better quality of life for some patients. However, they can also cause immune-related adverse effects, which may limit their use in certain patients12. Targeted therapies, such as sorafenib and lenvatinib, specifically inhibit pathways that promote cancer cell growth and angiogenesis13. These treatments have known efficacy to control disease progression and prolong survival8,14. Nonetheless, resistance to targeted therapies typically develops, and side effects of treatment are common15,16. Among the promising avenues in cancer immunotherapeutic strategies, the advent of chimeric antigen receptor (CAR) CAR T cells has revolutionized cancer treatment, especially in the treatment of hematologic malignancies such as lymphoma and multiple myeloma17,18,19.
CAR-expressing NK cells, combining the innate cytotoxic activity of NK cells with the precision targeting of CAR technology, represent an innovative and potentially transformative approach for solid tumors such as HCC20,21,22,23,24. CAR-engineered cells can specifically recognize and kill tumor cells expressing the target antigen while sparing normal tissues, thereby reducing the risk of off-target effects associated with conventional therapies25,26,27. CAR-NK cells produced from NK cells isolated from peripheral blood or cord blood are typically produced by transducing NK cells with CAR constructs that consist of an extracellular antigen-recognition domain, a transmembrane domain, and intracellular signaling domains necessary for activation and proliferation28,29,30.
The challenge of engineering a stable and homogenous population of functionally engineered NK cells for clinical treatment can be addressed by employing induced pluripotent stem cells (iPSCs)31,32. Engineering of human iPSCs with NK cell-optimized CARs provides an improved NK cell activation and proliferation signal and provides an inexhaustible and homogenous population of CAR-expressing NK cells as a standardized, "off-the-shelf therapy"20,33. In this protocol, genetic modification of iPSCs to generate CAR-expressing iPSC-derived NK cells involves integrating an NK cell optimized CAR construct that includes NK cell-derived transmembrane and signaling domains (NKG2D-2B4-CD3ζ) and an anti-glypican-3 (GPC3) scFv as described in our previous studies23. The genetically engineered iPSCs are then differentiated and expanded using an NK cell differentiation protocol developed previously34. These engineered CAR-expressing iPSC-derived NK cells have the ability to recognize and eliminate HCC and other tumor cells expressing specific tumor-associated antigens, such as Glypican 3 (GPC3), which is overexpressed in HCC and other malignancies35,36,37,38.
The application of iPSC-derived CAR-NK cells for the treatment of diverse cancers holds significant promise30,39,40; many of these iPSC-derived CAR-NK cells are currently in clinical trials41,42,43. To facilitate advancement in this area, this protocol enables efficient production of engineered iPSC-derived CAR-NK cells, from cell engineering to differentiation into mature NK cells and in vitro expansion.
Protokół
1. Feeder-free culture method of human iPSCs
NOTE: Thaw the frozen undifferentiated human iPSCs and culture them with the use of mTeSR 1-plus on Matrigel (henceforth referred to as basement membrane matrix [BMM]) pre-coated plates, as previously described23,34. It is very important to make sure the iPSCs are not differentiated before and after engineering. Freshly thawed iPSCs should be cultured for about 2-3 passages to exhibit pluripotency morphology consistent with the human pluripotent stem cells. The following instructions are used for passaging cells from one well of a 6-well plate.
- Before passaging iPSCs, coat the new 6 well plate with 1x BMM (1 mL/well) and incubate in a 37 °C incubator for 2-4 h before use.
- Prepare of 1x solubilized BMM.
- Thaw a 5 mL stock vial of BMM at 4 °C overnight.
- Quickly aliquot BMM using pre-chilled tips into pre-chilled microcentrifuge tubes.
- Store at -80 °C for up to 6 months.
- To prepare 1x BMM working solution, resuspend one BMM aliquot at 1:100 ratio into a cold DMEM/F12 medium. The 1x BMM working solution can be stored at -4 °C for 2 weeks.
- Coat the plate with sufficient volume to cover the surface (e.g., use 1 mL per well of 6-well plate).
- Incubate in 37 °C incubator for 2-4 h before use.
- Thaw a vial of frozen iPSCs (typically 1 × 106 cells/vial) in a water bath at 37 °C.
- Gradually add the thawed iPSCs into a 15 mL conical tube containing 5 mL of prewarmed mTeSR Plus medium, allowing the cells to acclimate by adding them drop by drop to minimize cellular shock.
- Centrifuge the cells at 300 × g for 5 min.
- Aspirate the supernatant and wash the cells once with 5 mL of PBS.
- Resuspend the cells in mTeSR Plus medium and plate them at a density of approximately 200,000 cells/3 mL/well in the BMM pre-coated 6-well plate.
- Passage iPSCs.
- Once the iPSCs reach 70-80% confluency, aspirate the media and perform single-cell passaging with TrypLE for 5 min at 37 °C (1 mL/well in 6 well plate).
- After 5 min, add 3 mL of 1x PBS to stop the dissociation reaction and wash the cells once with 3 mL of PBS.
- Centrifuge the cells at 300 × g for 5 min. After washing, resuspend the single cell iPSCs in mTeSR Plus medium, then plate onto BMM pre-coated 6 well plate.
NOTE: The total media volume required is 3 mL/well in a 6 well plate. The media needs to be changed every 2-3 days, and step 1.8 must be repeated when the cells become 70-80% confluent for at least 1-2 passages before engineering the iPSCs.
- Regularly check the pluripotency of the iPSCs. Stain the iPSCs with Allophycocyanin (APC) conjugated TRA-1-81 and PE-Cy7 conjugated stage-specific embryonic antigen-4 (SSEA-4) antibody for 30 min on ice, then analyze them using flow cytometry as described previously23,41.
NOTE: The iPSCs used in this experiment are adapted to TrypLE. iPSCs that have not previously been passaged with TrypLE typically require 8-10 passages to become fully adapted to TrypLE, which ensures the production of high-quality spin embryoid bodies (EBs).
2. Engineering of human iPSCs to express anti-GPC3 CAR using piggyBac vector
- Coat a new 6-well plate with BMM and incubate at 37 °C for at least 1 h (it can be longer as long as the liquid does not dry).
- Pre-warm mTeSR Plus with 10 µM Rho-associated, coiled-coil containing protein kinase (ROCK) Inhibitor (Y-27632) (from 1 mM stock prepared in sterile distilled water) at 37 °C.
- Turn on the Nucleofection system. Select the X unit, then select the locations of the wells to be transfected. Choose the P3 solution and manually input the code (CA-137).
- Harvest iPSCs at 70-80% confluency with 1 mL of TrypLE and incubate at 37 °C for 5 min. Check under the microscope to confirm the dissociation.
- Pipette 4-6 times to obtain a single-cell suspension, then neutralize with 5 mL of PBS and pellet at 300 × g for 5 min.
- Gently wash the cells once more to remove all traces of TrypLE.
- Resuspend in 1 mL of mTeSR Plus with Rock-Inhibitor and count the cells.
- To create anti-GPC3 CAR iPSCs, use 1 × 106 cells per transfection in a microcentrifuge tube and spin down at 300 × g for 5 min.
- While spinning, combine the CAR-expressing transposon DNA with super PiggyBac transposase expression vector in a 1.5 mL microcentrifuge tube at a ratio of 3:1 (e.g., 3 µg of plasmid containing the transposon to 1 µg of transposase plasmid).
- Include a green fluorescent protein (GFP) positive control (Table of Materials) using 0.5 µL of 1 mg/mL solution, and a non-transfected negative control to confirm the GFP expression.
- Resuspend the cell pellet carefully in 20 µL of room temperature (RT) P3 Nucleofector solution with the supplement provided in the nucleofection kit. Then, add this mixture to a 1.5 mL microcentrifuge tube containing plasmid DNA, resulting in a final volume of approximately 22-25 µL.
NOTE: Resuspend all the iPSCs to be transfected in P3 Nucleofector solution before adding GPC3 CAR plasmids or GFP control plasmid to the cells. - Transfer the cell/DNA suspension to a 20 µL 16-well strips. Ensure the sample covers the bottom of the cuvette without any bubbles.
- Gently tap 16-well strips on the bench top to remove any air bubbles and settle cells in the bottom.
- Place the 16-well strips containing iPSCs and CAR DNA mix into the Nucleofection system, select program CA-137, P3 solution, and then start the program. This program will end in 1 s and show a green tick.
- Add 80 µL of prewarmed mTeSR Plus with ROCK-Inhibitor medium to the well and carefully transfer the cells to the 6-well plate coated with BMM (final volume 1 mL per well).
- Briefly check the cells under the fluorescence microscope after 4 h for any GFP expression to confirm the success rate of transfection.
- Screen iPSCs for GFP expression to assess transfection efficiency 24 h post-transfection using flow cytometry23,41 to confirm the GFP expression.
- Incubate for 4 days at 37 °C, changing the media as required before proceeding to drug selection.
NOTE: Each nucleofection requires 0.2-2 x 106 iPSCs per reaction. Depending on the number of reactions, 24-well plates or 6-well plates can be used.
3. Clonal selection and pluripotency confirmation of GPC3-CAR iPSCs
- Perform a series of zeocin selections (1:10,000) on the transfected iPSCs to clonally select GPC3-CAR expressing iPSCs.
- To confirm the transfection efficiency, measure the GFP expression using a fluorescent microscope and flow cytometry23,41.
NOTE: The CAR construct is incorporated with a FLAG tag44,45 for the purpose of evaluating CAR expression both during the iPSC stage and following NK cell differentiation. - Maintain the transfected CAR iPSCs under drug selection until they achieve optimal CAR gene and GFP expression.
- To ensure homogeneous GFP and CAR expression, extend the duration of drug selection for 2-3 weeks.
- Perform cell sorting based on GFP or CAR expression using flow cytometry to obtain a homogeneous population of clonally selected iPSCs23.
- The CAR construct used here contains a zeocin drug selection marker. Select the GPC3 CAR gene transfected cells by adding zeocin (50 µg/mL of zeocin) to the culture medium.
- After zeocin selection, dilute the GPC3 CAR gene transfected iPSCs and plate them into a 96-well plate to achieve approximately one cell per well. This can be done through serial dilution or using a cell sorter.
NOTE: Use fluorescence-activated cell sorting (FACS) to isolate single transfected cells based on the expression of a fluorescent marker linked to the CAR construct. - Allow the single cells to expand into colonies in the 96-well plate.
- Feed the cells with fresh culture medium every 3-4 days once, and monitor the wells for colony growth.
- Screen the colonies for CAR expression. This can be done by polymerase chain reaction (PCR), quantitative polymerase chain reaction (qPCR), or Western blotting to verify the presence of the CAR construct23,47.
- Alternatively, use flow cytometry23,41 to detect the surface expression of the CAR protein.
NOTE: In this study, the CAR expression was quantified by FLAG tag expression using flow cytometry. - Additionally, perform karyotyping to identify any chromosomal abnormalities.
NOTE: This can be done by third-party organizations such as Wicell (wicell.org) or Thermo Fisher Scientific (thermofisher.com). - Once the CAR-positive clones are identified without any abnormalities, expand these clones in larger culture vessels such 6 well plate and T75 flask.
- Cryopreserve the clonally expanded CAR iPSCs using a cryopreservation medium in multiple vials for future use.
NOTE: The following points are recommended in this step: (i) Maintain optimal culture conditions for iPSCs to prevent differentiation. (ii) Allow 1-4 weeks to achieve a homogeneous population of CAR and GFP expression in iPSCs after drug selection. (iii) Continue to monitor the cells for stability of CAR expression and pluripotency markers. (iv) Use appropriate controls during the screening and verification steps. (v) After clonal selection and expansion, verify pluripotency by assessing TRA-1-81 and SSEA-4 expression on the CAR-expressing iPSCs.
4. Generation of hematopoietic progenitor cells from engineered CAR iPSCs by spin embryoid body (EB) Formation
NOTE: A spin EB or hematopoietic organoid protocol is used to produce hematopoietic progenitors23,34. The cells within the EBs differentiate into stromal cells to support lymphocyte development38, thereby eliminating the need for xeno-derived stromal cells like OP923,34,48. Below are the instructions for collecting cells from a single well of a 6-well plate for EB formation.
- Passage ~200,000 cells/well of 6-well BMM-pre-coated plate 2 days before spin EB formation. Ensure it reaches 70-80% confluence on the day of spin EBs setup. Generally, 1 well of a 6-well plate is sufficient for two plates of spin EB.
- Remove the culture medium and detach the cells by incubating with 1 mL of prewarmed TrypLE Select at 37 °C 5 min.
- Carefully dissociate cell aggregates to a single cell suspension using a 1 mL micropipette and transfer the cells to a 15 mL conical tube. Add 3 mL of 1x PBS to stop the dissociation reaction, and wash the cells once with 3 mL of PBS.
- Resuspend the cells in mTesR Plus medium and filter through a 70 µM cell strainer to remove aggregates.
- Spin down the cells to remove supernatant, and wash cells once again with 5 mL PBS. Then resuspend the cells in 1 mL of STEMdiff APEL medium (EB formation medium).
- Count and dilute the cells to appropriate density using the EB formation medium containing the following cytokines (SCF: 40 ng/mL, BMP4: 20 ng/mL, VEGF: 20 ng/mL) plus 10 µM ROCK inhibitor.
- Seed 3000-10000 cells/well in 100 µL of media (100,000 cells/mL) in the 96-well plates.
NOTE: The number of cells used in EB formation may vary depending on the iPSC line. It is recommended to test between 3,000 and 10,000 cells per well in 96-well plates. - Using a multichannel pipette, load the cell suspension into 96-well plates at 100 µL/well.
NOTE: For better embryoid bodies (EBs) formation, it is recommended to use ultra-low attachment round-bottom 96-well plates. - Centrifuge the 96-well plate at 300 × g for 5 min, then incubate the plates at 37 °C with 5% CO2 for 6 days.
NOTE: Preliminary studies have found 6 days are enough to give rise to >30% CD34+ cells for the CAR iPSC lines in this study. - Check EB quality by flow cytometry23,41.
- Prepare 4 mL of prewarmed 0.25% trypsin with 0.4% chicken serum (16 μL for 4 mL trypsin) for dissociating EBs.
- Transfer 10-30 EBs to a 15 mL conical tube.
- Add the trypsin/chicken serum solution to the EBs and incubate in a water bath for 10 min, vortexing every 30 s to ensure complete dissociation of EBs.
- Pipette the trypsinized EBs. Mix up and down several times to thoroughly dissociate the EBS.
- Add 5 mL of 1x PBS to stop the trypsinization process.
- Filter the cell solution through a 70 μm cell strainer in a new conical tube.
- Centrifuge the cells at 300 x g for 5 min.
- Aspirate the supernatant and resuspend in 3-5 mL of fresh media.
- Count the cells, stain with typical EB markers such as CD34, CD31, CD43, and CD45, and analyze using flow cytometry23,41 (Figure 3).
- Add the recommended volume of antibodies according to manufacturer instructions to each tube containing dissociated single cells of EB in 100 µL of flow buffer and incubate on ice for 30 min39,41.
- After incubation, wash the cells twice with flow buffer, add SYTOX blue live/dead stain, and analyze using flow cytometry.
NOTE: Typically, >30% CD34+ cells are obtained from the total population of dissociated Spin embryoid bodies on day 6 using the iPSCs used in this study, although this percentage can vary depending on the iPSC source and culture conditions.
5. Differentiation of GPC3 CAR iPSC derived NK cells from Spin EBs
NOTE: The GPC3 CAR-expressing Spin EBs can be transferred into either 24-well plates or 6-well plates coated with 2% gelatin or without coating. For medium changes, 6-well plates are more suitable, while 2% gelatin coating enhances EBs attachment.
- Prepare gelatin-coated 6-well plates by following the steps below.
- Prepare a 2% (w/v) gelatin solution by dissolving gelatin in tissue culture-grade water.
- Sterilize the solution by autoclaving at 121 °C, 15 psi for 30 min.
- Coat the surface of each well with 5-10 µL of gelatin solution per cm2 (~1 mL per well of a 6-well plate).
- Incubate the plates at 37 °C for 2-4 h before use. After incubation, aspirate any remaining gelatin solution before proceeding with EBs transfer.
- Prepare NK cell differentiation medium: Add 56.6% DMEM+ GlutaMAX-I, 28.3% F12+ GlutaMAX-I, 15% heat-inactivated human AB serum, 1% P/S, 2 mM L-glutamine, 1 µM β-mercaptoethanol, 5 ng/mL sodium selenite, 50 µM ethanolamine, and 20 mg/L ascorbic acid. Store at 4 °C in the dark.
- Add 3 mL of NK differentiation medium containing the following cytokines to each well of the gelatin-coated 6-well plates (IL-3: 5 ng/mL, SCF: 20 ng/mL, IL-7: 20 ng/mL, and IL-15: 10 ng/mL).
- On day 6, transfer Spin EBs directly into the prepared gelatin-coated 6-well plates containing NK differentiation medium by following the steps below.
- Carefully transfer EBs from 96-well plates to a 10 cm dish, using a 10 mL serological pipette to remove most of the medium.
- Add 3 mL of NK differentiation medium containing the above cytokines to each well of the gelatin-coated 6-well plate.
- Distribute 16-20 EBs into each well of the 6-well plate using a 1 mL micropipette.
NOTE: On average, the EBs from a single 96-well plate can be used to seed one 6-well plate for the NK differentiation plate.
- Perform medium changes every 5-7 days. After the first week of NK cell differentiation, exclude IL-3 from the media.
NOTE: After 14 days, the medium needs to be changed every 3-4 days. - Continue medium changes for 3-4 weeks. Evaluate the expression of NK cell markers (CD45+CD56+) by flow cytometry24,42 on suspension cells.
- When >80% of suspension cells express CD45+CD56+, harvest the cells by passing them through a 70 µm filter to remove any clumps.
- Co-culture harvested NK cells with 100 gray irradiated aAPCs34,48 and culture in NK expansion medium containing IL-2 (100ng/mL) and IL-15 (10 ng/mL).
6. Expansion of anti-GPC3 CAR iPSC derived NK cells
NOTE: Typically, a yield of 2-20 × 106 NK cells can be obtained from a single 6-well plate, even before expansion. To facilitate further expansion of NK cells for downstream applications, artificial antigen-presenting cells (aAPCs) are employed to generate >1 × 109 NK cells.
- Utilize engineered K562 cells expressing 41BBL and membrane-bound IL-21 (41BBL-mbIL-21)48 as aAPCs to induce NK cell expansion.
- Prior to co-culturing aAPCs with NK cells, irradiate aAPCs with 100 gray and preserve them as frozen stocks.
- Harvest the suspension cells from the 6-well plate and culture the NK cells in NK expansion medium supplemented with 50 U/mL IL-2 and IL-15 (10 ng/mL; to be added freshly) at a density of 5 × 105 cells/mL.
- Add thawed irradiated aAPCs to NK cells at a 1:1 ratio.
- Replenish the culture medium every 3-4 days, adding fresh IL-2 and IL-15 at a concentration of 50 U/mL and 10 ng/mL, respectively.
- Ensure that CAR iPSC-derived NK cells maintain the capacity for expansion over a period of >3 months, maintaining both cell viability and cytolytic activity.
7. Phenotypic and functional characterization of GPC3 CAR iPSC derived NK cells
NOTE: The characterization of GPC3 CAR iPSC-derived NK cells involves a comprehensive assessment of their phenotypic profile and functional activity.
- Evaluate the NK activation receptors, including CD45, CD56, CD94, CD16, NKp30, NKp44, NKp46, NKG2D, DNAM, TRAIL, and FasL, expression on the anti-GPC3 CAR iPSC derived NK cells through flow cytometry24,42 to determine the CAR NK cell activity and maturation status (Figure 4).
- Assess the in vitro functional activity of GPC3 CAR iPSC derived NK cells using flow cytometry-based Caspase-3/7 assays, CD107a granule release assays, IFN-γ and TNF-α cytokine release assays against various tumor targets.
- For the Caspase3/7 assay, follow the steps below.
- Count the target cells and pre-stain them with cell tracing fluorescent dye at a final concentration of 5 µM in PBS for 15 min at 37 °C.
- Wash stained target cells in a complete culture medium before co-culture with CAR iPSC-derived NK cells at various effector to target (E:T) ratios at 37 °C.
- After 3 h and 30 min of co-culture, add Caspase-3/7 green detection reagent for another 30 min of incubation, resulting in a total incubation time of 4 h.
- Add 1 µL of dead cell staining solution during the final 5 min of staining, and mix gently.
- Analyze the stained cells using flow cytometry23,41(Figure 5).
- Implement this protocol to ensure a comprehensive understanding of the phenotypic characteristics and functional activity of GPC3 CAR iPSC-derived NK cells, enabling robust assessment of their therapeutic potential.
NOTE: It typically takes 4-5 weeks to obtain >90% CD45+CD56+ cells from spin EB based GPC3 CAR iPSC derived NK cell differentiation and NK expansion conditions using GPC3 CAR iPSC lines. Some iPSC cell lines might only require 3 weeks, and some lines may take longer (about 4-5 weeks).
8. Troubleshooting items and solutions
NOTE: Troubleshooting the differentiation, expansion, and functional testing of iPSC-derived GPC3 CAR NK cells using the spin embryoid body (EB) method can involve several steps. Below are some common issues one may encounter, along with suggested solutions:
- Symptoms of inconsistent EB formation
- Check if the EBs are either too small or too large, with irregular shapes.
- Solution for inconsistent EB formation
- Adjust the centrifuge speed and duration used to form EBs. A speed of around 200-300 × g for 5-10 min is typical.
- Ensure that the appropriate U-bottom plates or ultra low-attachment culture dishes are used to facilitate EB formation.
- Confirm that the iPSCs are homogeneous and free of differentiated cells that might affect EB formation.
- Symptoms of poor differentiation of iPSCs to NK cells
- Check for low expression of NK cell markers (e.g., CD56, NKp46).
- Check for failure to form EBs or poorly formed EBs.
- Solution for poor differentiation of iPSCs to NK cells
- Ensure that the concentrations of essential growth factors (e.g., VEGF, BMP4, Y-27632, FLT3, IL-15, IL-7, SCF, and IL-3) are optimal for EB formation and NK cell differentiation. Adjust the concentrations based on previous studies23,34,41.
- Ensure that the initial seeding density of iPSCs is appropriate. Typically, a density of 8000-10,000 cells/100 µL is recommended for spin EB formation.
- Verify that the differentiation medium is correctly formulated, including necessary supplements (e.g., serum or serum-free alternatives, other cytokines).
- Allow sufficient time for EB formation, a minimum 6 days to up to 12 days before switching to NK cell differentiation medium.
- Symptoms of poor expansion of NK cells
- Check for limited cell yield during expansion.
- Check for reduced viability of expanded NK cells.
- Solutions for poor expansion of NK cells
- Increase the concentration of IL-15 and IL-2 during the expansion phase. This can significantly enhance NK cell proliferation.
- Consider using feeder cells such as irradiated aAPCs to improve NK cell survival and expansion.
- Monitor cell density regularly and passage cells before they reach overcrowding to maintain optimal growth conditions.
- Symptoms of suboptimal functional testing results
- Check for reduced cytotoxicity against target cells.
- Check for low cytokine secretion levels (e.g., IFN-γ, TNF-α).
- Solutions for suboptimal functional testing results
- Optimize the target-to-effector ratio in cytotoxicity assays. Start with a 10:1 ratio and adjust based on results.
- Ensure that NK cells are adequately activated before functional assays. This may include pre-stimulation with cytokines or co-culturing with irradiated aAPCs.
- Verify the sensitivity of the target cell line to NK cell-mediated lysis. Use known sensitive targets as controls.
- Extend the duration of the functional assays to allow sufficient time for NK cell activity to manifest.
- Symptoms of variable CAR expression levels
- Check for inconsistent CAR expression among NK cells.
- Solutions for variable CAR expression levels
- Verify the efficiency of the CAR transfection method (e.g., viral transduction, electroporation). Adjust the MOI (multiplicity of infection) or electroporation conditions if needed.
- Implement appropriate selection markers to enrich CAR+ NK cells. Use antibiotic selection or fluorescent marker sorting as necessary.
Wyniki
Schematic diagram of anti-GPC3-CAR iPSC derived NK cells differentiation and expansion
The schematic diagram illustrates the in vitro differentiation of anti-GPC3-CAR engineered NK cells derived from human induced pluripotent stem cells (iPSCs) and a schematic representation of piggyBac vector carrying GPC3 CAR construct. Initially, unmodified iPSCs are transfected with a piggyBac vector encoding the GPC3 CAR (chimeric antigen receptor). Following transfection, these GPC3 CAR-expressing iPSCs are clonally expanded and then differentiated into functional CAR iPSC-derived NK cells. These engineered NK cells are subsequently subjected to in vitro and in vivo functional assays to evaluate their antitumor activity against various tumor cell lines (Figure 1).
Microscopic analysis of the NK differentiation process of WT and anti-GPC3-CAR engineered iPSCs at various stages
The GPC3 CAR-engineered iPSCs are differentiated into anti-GPC3-CAR-engineered iPSC NK cells. The formation of spin embryoid bodies (EBs) from GPC3 CAR engineered iPSCs, to the differentiation of GPC3-CAR iPSC NK cells were presented as a series of microscopic images documenting the differentiation stages of anti-GPC3-CAR engineered iPSCs into functional GPC3 CAR iPSC NK cells. The undifferentiated wild-type (WT) and GPC3 CAR iPSCs were cultured in mTeSR Plus medium (Figure 2A). These WT and anti-GPC3-CAR iPSCs are then dissociated into single cells and cultured in STEMdiff APEL medium containing SCF (40 ng/mL), BMP4 (20 ng/mL), VEGF (20 ng/mL) and 10 µM ROCK inhibitor for 6 days in an ultra-low attachment U-bottom 96 well plate. The formation of spin EBs was captured on day 6 from both WT and GPC3 CAR iPSCs (Figure 2B). These spin EBs are further transferred to 6 well plate containing NK differentiation medium with the following cytokines IL-3 (5 ng/mL), SCF (20 ng/mL), IL-7 (20 ng/mL), and IL-15 (10 ng/mL). The differentiation process of WT and GPC3 CAR iPSC-derived NK cells at various time points from day 3 to day 28, were captured using a microscope at 100x magnification (Figure 2C).
Phenotype of WT and anti-GPC3 CAR iPSC derived EBs (day 6) and WT and GPC3 CAR iPSC derived NK cells (day 35)
The WT and GPC3 CAR iPSC-derived cells are phenotypically characterized at different stages of NK differentiation process. Panel A shows the expression of typical hematopoietic antigens CD34, CD31, CD43, and CD45 from the spin EBs of WT and GPC3 CAR iPSCs collected on day 6 were measured by flow cytometry (Figure 3A). Panel b presents the phenotype of WT and GPC3 CAR iPSC-derived NK cells harvested from the NK differentiation medium on day 35, highlighting the NK-specific markers (CD45, CD56, CD16, and NKG2D) associated with mature NK cells were measured by flow cytometry (Figure 3B).
Phenotype of expanded WT and anti-GPC3-CAR iPSC derived NK cells
After the Differentiation of WT and anti-GPC3 CAR iPSC-derived NK cells, the differentiated NK cells are harvested and expanded using irradiated aAPCs in a Gibco NK Xpander medium containing IL-2 (100 U/mL) and IL-15 (10 ng/mL). The expanded WT and anti-GPC3 CAR iPSC NK cells activation receptors such as CD94, CD16, NKp30, NKp44, NKp46, NKG2D, CD226, FasL, and TRAIL were measured by using flow cytometry (Figure 4A). After confirming the NK activation phenotype on the expanded WT and anti-GPC3 CAR iPSC NK cells, we evaluated the surface expression of GPC3 antigen on various tumor cell lines, including HepG2, SNU-449, SKOV3, and CAL27, via flow cytometry (Figure 4B). We have also confirmed the CAR expression on expanded anti- GPC3 CAR iPSC-derived NK cells by flow cytometric analysis.
Functional activity of WT and anti-GPC3 CAR iPSC derived NK cells against HCC and other tumor cell lines
After confirming the phenotype of WT and anti-GPC3 CAR iPSC-derived NK cells, we evaluated the functional antitumor activity against various tumor cell lines. Caspase3/7-based killing assays were conducted to assess the cytotoxic activity of WT and anti-GPC3 CAR iPSC-derived NK cells against HepG2, SNU-449, CAL27, and SKOV3 cell lines at various effector-to-target ratios. The results indicate the enhanced antitumor efficacy of anti-GPC3 CAR iPSC-derived NK cells compared to WT iPSC NK cells, showcasing their potential in targeting GPC3-expressing tumor cells via CAR specificity and other NK activation mechanisms (Figure 5A-D).

Figure 1: Schematic diagram of anti-GPC3-CAR iPSC derived NK differentiation, expansion and clinical application. (A) Overview of the process for generating anti-GPC3 CAR iPSC-derived NK cells for preclinical and clinical use. Unmodified iPSCs are transfected with a piggyBac vector carrying anti-GPC3-CAR gene. After successful transfection, anti-GPC3-CAR-expressing iPSCs are clonally expanded and differentiated into functional NK cells. These anti-GPC3 CAR iPSC-derived NK cells are subjected to both in vitro and in vivo functional assays to assess their cytotoxic activity against various GPC3-expressing tumor cell lines39. Upon validation of their efficacy and safety, these CAR iPSC-derived NK cells are further developed as a potential "off-the-shelf" therapy for clinical applications in cancer treatment. (B) Schematic representation of the piggyBac vector containing the anti-GPC3 CAR construct used for transfecting iPSCs. (C) Typical timeline of iPSC-derived NK cell differentiation, expansion, harvesting, and performing functional assays. Please click here to view a larger version of this figure.
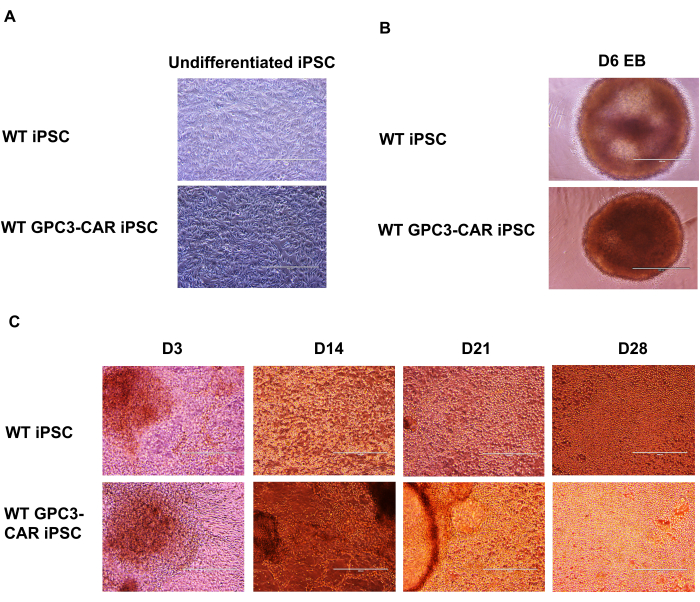
Figure 2: Microscopic images of CAR iPSC NK differentiation from anti-GPC3-CAR engineered iPSCs at different stages. (A) Undifferentiated WT and GPC3 CAR iPSC cultured in mTeSR plus medium. (B) WT and GPC3 CAR iPSC spin embryoid body on day 6. (C) WT and GPC3 CAR iPSC derived NK cell differentiation at different days from D3 to D28. Magnification of 100x for a to c. Please click here to view a larger version of this figure.
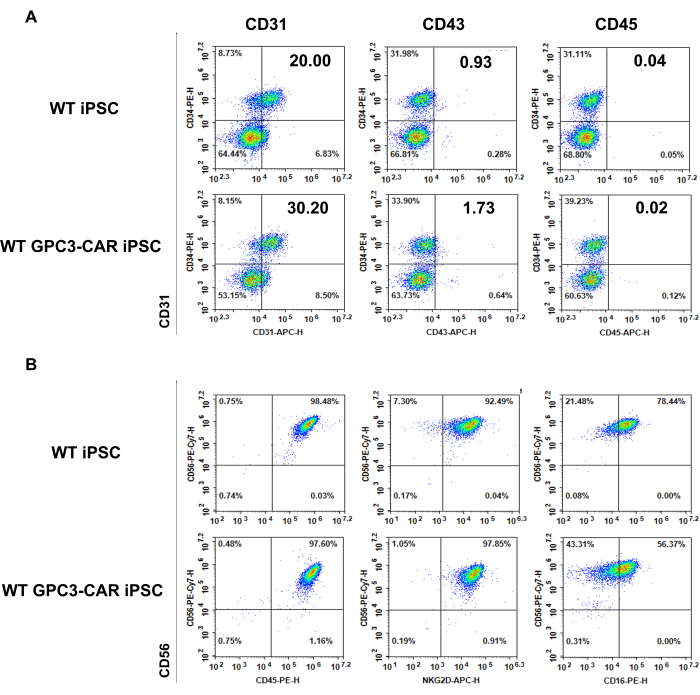
Figure 3: Phenotype of WT and anti-GPC3 CAR iPSC derived day 6 EB and differentiated WT and GPC3 CAR iPSC derived NK cells on Day 35. (A) Typical hematopoietic antigens expressed on WT and GPC3 CAR iPSCs on day 6 of Differentiation of hematopoietic Differentiation. (B) Phenotype of WT and GPC3 CAR iPSC derived NK cells are harvested from NK differentiation medium on day 35. Please click here to view a larger version of this figure.

Figure 4: Phenotype of WT and anti-GPC3-CAR iPSC derived NK cells after expansion. (A) WT and GPC3 CAR iPSC derived NK cells are expanded in Gibco NK Xpander medium, and NK maturation markers were assessed by using flow cytometry. (B) Surface expression of GPC3 antigen on various tumor cell lines including HepG2, SNU-449, SKOV3 and CAL27 were measured by using flow cytometry. (C) Representative histogram showing the CAR expression on the GPC3 CAR iPSC derived NK cells after expansion. The CAR construct incorporated with FLAG tag and GFP to measure the CAR by flow cytometry. Please click here to view a larger version of this figure.

Figure 5: Functional activity of WT and anti-GPC3 CAR iPSC derived NK cells against HCC and other tumor cell lines. (A-D) Antitumor activity of WT and GPC3 CAR iPSC-derived NK cells were tested against various tumor cell lines, including HepG2, SNU-449, CAL27, and SKOV3. The X-axis represents the E:T ratios of the WT and anti-GPC3 CAR iPSC-derived NK cells tested against HepG2, SNU-449, CAL27, and SKOV3. The specific E: T ratios indicated on the x-axis are 10:1, 5:1, 2.5:1, 1:1, 0.5:1, and 0.25:1. Please click here to view a larger version of this figure.
Dyskusje
This protocol outlines a standardized and reproducible approach for generating CAR-expressing, iPSC-derived NK cells from a consistent cell source aimed at facilitating targeted "off-the-shelf" cancer immunotherapy. Multiple preclinical and clinical studies have shown the efficacy of adoptive NK cell-based immunotherapy in treating cancers while minimizing toxicities, like graft versus host diseases (GvHD) or cytokine-release syndrome (CRS)23,42, 49,50,51,52,53,54,55,56,57. This approach utilizes an efficient and well-defined system to produce homogeneous and well-characterized CAR iPSC-derived NK cells can be scaled for clinical application. Additionally, iPSC-derived NK cells engineered to express NK-optimized CAR retain typical NK cell phenotypes and cytolytic functions 23,39,42,50,51,52,58.
Using human iPSCs for CAR iPSC-derived NK cell production offers a more efficient method for genetic modification, including CAR expression, compared to primary NK cells isolated from peripheral blood31,59,60. In addition to CAR expression, further enhancements to the antitumor activity of these cells can be achieved through modifications such as the deletion of inhibitory receptors or the introduction of cytokine expression41,39,61. This can be achieved through a single genetic modification event, eliminating the need for patient-specific modifications as seen in current CAR-T cell therapies31,62,63. Additionally, NK cell-based therapies with novel CAR-expressing cells can potentially be used in treating chronic infectious diseases30,64,65,66,67,68.
This protocol collectively demonstrates the potential of using human iPSCs to create homogeneous populations of CAR-expressing NK cells to enhance in vitro and in vivo antitumor activity. NK cell-optimized CARs enable antigen-specific activation of signaling pathways, improving the function of these cells. Modifications and troubleshooting are essential for optimizing the yield and functionality of CAR NK cells. Adjusting the cytokine cocktail and timing can improve differentiation efficiency. Ensuring the stability and expression of the CAR construct in NK cells may require optimizing transfection methods or vector design. Additional troubleshooting could address issues such as low transduction efficiency, cell viability, and functional activity of CAR iPSC derived NK cells, and may involve iterative testing of different culture conditions or genetic modifications to enhance persistence, cytotoxicity, and safety.
The significance of our method lies in its ability to generate a potentially unlimited supply of CAR NK cells from a standardized iPSC source. iPSC-derived CAR NK cells offer a stable platform for multiple gene edits, reducing variability and improving the consistency of the engineered cells. This method also enables the creation of "off-the-shelf" NK cell therapies that are ready for immediate use, bypassing the need for individualized cell sourcing and processing.
In addition, this protocol has certain limitations in maintaining the stability of CAR expression when using piggyBac vectors, as the transient nature of transgene integration may result in variable expression levels over time. Furthermore, the reliance on aAPCs and specific cytokines for expansion can complicate the scalability of these processes for clinical applications. Lastly, potential immune responses against the piggyBac elements may pose risks in therapeutic settings.
In conclusion, the differentiation and expansion of CAR-NK cells from human iPSCs hold significant promise for treating hepatocellular carcinoma (HCC) and other malignancies. This innovative approach leverages the regenerative potential of iPSCs and the innate immune properties of NK cells to create a potent and targeted cancer immunotherapy. Advances in this field could lead to more effective and widely available NK cell-based therapies, offering new hope for patients with HCC and other hard-to-treat diseases.
Ujawnienia
DSK is a co-founder and advisor to Shoreline Biosciences and has an equity interest in the company. DSK also consults for Therabest and RedC Bio for which he receives income and/or equity. The terms of these arrangements have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Podziękowania
We thank all Kaufman lab members for their support, scientific insights and discussions. These studies were supported by the NIH/NCI grants U01CA217885, P30CA023100 (administrative supplement) and the Sanford Stem Cell Institute at UCSD. JT: writing and revision of manuscript. DSK: reviewed and edited the manuscript.
Materiały
| Name | Company | Catalog Number | Comments |
| aAPC | Dean A. Lee lab | N/A | |
| a-MEM culture medium | Fisher Scientific | Cat#12634 | |
| APC anti-DYKDDDDK (FLAG Tag) | BD Biosciences | Cat#637308 | |
| APC-anti-human TRA-1-81 | ThermoFisher | 17-8883-42 | |
| APC-CD16 | BD Biosciences | Cat#302015 | |
| APC-CD43 | BD Biosciences | Cat# 560198 | |
| APC-CD45 | BD Biosciences | Cat# 555485 | |
| APC-GPC3 | BD Biosciences | Cat#DB100B | |
| APC-NKG2D | BD Biosciences | Cat# 558071 | |
| CellEvent Caspase-3/7 Green Detection Reagent | Thermo fisher | Cat#C10423 | |
| CellTrace Violet Cell Proliferation Kit | Thermo fisher | Cat#C34571 | Cell tracing fluorescent dye |
| CryoStor solution | Stem Cell Technologies | https://www.stemcell.com/products/cryostor-cs10.html | Cryopreservation medium |
| CTS NK Xpander | Gibco | A5019001 | |
| CTS NK-Xpander Medium | Life Technologies | Cat#A5019001 | |
| DMEM | Gibco | 11965084 | |
| DMEM, high glucose, GlutaMAX Supplement, pyruvate | Gibco | 10569010 | |
| EasySep Human NK Cell Enrichment Kit | StemCell Technologies, Inc. | Cat#19055 | |
| Ethanolamine | Sigma Aldrich | E9508 | |
| Fetal bovine serum | Fisher Scientific | Cat# 10437010 | |
| FITC-CD94 | BD Biosciences | Cat#555888 | |
| GlutaMAX Supplement | Gibco | 35050061 | |
| GolgiPlug | BD Biosciences | Cat#555029 | |
| GolgiStop | BD Biosciences | Cat#554724 | |
| Ham's F-12 Nutrient Mix, GlutaMAX Supplement | Gibco | 31765035 | |
| Horse serum | Fisher Scientific | Cat#16050130 | |
| Human Serum | AB Sigma-Aldrich | Cat#BP2525100 | |
| Human Stem Cell NucleofectorTM Kit | Lonza | Cat# VPH-5012 | |
| Human: HePG2 cells | ATCC | Cat#HB-8065 | |
| Human: HePG2-td-tomato-luc cells | Dan S. Kaufman lab | N/A | |
| Human: iPS cells | Dan S. Kaufman lab | N/A | |
| Human: SNU-449 cells | ATCC | Cat#CRL-2234 | |
| Human: SNU-449-td-tomato-luc cells | Dan S. Kaufman lab | N/A | |
| IncuCyte Caspase-3/7 Green Apoptosis Assay | Essenbioscience | Cat#4440 | |
| L-Ascorbic acid | Sigma Aldrich | A5960 | |
| MP Biomedicals Human Serum, Type AB | MP Biomedicals | ICN2938249 | |
| mTeSR plus | StemCell Technologies, Inc. | 100-0276 | |
| NovoExpress software | ACEA Biosciences | https://www.agilent.com/en/product/research-flow-cytometry/flow-cytometry-software/novocyte-novoexpress-software-1320805 | |
| PE/Cy7 anti-human SSEA-4 Antibody | Biolegend | 330420 | |
| PE-CD16 | BD Biosciences | Cat#560995 | |
| PE-CD226 | BD Biosciences | Cat#559789 | |
| PE-CD34 | BD Biosciences | Cat# 555822 | |
| PE-CD45 | BD Biosciences | Cat# 555483 | |
| PE-CD94 | BD Biosciences | Cat#555888 | |
| PE-cy7-CD56 | BioLegend | Cat# 318318 | |
| PE-FAS Ligand | BD Biosciences | Cat#564261 | |
| PE-NKp30 | BD Biosciences | Cat# 558407 | |
| PE-NKp44 | BD Biosciences | Cat#558563 | |
| PE-NKp46 | BD Biosciences | Cat#331908 | |
| PE-NKp46 | BD Biosciences | Cat#557991 | |
| Peripheral blood buffy coat | San Diego Blood Bank (https://www. sandiegobloodbank.org/) | N/A | |
| PE-TRAIL | BD Biosciences | Cat#565499 | |
| pKT2-mCAG-IRES-GFP-ZEO | Branden Moriarity lab | N/A | |
| pMAX-GFP plasmid | Lonza | N/A | GFP positive control |
| Prism 9 | Graphpad | Version 9 | |
| pSpCas9 | GenScript | PX165 | |
| RBC Lysis Buffer (10x) | Biolegend | Cat#420301 | |
| Recombinant human bFGF basic | R&D Systems | Cat#4114-TC | |
| Recombinant human BMP-4 | PeproTech | Cat#120-05 | |
| Recombinant human FLT-3 Ligand | PeproTech | Cat# 300-19 | |
| Recombinant human IL-15 | PeproTech | Cat# 200-15 | |
| Recombinant human IL-2 | PeproTech | Cat# 200-02 | |
| Recombinant human IL-3 | PeproTech | Cat#200-03 | |
| Recombinant human IL-7 | PeproTech | Cat# 200-07 | |
| Recombinant Human Nodal Protein | R&D Systems | Cat#3218-ND-025 | |
| Recombinant human SCF | PeproTech | Cat# 300-07 | |
| Recombinant Human TGF-β1 | PeproTech | Cat#100-21 | |
| Recombinant human VEGF | PeproTech | Cat# 100-20 | |
| RPMI1640 | Gibco | 11875093 | |
| Sodium selenite | Sigma Aldrich | 214485 | |
| STEMdiff APEL 2 Medium | StemCell Technologies, Inc. | 5270 | EB formation medium |
| STEMdiff APEL2 Medium | StemCell Technologies, Inc. | Cat#05270 | |
| Super piggyBac Transposase expression vector | SBI | Cat#PB210PA-1 | |
| SYTOX AADvanced Dead Cell Stain Kit | ThermoFisher Scientific | S10274, S10349 | Dead cell staining solution kit |
| β-mercaptoethanol | Gibco | 21985023 |
Odniesienia
- Chan, I. S., Ewald, A. J. The changing role of natural killer cells in cancer metastasis. J Clin Invest. 132 (6), e143762(2022).
- Yu, Y. The function of NK cells in tumor metastasis and NK cell-based immunotherapy. Cancers (Basel). 15 (8), 2323(2023).
- Paul, S., Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 8, 1124(2017).
- Myers, J. A., Miller, J. S. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 18 (2), 85-100 (2021).
- Llovet, J. M., et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 7, 6(2021).
- Yang, Y., Xiong, L., Li, M., Jiang, P., Wang, J., Li, C. Advances in radiotherapy and immunity in hepatocellular carcinoma. J Transl Med. 21 (1), 526(2023).
- Rich, N. E., Yopp, A. C., Singal, A. G. Medical management of hepatocellular carcinoma. J Oncol Pract. 13 (6), 356-364 (2017).
- Niu, M., Yi, M., Li, N., Wu, K., Wu, K. Advances of Targeted Therapy for Hepatocellular Carcinoma. Front Oncol. 11, 719896(2021).
- Yu, S. J. Immunotherapy for hepatocellular carcinoma: Recent advances and future targets. Pharmacol Ther. 244, 108387(2023).
- Ntellas, P., Chau, I. Updates on systemic therapy for hepatocellular carcinoma. Am Soc Clin Oncol Educ Book. 44, e430028. 44, e430028(2024).
- Liu, T. -H., Shen, Y. -C., Cheng, A. -L. Immune checkpoint inhibitors for hepatocellular carcinoma - A game changer in treatment landscape. J Formos Med Assoc. 121 (8), 1371-1383 (2022).
- Sun, Q., Hong, Z., Zhang, C., Wang, L., Han, Z., Ma, D. Immune checkpoint therapy for solid tumours: clinical dilemmas and future trends. Signal Transduct Target Ther. 8 (1), 320(2023).
- Huang, A., Yang, X. -R., Chung, W. -Y., Dennison, A. R., Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 5 (1), 146(2020).
- Girardi, D. M., Pacífico, J. P. M., Guedes de Amorim, F. P. L., Dos Santos Fernandes, G., Teixeira, M. C., Pereira, A. A. L. Immunotherapy and targeted therapy for hepatocellular carcinoma: A literature review and treatment perspectives. Pharmaceuticals (Basel). 14 (1), 28(2020).
- Laface, C., et al. Targeted therapy for hepatocellular carcinoma: Old and new opportunities. Cancers (Basel). 14 (16), 4028(2022).
- Shyam Sunder, S., Sharma, U. C., Pokharel, S. Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management. Signal Transduct Target Ther. 8 (1), 262(2023).
- Cappell, K. M., Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat Rev Clin Oncol. 20 (6), 359-371 (2023).
- June, C. H., O'Connor, R. S., Kawalekar, O. U., Ghassemi, S., Milone, M. C. CAR T cell immunotherapy for human cancer. Science. 359 (6382), 1361-1365 (2018).
- Schuster Stephen, J., et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 377 (26), 2545-2554 (2017).
- Schmidt, D., et al. Engineering CAR-NK cells: how to tune innate killer cells for cancer immunotherapy. Immunother Adv. 2 (1), Itac003(2022).
- Zhang, B., et al. Chimeric antigen receptor-based natural killer cell immunotherapy in cancer: from bench to bedside. Cell Death Dis. 15 (1), 50(2024).
- Wang, K., et al. Reprogramming natural killer cells for cancer therapy. Mol Ther. 32 (9), 2835-2855 (2024).
- Li, Y., Hermanson, D. L., Moriarity, B. S., Kaufman, D. S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance antitumor activity. Cell Stem Cell. 23 (2), 181-192.e5 (2018).
- Liu, E., Marin, D., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 382 (6), 545-553 (2020).
- Chen, L., Xie, T., Wei, B., Di, D. L. Current progress in CAR-T cell therapy for tumor treatment. Oncol Lett. 24 (4), 358(2022).
- Rafiq, S., Hackett, C. S., Brentjens, R. J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 17 (3), 147-167 (2020).
- Sterner, R. C., Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11 (4), 69(2021).
- Albinger, N., Hartmann, J., Ullrich, E. Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany. Gene Ther. 28 (9), 513-527 (2021).
- Dagher, O. K., Posey, A. D. Forks in the road for CAR T and CAR NK cell cancer therapies. Nat Immunol. 24 (12), 1994-2007 (2023).
- Xie, G., Dong, H., Liang, Y., Ham, J. D., Rizwan, R., Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. 59, 102975(2020).
- Cichocki, F., van der Stegen, S. J. C., Miller, J. S. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood. 141 (8), 846-855 (2023).
- Goldenson, B. H., Hor, P., Kaufman, D. S. iPSC-derived natural killer cell therapies - Expansion and targeting. Front Immunol. 13, 841107(2022).
- Zhu, H., Kaufman, D. S. Engineered human pluripotent stem cell-derived natural killer cells: the next frontier for cancer immunotherapy. Blood Sci. 1 (1), 4-11 (2019).
- Knorr, D. A., et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2 (4), 274-283 (2013).
- Shimizu, Y., Suzuki, T., Yoshikawa, T., Endo, I., Nakatsura, T. Next-generation cancer immunotherapy targeting Glypican-3. Front Oncol. 9, 248(2019).
- Schepers, E. J., Glaser, K., Zwolshen, H. M., Hartman, S. J., Bondoc, A. J. Structural and functional impact of posttranslational modification of Glypican-3 on liver carcinogenesis. Cancer Res. 83 (12), 1933-1940 (2023).
- Batra, S. A., et al. Glypican-3-specific CAR T cells coexpressing IL15 and IL21 have superior expansion and antitumor activity against hepatocellular carcinoma. Cancer Immunol Res. 8 (3), 309-320 (2020).
- Gao, W., et al. Immunotoxin targeting glypican-3 regresses liver cancer via dual inhibition of Wnt signalling and protein synthesis. Nat Commun. 6, 6536(2015).
- Thangaraj, J. L., Coffey, M., Lopez, E., Kaufman, D. S. Disruption of TGF-β signaling pathway is required to mediate effective killing of hepatocellular carcinoma by human iPSC-derived NK cells. Cell Stem Cell. 31 (9), 1327-1343.e5 (2024).
- Maddineni, S., Silberstein, J. L., Sunwoo, J. B. Emerging NK cell therapies for cancer and the promise of next generation engineering of iPSC-derived NK cells. J Immunother Cancer. 10 (5), e004693(2022).
- Zhu, H., et al. Metabolic reprograming via deletion of CISH in human iPSC-derived NK cells promotes in vivo persistence and enhances antitumor activity. Cell Stem Cell. 27 (2), 224-237.e6 (2020).
- Strati, P., et al. Preliminary results of a phase I trial of FT516, an off-the-shelf natural killer (NK) cell therapy derived from a clonal master induced pluripotent stem cell (iPSC) line expressing high-affinity, non-cleavable CD16 (hnCD16), in patients (pts) with relapsed/refractory (R/R) B-cell lymphoma (BCL). J Clin Oncol. 39, 7541-7541 (2021).
- Hong, D., et al. Preliminary results of an ongoing phase I trial of FT500, a first-in-class, off-the-shelf, induced pluripotent stem cell (iPSC) derived natural killer (NK) cell therapy in advanced solid tumors. J Immunother Cancer. 8 (3), A231-A232 (2020).
- Zah, E., et al. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat Commun. 11 (1), 2283(2020).
- Ahn, S., et al. Cancer immunotherapy with T cells carrying bispecific receptors that mimic antibodies. Cancer Immunol Res. 7 (5), 773-783 (2019).
- Ludwik, K. A., Telugu, N., Schommer, S., Stachelscheid, H., Diecke, S. ASSURED-optimized CRISPR protocol for knockout/SNP knockin in hiPSCs. STAR Protoc. 4 (3), 102406(2023).
- Zhu, H., Kaufman, D. S. An improved method to produce clinical scale natural killer cells from human pluripotent stem cells. Methods Mol Biol. 2048, 107-119 (2019).
- Denman, C. J., et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 7 (1), e30264(2012).
- Leivas, A., et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 11 (8), 146(2021).
- Ramdial, J. L., et al. A phase II clinical trial of "Off-the-Shelf" NK cells with allogeneic stem cell transplantation to decrease disease relapse in patients with high-risk myeloid malignancies. Blood. 140 (Supplement 1), 7484-7485 (2022).
- Bachanova, V., et al. Initial clinical activity of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood. 136, 8(2020).
- Bae, W. K., et al. A phase I study of locoregional high-dose autologous natural killer cell therapy with hepatic arterial infusion chemotherapy in patients with locally advanced hepatocellular carcinoma. Front Immunol. 13, 879452(2022).
- Marin, D., et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat Med. 30 (3), 772-784 (2024).
- Fehniger, T. A., et al. A phase 1 trial of CNDO-109-activated natural killer cells in patients with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant. 24 (8), 1581-1589 (2018).
- Nguyen, R., et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J Immunother Cancer. 7 (1), 81(2019).
- Miller, J. S., et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 105 (8), 3051-3057 (2005).
- Liu, E., et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 382 (6), 545-553 (2020).
- Daher, M., Melo Garcia, L., Li, Y., Rezvani, K. CAR-NK cells: the next wave of cellular therapy for cancer. Clin Transl Immunology. 10 (4), e1274(2021).
- Lin, X., Sun, Y., Dong, X., Liu, Z., Sugimura, R., Xie, G. IPSC-derived CAR-NK cells for cancer immunotherapy. Biomed Pharmacother. 165, 115123(2023).
- Bachanova, V., et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood. 138, 823(2021).
- Saetersmoen, M. L., Hammer, Q., Valamehr, B., Kaufman, D. S., Malmberg, K. J. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin Immunopathol. 41 (1), 59-68 (2019).
- Gerew, A., et al. Deletion of CISH and TGFβR2 in iPSC-derived NK cells promotes high cytotoxicity and enhances in vivo tumor killing. Blood. 138, 2780(2021).
- Goldenson, B. H., Hor, P., Kaufman, D. S. iPSC-derived natural killer cell therapies - Expansion and targeting. Front Immunol. 13, 841107(2022).
- Karvouni, M., Vidal-Manrique, M., Lundqvist, A., Alici, E. Engineered NK cells against cancer and their potential applications beyond. Front Immunol. 13, 825979(2022).
- Ayuso, J. M., et al. Microphysiological model reveals the promise of memory-like natural killer cell immunotherapy for HIV± cancer. Nat Commun. 14 (1), 6681(2023).
- Miller, J. S., et al. Safety and virologic impact of haploidentical NK cells plus interleukin 2 or N-803 in HIV infection. J Infect Dis. 229 (5), 1256-1265 (2024).
- Gutiérrez-Hoya, A., Soto-Cruz, I. NK cell regulation in cervical cancer and strategies for immunotherapy. Cells. 10 (11), 3104(2021).
- Lisco, A., et al. Treatment of relapsing HPV diseases by restored function of natural killer cells. N Engl J Med. 385 (10), 921-929 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone