Method Article
Ex Vivo Porcine Experimental Model for Studying and Teaching Lung Mechanics
W tym Artykule
Podsumowanie
We present an ex vivo pig lung model for the demonstration of pulmonary mechanics and alveolar recruitment maneuvers for teaching purposes. The lungs can be used for more than one day (up to five days) with minimal changes in pulmonary mechanics variables.
Streszczenie
Mechanical ventilation is widely used and requires specific knowledge for understanding and management. Health professionals in this field may feel insecure and lack knowledge because of inadequate training and teaching methods. Therefore, the objective of this article is to outline the steps involved in generating an ex vivo porcine lung model to be used in the future, to study and teach lung mechanics. To generate the model, five porcine lungs were carefully removed from the thorax following the guidelines of the Animal Research Ethics Committee with adequate care and were connected to the mechanical ventilator through a tracheal cannula. These lungs were then subjected to the alveolar recruitment maneuver. Respiratory mechanics parameters were recorded, and video cameras were used to obtain videos of the lungs during this process. This process was repeated for five consecutive days. When not used, the lungs were kept refrigerated. The model showed different lung mechanics after the alveolar recruitment maneuver every day; not being influenced by the days, only by the maneuver. Therefore, we conclude that the ex vivo lung model can provide a better understanding of lung mechanics and its effects, and even of the alveolar recruitment maneuver through visual feedback during all stages of the process.
Wprowadzenie
Mechanical ventilation (MV) is widely used in intensive care units (ICUs) and surgical centers. Its monitoring is essential to help recognize asynchronies and prevent injuries for all patients, especially when the patient has serious lung injuries1,2,3,4,5,6. Monitoring respiratory mechanics can also contribute to the clinical understanding of the disease progression and therapeutic applications, such as the use of positive end-expiratory pressure (PEEP) or the alveolar recruitment maneuver (ARM). However, the use of these techniques requires a proficient understanding of curves and basic lung mechanics3,4.
Students, residents, and medical professionals feel insecure about MV management, from turning on the ventilator and initial adjustments to monitoring plateau and driving pressures, and this insecurity is associated with a lack of knowledge and adequate prior training7,8,9,10. We observed that professionals who participated in simulations and used a lung model reported greater confidence, understanding of the parameters, and understanding of the components of lung mechanics8,11,12.
Models for studying and training MV with test lungs, bellows, and pistons can simulate different pressures and volumes, as well as different lung mechanics conditions13,14,15. Computational and software models also contribute to the study of cardiopulmonary interaction by generating simulations that can be used to teach the principles of MV11 to health professionals16,17.
While computational models may present difficulties in representing pulmonary hysteresis16, models with test lung and bellows13,14,15can produce pressure-volume curves similar to the physiological curve and demonstrate pulmonary dynamics. As an advantage, the ex vivo porcine lung presents similar anatomy to humans18, also producing MV curves, pulmonary hysteresis, and providing visual feedback of the lungs inside the acrylic box during the lung mechanics analysis. Visual models are important and can help understand difficult-to-imagine components and concepts. Thus, ex vivo lung models represent a practical way of teaching.
Studies with ex vivo porcine lungs, such as those on MV with positive and negative pressure19,20,21, analysis of aerosol distribution22,23, pediatric simulations24, and lung perfusion25 can improve the knowledge on MV. Recent studies analyzing models in positive and negative pressure have shown that positive-pressure ventilation can lead to abrupt recruitment with greater local deformation, greater distension, hysteresis curve differences, and possible tissue lesions compared to negative pressure pressure19,20,21. Nevertheless, positive-pressure models are necessary because patients are under positive pressure during MV pressure19,20,21. The development of a lung model for preclinical studies opens possibilities for new research and applications, including MV teaching and training.
Here, we present an ex vivo porcine lung model for studying and training purposes. Our primary objective is to describe the steps for the generation of this ex vivo porcine lung model under positive-pressure MV. It can be used in the future to study and teach lung mechanics.
Protokół
The protocol was approved by the Animal Research Ethics Committee of our Institution (protocol no. 1610/2021).
1. Anesthesia and animal preparation
- Initially, place the animal on a scale and check the weight to adjust the medications and sedation necessary for the procedure.
- Administer ketamine 5 mg/kg and midazolam 0.25 mg/kg intramuscularly.
- Puncture the marginal ear vein with a 20 G venous catheter and administer intravenous propofol (5 mg/kg) for anesthesia induction.
- Administer 3 mL of heparin intravenously into the access to the marginal ear vein to assist in the cardiopulmonary extraction and perfusion.
- After anesthesia, perform orotracheal intubation with a 6.5 mm orotracheal cannula (OTC) and fix the OTC with adhesive tape, leaving it firmly fixed to avoid displacement during the procedure.
NOTE: The depth of sedation is checked by monitoring hemodynamic parameters and using a gas analyzer, such as mean arterial pressure, heart rate, and inspired/expired isoflurane concentration.
2. Intraoperative mechanical ventilation
- Connect the animal via the OTC to MV, maintaining sedation with 1.5% isoflurane at 50% of the inspired fraction of oxygen (FiO2) and fentanyl 10 mcg/kg bolus + 10 mcg/kg/h continuous infusion.
- Tap on the mechanical ventilator screen and select the volume-controlled ventilation (VCV) mode, select the tidal volume (TV) button, and turn the scroll wheel until the tidal volume value corresponds to 8 mL/kg.
- Tap on the mechanical ventilator screen. Select the FiO2 and turn the scroll wheel until the value of 50% is reached.
- Tap on the mechanical ventilator screen and select the respiratory rate (RR). Turn the wheel until it reaches the ideal value to maintain an end-expired CO2 of 35-45 mmHg measured by capnography coupled to the mechanical ventilator.
NOTE: The depth of sedation is checked by monitoring hemodynamic parameters and using a gas analyzer such as mean arterial pressure, heart rate, and inspired/expired isoflurane concentration.
3. Tissue dissection and OTC exchange
- Make a medial sternal incision from 2 cm above the manubrium to 2 cm below the xiphoid process of the sternum to access the thoracic cavity. Position the rib retractors, expanding the field of view during the procedure.
- Use a scalpel to make a horizontal tracheal incision at the height of the cricoid cartilage (just at the first tracheal rings) wide enough to introduce a new tracheal cannula.
- Deflate the OTC cuff that is inside the airway and pull slowly to remove it. Meanwhile, insert the new OTC into the incision made in the trachea after removing the old OTC. Leakage may occur due to the deflated cuff, ceasing when repositioning the new OTC.
- Inflate the newly inserted tracheal tube cuff by connecting a 20 mL syringe to the pilot balloon. The syringe delivers air under pressure and inflates the pilot balloon and cuff. Once the cuff inflates, remove the syringe.
- Tie the new tracheal cannula directly to the trachea with 2-0 polyester to prevent leakage and movement while placing the lung in the plexiglass ventilation box.
- With the scalpel, dissect the tissues to remove the cardiopulmonary organs from the thorax.
4. Animal euthanasia
- Increase the isoflurane concentration to 5% and administer 10 mL of 19.1% potassium chloride. Subsequently, check the absence of vital signs.
NOTE: This procedure was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
5. Cardiopulmonary extraction
- After euthanasia, dissect the respiratory ligament to remove the lungs.
- After the tissue dissection, clamp the OTC with the appropriate Kelly forceps during the end of inspiration, keeping the lungs inflated.
- Disconnect the OTC from the mechanical ventilator, but keep it clamped.
- Section the aortic artery, position the aspirator inside the thoracic cavity to remove the extravasated blood, maintain visualization of the cavity while finishing the dissections, and free the organs to be removed from the thoracic cavity.
NOTE: The inferior pulmonary ligament should be carefully released to avoid pulmonary laceration. - Remove the heart and lung from the ribcage with the OTC clamped, without separating them, and place them on a tray.
6. Cardiopulmonary preparation
- With the lung on a tray, cannulate the pulmonary artery with a large-bore single-lumen catheter and connect it to the infusion set to continuously administer 2,000 mL of cold 0.9% saline solution (SS) or until clear liquid flows from the aorta.
NOTE: SS should be administered at a normal rate, avoid squeezing the intravenous (IV) bag. - After clearing the flow, suture the aortic artery with 2-0 polyester and administer another 100 mL of 0.9% SS. Close the single-lumen catheter outlet as the liquid will remain inside until the end of the experiment.
- Unclamp the OTC, note that the lungs will deflate and remain closed, ready to receive MV and the ARM.
7. MV inside an acrylic box
- After preparation, open the acrylic box and position the lungs vertically inside the box. Pass the OTC through the hole in the lid and connect the tracheal cannula to the mechanical ventilator.
NOTE: Make sure the tracheal cannula is firmly secured in the trachea. - Select the Start Ventilation button.
- Tap on the mechanical ventilator screen and select the mechanical ventilator for VCV.
- Tap on the VCV mode settings screen, and select the TV button, turn the wheel until it reaches the value of 6 mL/kg. Do the same to adjust PEEP to 5 cm H2O, FiO2 to 21%, RR to 15 breaths per minute, and inspiratory pause time to 10%.
8. ARM
- To start the recruitment, increase PEEP from 5 cm H2O to 6 cm H2O and then increase it in step-by-step increments of 2 cm H2O until reaching 14 cm H2O.PEEP is increased using the on-screen button below the PEEP value displayed on the screen. Turn the wheel to increase the value.
- For each PEEP, write down the peak pressure, plateau pressure, dynamic compliance, and airway resistance values displayed on the mechanical ventilator screen. Write down the driving pressure, which is the plateau pressure value minus the PEEP value adjusted at that time.
- After reaching 14 cm H2O, reduce PEEP in step-by-step decrements of 2 cm H2O until reaching 6 cm H2O, then reduce it to 5 cm H2O. PEEP is reduced using the on-screen button below the PEEP value displayed on the screen. Turn the wheel to decrease the value.
- For each PEEP, write down the peak pressure, plateau pressure, dynamic compliance, and airway resistance values displayed on the mechanical ventilator screen. Write down the driving pressure, which is the plateau pressure value minus the PEEP value adjusted at that time.
NOTE: Maintain each PEEP value for 10 min during the increment and for 5 min at each step during the decrement.
- For each PEEP, write down the peak pressure, plateau pressure, dynamic compliance, and airway resistance values displayed on the mechanical ventilator screen. Write down the driving pressure, which is the plateau pressure value minus the PEEP value adjusted at that time.
9. Cardiopulmonary maintenance
- At the end of the recruitment stage, gently clamp the tracheal cannula with the clamp during inspiration, keeping the lungs inflated. Open the acrylic box.
- Remove the lungs from the acrylic box and carefully place them in a glass container.
NOTE: Make sure the tracheal cannula is firmly secured in the trachea. - Pour 500 mL of 0.9% SS.
- Store it in the refrigerator in a plastic-wrapped glass container at a temperature of 2 to 8 °C for 24 h.
- Repeat the steps 7, 8, and 9 for five consecutive days.

Figure 1: Study flowchart. Please click here to view a larger version of this figure.
Wyniki
We used five female pigs weighing between 23.4-26.9 kg and followed the described protocol for cardiopulmonary extraction and lung mechanics analysis. Our intention is that the model is useful for the study of lung mechanics by analyzing peak pressure, plateau pressure, resistance, driving pressure, and dynamic compliance variables collected directly from the mechanical ventilator screen. The model flowchart is shown in Figure 1.
The lungs were analyzed for five consecutive days, repeating the entire process described in items 7.2, 8.1, 8.2, 9.1, 9.2, and 9.3 of the protocol. We tried to show how lung variables behaved pre- and post-recruitment and to verify the durability of the ex vivo pulmonary model in the established period.
Significant differences (p < 0.05) were observed for all variables between pre- and post-ARM. The peak pressure, plateau pressure (Figure 2), and driving pressure (Figure 3) decreased after the maneuver (p = 0.0005), while dynamic compliance (p = 0.0007) increased (Figure 4), demonstrating open collapsed alveoli and lung area gain. Resistance (Figure 5) also increased after recruitment (p = 0.0348). None of the variables were significantly influenced by the day.
Based on these results, we showed that the model is effective in demonstrating visual lung mechanics changes through the ARM (Figure 6) and in studying and teaching lung mechanics (Figure 7). In addition, we showed that the model can be used for at least five consecutive days. As we did not evaluate the model beyond this period, we cannot confirm the final durability of the lung model.

Figure 2: Pressures. (A) Peak pressure. The pre-ARM Ppeak ranged from 21 ± 3.2 to 23 ± 2.3 cmH2O, while the post-ARM Ppeak ranged between 9 ± 0.6 and 12.6 ± 1.4 cmH2O in the five lungs. The two-way ANOVA statistical analysis was used to calculate the p-value of 0.0005, which was considered significant. (B) Plateau pressure. The pre-ARM Pplateau ranged from 21 ± 3.2 to 22 ± 2.3 cmH2O, while the post-ARM Pplateau ranged between 8.8 ± 0.4 and 11.6 ± 1.6 cmH2O in the five lungs. The two-way ANOVA statistical analysis was used to calculate the p-value of 0.0005, which was considered significant. Please click here to view a larger version of this figure.
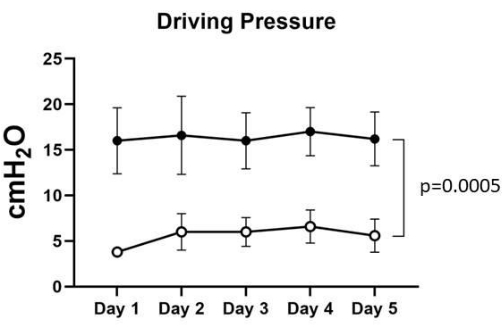
Figure 3: Driving pressure. The pre-ARM Driving pressure ranged from 16 ± 3.2 to 17 ± 2.3 cmH2O, while the post-ARM Driving pressure ranged between 3.8 ± 0.4 and 6.6 ± 1.6 cmH2O in the five lungs. The two-way ANOVA statistical analysis was used to calculate the p-value of 0.0005, which was considered significant. Please click here to view a larger version of this figure.

Figure 4: Dynamic compliance. The pre-ARM Dynamic compliance ranged from 9.1 ± 1.2 to 10.2 ± 2.6 mL/cmH2O, while the post-ARM Dynamic compliance ranged between 23.6 ± 3.5 and 43.8 ± 11.3 mL/cmH2O in the five lungs. The two-way ANOVA statistical analysis was used to calculate the p-value of 0.0007, which was considered significant. Please click here to view a larger version of this figure.
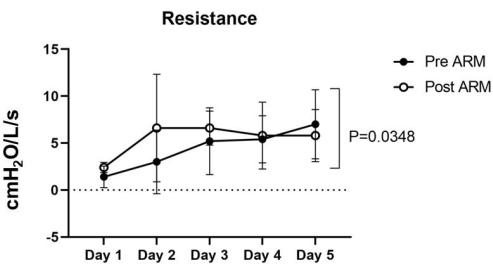
Figure 5: Resistance. The pre-ARM Resistance ranged from 1.4 ± 1.0 to 7 ± 3.2 cmH2O/L/seg, while the post-ARM Resistance ranged between 2.4 ± 0.4 and 6.6 ± 5.1 cmH2O/L/seg in the five lungs. The two-way ANOVA statistical analysis was used to calculate the p-value of 0.0348, which was considered significant. Please click here to view a larger version of this figure.
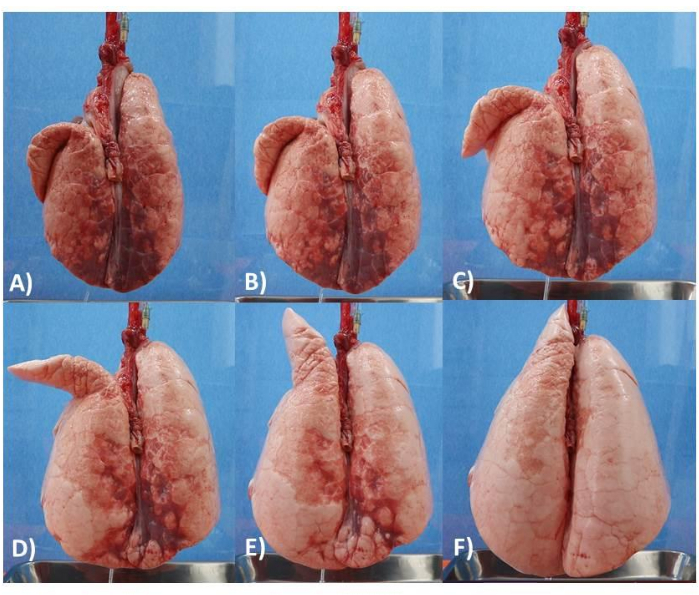
Figure 6: Lung model. (A) Lung with PEEP of 5 cm. (B) Lung with PEEP of 6 cm. (C) Lung with PEEP of 8 cm. (D) Lung with PEEP of 10 cm. (E) Lung with PEEP of 12 cm. (F) Lung with PEEP of 14 cm. Please click here to view a larger version of this figure.
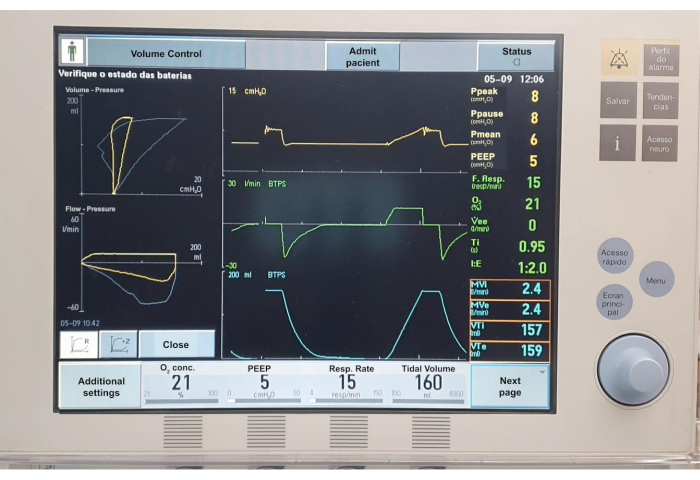
Figure 7. Mechanical ventilation charts. Please click here to view a larger version of this figure.
Dyskusje
The described protocol is useful for producing an ex vivo porcine lung model under positive-pressure MV. It can be used for studying and teaching lung mechanics through visual feedback from the lungs during recruitment and analysis of the curves and values projected on the device screen. To achieve this result, pilot studies are needed to understand the behavior of the lungs outside the rib cage and to identify the need for adaptations.
We identified that the critical point was the formation of bubbles, fistulas, and lesions in the pleura that were visualized when connecting the mechanical ventilator, with a difference between inspired and expired TV and changes in the volume curve. Thus, one of the first protocol modifications was to use a wide surgical opening of the thorax, with diaphragm incision at the beginning of the procedure during the dissection of the cardiopulmonary organs, which can improve visualization of the structures and help the careful release of the inferior pulmonary ligament, maintaining lung integrity. Furthermore, manual inflation of the pilot lungs after the structures were dissected showed that this inflation exceeds the pressure limits and contributes to the formation of blisters and fistulas. Some studies using ex vivo lungs presented the possibility of using fibrin glue for leaks, with positive results; although we did not use this approach in the study, it could be an alternative to improve the model26,27. Another relevant point is that the lungs were removed and completely deflated in the pilot study, keeping them totally collapsed from organ preparation to MV initiation, which made it difficult to open the lungs to MV and increased the possibility of fistula formation. Hence, we started to clamp the OTC and keep the lungs inflated during the dissection until SS was administered. Afterward, the OTC was released, deflated, and connected the lungs to the mechanical ventilator to start the ARM, and an analysis of lung mechanics was performed to demonstrate the pulmonary hysteresis curve. This did not compromise lung recruitment or the analysis of lung mechanics because anesthetized patients have atelectasis and reduced lung compliance even during MV28,29,30,31.
In the pilot study, an initial PEEP of 5 cm H2O was used and increased in 5 cm H2O increments up to 25 cm H2O32,33. However, the peak and plateau pressures reached values greater than 40 and 30 cm H2O, respectively, with fistula formation. Thus, a gradual increase in 2 cm H2O increments was performed to better analyze the behavior of pressures over time and to understand PEEP limits in our ex vivo lung model. There was no difference in mortality between sustained and incremental inflation, but incremental inflation is the most used and can facilitate the stepwise analysis of lung mechanics34. As for the use of negative pressure20,21, the model was tested only under positive pressure because patients on MV are subjected to positive pressure. We do not rule out the use of negative pressure in the future, but it would require acrylic case changes.
The literature presents some models produced with a test lung, pistons, and an ex vivo model13,14 that were placed in hermetically sealed boxes that simulated the ribcage. Our model was placed in a conventional acrylic box, which, despite reducing the possibility of applying negative pressure, can facilitate the production of the model. Another model produced for preclinical studies18 is similar to ours, but the lungs were positioned horizontally while ours were maintained vertically, receiving the action of gravity without the support of the organs and ribcage. These lungs were used during experiments within 48 hours after euthanasia18,19,20,21,35. Our model was used for a total of 120 h, being kept at a temperature of 2-8 °C during the 24 h of the experiment, showing the positive results described in the representative results section.
The gap in teaching and training was not addressed at this first moment, but the model is effective for analyzing lung mechanics and can be used as a tool for research and teaching. In addition, we did not aim to study perfusion solutions, but in the same way that we infused SS in step 6.1, perfusion and preservation solutions can be used, opening new possibilities for studies with the same model presented.
This technique has some limitations: 1) knowledge of animal anatomy to ensure that the lungs are removed properly; 2) the model was not evaluated beyond five days; 3) the model appears to be appropriate for teaching ventilation but has not been tested in a teaching context; 4) it is an animal model, so it is important to consider its applicability limitations in humans.
Ujawnienia
The authors declare no conflicts of interest.
Podziękowania
We thank all colleagues and professionals who contributed to and supported the construction of this ex vivo porcine lung model protocol.
This study had no funding sources.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.9% Saline solution | 2500ml | ||
| Anesthesia machine - Primus | Drager | REF 8603800-18 | Anesthesia work station used in the procedure |
| Aspirator | For blood aspiration from thorax | ||
| Bedside Monitor - Life Scope | Nihon Kohden | BSM-7363 | Multiparameter monitor used during the procedure |
| Bonney Tissue Forceps | Any tissue forceps is suitable | ||
| Disposable scalper, #23 | Any scalper is suitable | ||
| Disposable syringe needles, 18G x 1 1/2", 23G x 1" | BD | 302814 | Widely available |
| Disposable syringes, 10ml | Widely available | ||
| Electrosurgical unit - SS-501 | WEM | For cutting and coagulation during thorax incision | |
| Fentanyl | 10 mcg/kg bolus + 10 mcg/kg/hour continuous infusion | ||
| Finochietto retractor | Any finochietto retractor is suitable | ||
| heparin | 3ml | ||
| Infusion set | Any infusion set is suitable | ||
| Isoflurane | 1.5% | ||
| Kelly Forceps Curved | Any kelly forceps is suitable | ||
| Ketamine | 5mg/kg | ||
| Lactated Ringer solution | 500ml | ||
| Mechanical ventilator - Servo I | Maquet | REF 6449701 | Mechanical ventilator used in the procedure |
| Metzenbaum Scissor (Straight and curved) | Any metzenbaum scissor is suitable | ||
| Midazolam | 0.25mg/kg | ||
| Orotracheal intubation cannula, #6.5 | Rusch | 112282 | Widely available |
| Plexiglass | Custom made plexiglass box: 30x45x60cm | ||
| Polyester suture, 2-0 | Widely available | ||
| Potassium choride | 10 ml, 19.1% potassium chloride. | ||
| propofol | 5mg/kg | ||
| Three way stopcock | Widely available | ||
| Venous catheter, G20 x 1" | BD | 38183314 | Widely available |
Odniesienia
- Roberto, C., Carvalho, R., Toufen Jr, C., Franca, S. A. Mechanical Ventilation: Principles, graphic analysis and ventilation modalities. Jornal Brasileiro de Pneumologia. 33 (2), 54-55 (2007).
- Barbas, C. S. V., et al. Brazilian recommendations for mechanical ventilation 2013. Part I. Revista Brasileira de Terapia Intensiva. 26 (2), 89-121 (2014).
- Walter, J. M., Corbridge, T. C., Singer, B. D. Invasive mechanical ventilation. Southern Medical Journal. 111 (12), 746-753 (2018).
- Faustino, E. A. Concepts and monitoring of pulmonary mechanics in patients under ventilatory support in the intensive care unit. Revista Brasileira de Terapia Intensiva. 19 (2), 161-169 (2007).
- Holanda, M. A., Vasconcelos, R. S., Ferreira, J. C., Pinheiro, B. V. Patient-ventilator asynchrony. Jornal Brasileiro de Pneumologia. 44 (2), 321-333 (2018).
- Rezoagli, E., Laffey, J. G., Bellani, G. Monitoring lung injury severity and ventilation intensity during mechanical ventilation. Seminars in Respiratory and Critical Care Medicine. 43 (3), 346-368 (2022).
- Tallo, F. S. Evaluation of self-perception of mechanical ventilation knowledge among Brazilian final-year medical students, residents, and emergency physicians. Clinics. 72 (2), 65-70 (2017).
- Schroedl, C. J., et al. Impact of simulation-based mastery learning on resident skill managing mechanical ventilators. American Thoracic Society Scholar. 2 (1), 34-48 (2021).
- Wilcox, S. R., et al. Academic emergency medicine physicians' knowledge of mechanical ventilation. The Western Journal of Emergency Medicine. 17 (3), 271-279 (2016).
- Cox, C. E., et al. Effectiveness of medical resident education in mechanical ventilation. American Journal of Respiratory and Critical Care Medicine. 167 (1), 32-38 (2003).
- Keegan, R., Henderson, T., Brown, G. Use of the virtual ventilator, a screen-based computer simulation, to teach the principles of mechanical ventilation. Journal of Veterinary Medical Education. 36 (4), 436-443 (2009).
- Spadaro, S., et al. Simulation training for residents focused on mechanical ventilation: A randomized trial using mannequin-based versus computer-based simulation. Simulation in Healthcare. 12 (6), 349-355 (2017).
- Chase, J. G., Yuta, T., Mulligan, K. J., Shaw, G. M., Horn, B. A novel mechanical lung model of pulmonary diseases to assist with teaching and training. BMC Pulmonary Medicine. 6 (21), 1-11 (2006).
- Kuebler, W. M., Mertens, M., Pries, A. R. A two-component simulation model to teach respiratory mechanics. Advances in Physiology Education. 31 (2), 218-222 (2007).
- Heili-Frades, S., Peces-Barba, G., Rodríguez-Nieto, M. J. Design of a lung simulator for learning lung mechanics in mechanical ventilation. Archivos de Bronconeumología. 43 (12), 674-679 (2007).
- Ngo, C., Dahlmanns, S., Vollmer, T., Misgeld, B., Leonhardt, S. An object-oriented computational model to study cardiopulmonary hemodynamic interactions in humans. Computer Methods and Programs in Biomedicine. 159, 167-183 (2018).
- Lazzari, C. D., Genuini, I., Pisanelli, D. M., D'Ambrosi, A., Fedele, F. Interactive simulator for e-Learning environments: a teaching software for health care professionals. Biomedical Engineering Online. 13 (172), 1-18 (2014).
- Perinel, S., et al. Development of an ex vivo human-porcine respiratory model for preclinical studies. Scientific Reports. 7, 1-6 (2017).
- Aboelnazar, N. S., et al. Negative pressure ventilation decreases inflammation and lung edema during normothermic ex-vivo lung perfusion. The Journal of Heart and Lung Transplantation. 37 (4), 520-530 (2018).
- Sattari, S., et al. Introducing a custom-designed volume-pressure machine for novel measurements of whole lung organ viscoelasticity and direct comparisons between positive- and negative-pressure ventilation. Frontiers in Bioengineering and Biotechnology. 8, 1-12 (2020).
- Sattari, S., et al. Positive- and negative-pressure ventilation characterized by local and global pulmonary mechanics. American Journal of Respiratory and Critical Care Medicine. 207 (5), 577-586 (2023).
- Montigaud, Y., et al. Development of an ex vivo preclinical respiratory model of idiopathic pulmonary fibrosis for aerosol regional studies. Scientific Reports. 9 (1), 17949 (2019).
- Montigaud, Y., et al. Aerosol delivery during invasive mechanical ventilation: development of a preclinical ex vivo respiratory model for aerosol regional deposition. Scientific Reports. 9 (1), 17930 (2019).
- Montigaud, Y., et al. Development of an ex vivo respiratory pediatric model of bronchopulmonary dysplasia for aerosol deposition studies. Scientific Reports. 9 (1), 5720 (2019).
- Buchko, M. T., et al. A low-cost perfusate alternative for ex vivo. lung perfusion. transplantation proceedings. 52 (10), 2941-2946 (2020).
- Kondo, N. Development of an effective method utilizing fibrin glue to repair pleural defects in an ex-vivo pig model. Journal of Cardiothoracic Surgery. 15 (1), 110 (2020).
- Gasek, N., et al. Development of alginate and gelatin-based pleural and tracheal sealants. Acta Biomaterialia. 131, 222-235 (2021).
- Li, X., et al. Effects of individualized positive end-expiratory pressure combined with recruitment maneuver on intraoperative ventilation during abdominal surgery: a systematic review and network meta-analysis of randomized controlled trials. Journal of Anesthesia. 36 (2), 303-315 (2022).
- Hu, M. C., Yang, Y. L., Chen, T. T., Lee, C. I., Tam, K. W. T. Recruitment maneuvers to reduce pulmonary atelectasis after cardiac surgery: A meta-analysis of randomized trials. The Journal of Thoracic and Cardiovascular Surgery. 164 (1), 171-181 (2020).
- Hu, M. C., et al. Recruitment maneuvers in patients undergoing thoracic surgery: a meta-analysis. General Thoracic and Cardiovascular Surgery. 69 (12), 1553-1559 (2021).
- Zeng, C., Lagier, D., Lee, J. W., Melo, M. F. V. Perioperative pulmonary atelectasis: Part I. Biology and mechanisms. Anesthesiology. 136 (1), 181-205 (2022).
- Niman, E., et al. Lung recruitment after cardiac arrest during procurement of atelectatic donor lungs is a protective measure in lung transplantation. Journal of Thoracic Disease. 14 (8), 2802-2811 (2022).
- Calvo, R. N., et al. Comparison of the efficacy of two alveolar recruitment maneuvers in improving the lung mechanics and the degree of atelectasis in anesthetized healthy sheep. Research in Veterinary Science. 150 (5), 164-169 (2022).
- Pensier, J., et al. Effect of lung recruitment maneuver on oxygenation, physiological parameters and mortality in acute respiratory distress syndrome patients: a systematic review and meta-analysis. Intensive Care Medicine. 45 (12), 1691-1702 (2019).
- Mariano, C. A., Sattari, S., Quiros, K. A. M., Nelson, T. M., Eskandari, M. Examining lung mechanical strains as influenced by breathing volumes and rates using experimental digital image correlation. Respiratory Research. 23 (1), 92 (2022).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone