Method Article
Intra-Cardiac Injection of Human Prostate Cancer Cells to Create a Bone Metastasis Xenograft Mouse Model
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Here, we present a protocol for the intra-cardiac injection of human prostate cancer cells to generate a mouse model with bone metastasis lesions.
Streszczenie
As the most common male malignancy, prostate cancer (PC) ranks second in mortality, primarily due to a 65%-75% bone metastasis rate. Therefore, it is essential to understand the process and related mechanisms of prostate cancer bone metastasis for developing new therapeutics. For this, an animal model of bone metastasis is an essential tool. Here, we report detailed procedures to generate a bone metastasis mouse model via intra-cardiac injection of prostate cancer cells. A bioluminescence imaging system can determine whether prostate cancer cells have been accurately injected into the heart and monitor cancer cell metastasis since it has great advantages in monitoring metastatic lesion development. This model replicates the natural development of disseminated cancer cells to form micro-metastases in the bone and imitates the pathological process of prostate cancer bone metastasis. It provides an effective tool for further exploration of the molecular mechanisms and the in vivo therapeutic effects of this disease.
Wprowadzenie
Prostate cancer is the most frequent cancer in men in 112 countries and ranks second for mortality in higher human development index countries1,2. Most deaths in prostate cancer patients are caused by metastasis, and about 65%-75% of the cases will develop bone metastasis3,4. Therefore, prevention and treatment of prostate cancer bone metastases are urgently needed to improve the clinical outcome of prostate cancer patients. The animal model of bone metastasis is an indispensable tool for exploring the multistage process and molecular mechanisms involved in each stage of prostate cancer bone metastasis, thus identifying therapeutic targets and developing novel therapeutics5.
The most common methods to generate experimental animal models of prostate cancer bone metastasis include the orthotopic, intra-diaphysis (such as intra-tibial), and intra-cardiac injection of prostate cancer cells. The bone metastasis model with orthotopic injection is generated by directly injecting prostate cancer cells into the prostate of a mouse6,7. This experimental animal model has very similar clinical characteristics to prostate cancer bone metastasis. However, the metastasis mainly happens in the axillary lymph node and the lung rather than in the bone8,9.The intra-tibial injection model for prostate cancer directly injects prostate cancer cells into the tibia with a hightumor formation rate in the bone (tibia)10,11; however, the bone cortex and bone marrow cavity are easily damaged. Additionally, the tibial injection method cannot stimulate the pathological process of prostate cancer bone metastasis in which the cancer cells colonize the bone through circulation. To investigate the circulation, vascular extravasation, and distant metastasis with a higher bone metastasis rate of cancer cells, an intra-cardiac injection technique has been developed by directly injecting prostate cancer cells into the left ventricle of the mouse8,12,13. This makes it a valuable animal model for bone metastasis research8. The intra-cardiac injection method shows a bone metastasis rate of about 75%9,14, much higher than the orthotopic injection method. Therefore, the intra-cardiac injection is an ideal method to generate an animal model with prostate cancer bone metastasis.
This work aims to describe the process of establishing a mouse model of prostate cancer bone metastases, allowing readers to visualize the model establishment. The current work provides detailed processes, precautions, and illustrative pictures to generate a bone metastasis xenograft model via intra-cardiac injection of human prostate cancer cells in athymic mice. This method provides an effective tool for further exploring the molecular mechanisms and the in vivo therapeutic effects of prostate cancer bone metastasis.
Protokół
Six to eight week old male BALB/c athymic mice (n = 10) were housedin individually ventilated mice cages (5 mice/cage) in a specific-pathogen-free (SPF) animal room under the conditions of 12 h light/dark cycle, with free access to SPF feed and sterile water. Mice were adaptively fed for a week before the experiments.All animal experiments were approved by the animal welfare committee of Shanghai University of Traditional Chinese Medicine.
1. Cell preparation
- On the day of prostate cancer cell injection, wash 80%-90% confluent luciferase labeled PC-3 cells (PC-3-luciferase) cultured in a 10 cm cell culture dish with pre-cold sterile PBS (pH 7.4) twice. Trypsinize for 3 min with 1.5 mL of 0.25% trypsin, and collect the cells into a 15 mL centrifuge tube after quenching the trypsin with 6 mL of F-12 medium containing 10% serum.
NOTE: The PC-3-luciferase cell line is derived from the PC-3 cell line after being transfected with the pLV-luciferase vector15. - Use an automatic cell counter and calculate the cell concentration by transferring 20 µL of the cell suspension to the cell counting plate (see Table of Materials).
- Centrifuge the cells at 800 x g at room temperature (RT) for 5 min.
- Use a pipette to discard the supernatant and resuspend the cell pellet in F-12 medium (see Table of Materials) to a final cell density of 1 x 107 cells/mL.
- Keep the cells on ice at all times until injection.
- Bring the cells to the surgery room and finish the cell injection within 2 h.
NOTE: Prepare additional cell suspensions (usually doubled volumes as required) to ensure accurate injection doses (for example, to avoid the dead spaces inside the syringes).
2. Surgery for intra-cardiac injection of the human prostate cancer cells
NOTE: The surgical apparatus used for intra-cardiac injection is a 1 mL syringe (Figure 1). Provide thermal support throughout the procedure until the animal’s recovery from anesthesia.
- Keep the mouse in the SPF animal room. Perform all the procedures with sterilized apparatuses within an aseptic cabinet.
- Anesthetize the mouse using 2% isoflurane with 98% oxygen under a 2 L/min oxygen flow rate.
NOTE: Smear a small quantity of ophthalmic ointment on the mouse's eyes to avoid dryness during the anesthesia.Perform the whole surgery in a well-ventilated area. Make sure that the mouse is deeply anesthetized by pinching the mouse's toes before cell injection. If responses (e.g., a jerk or twitch) remain, wait for additional time for the anesthesia to take effect. - Place the mouse in a supone position and immobilize both upper limbs perpendicularly stretched away from the midline (Figure 2A).
- Immobilize the mouse from the abdomen down with surgical adhesive tape. Avoid pressing hard and displacing the internal organs (Figure 2B).
NOTE: Maintaining the topographic anatomy (i.e., fixing with adhesive tape) is essential since the intra-cardiac injection is blind (i.e., the accurate injection site on the heart is invisible to the naked eye). - Disinfect the skin of the thorax with a 70% ethanol swab.
- Identify the mouse xiphoid process in the depression at the lowest end of the mid-sternum by palpating down the middle of the anterior chest wall. Identify the mouse jugular notch in the central depression at the upper border of the manubrium by palpating up from the middle of the anterior chest wall.
- Label the most inferior point of the xiphoid process and jugular notch with a marker pen (Figure 2A). Make a third mark in the middle of these two landmarks and slightly to the right (animal's left) just over the heart in the third intercostal space.
NOTE: The site of injection is 1-2 mm left of the midline, and the injection point is between the third and fourth ribs of the mouse (Figure 2A). - Load 200 µL of the cell suspension into a 1 mL syringe.
NOTE: Keep an air bubble in the syringe, as shown in Figure 2C, to ensure that the blood is pulsating. The cell suspension must be thoroughly mixed before loading. - Vertically insert a 26 G needle through the injection site (Figure 2D).
- Keep the hand holding the syringe stable on the table, or use the other hand to hold it stable. Ensure that the long axis of the syringe is perpendicular to the injection site, and insert the syringe about 2 mm subcutaneously.
- Bright-red blood pulsation is visible in the needle hub and/or cell suspension when the needle tip is correctly inserted into the left ventricle. If the blood pulsation is invisible, the needle tip is not in the heart. Retract and replace the needle (in case the blood clotting inside the needle stops blood pulsation). Insert the needle once again.
- Inject the cells. Inject the cell suspension (100 µL) very slowly over 40-60 s and keep the hand holding the syringe stable all the time.
NOTE: Keep a close eye on the cell suspension in the syringe, and do not inject the air bubble inside the syringe into the heart. - Apply pressure to the injection site with a dry cotton swab for 15 s to ensure hemostasis when the needle is retracted.
- Return the mouse to the clean cage and monitor the animal until it completely recovers from anesthesia.
- Image the mouse with an in vivo bioluminescence system within 24 h post-injection to ensure the cancer cells have entered the systemic circulation.
NOTE: Correctly injected cancer cells enter the arterial circulation via the left heart ventricle, which is seen by the bioluminescence signaling visible all over the body (for example, see Figure 3A). - Perform in vivo bioluminescence imaging.
- Turn on the instrument and the computer. Open the software when the power indicator on the instrument and computer brightens, and then open the Image Acquisition window.
- Identify the in vivo imaging system connected to the computer in the device pane. Place the mouse to be imaged into the imaging chamber in a supine position. Then close the door of the imaging chamber.
- Set the parameters on the Setting pane provided in the Image Acquisition window.
- Set the parameters with Lens > Zoom > 1x, Lens > Focus > 108, and Lens > Iris > F 2.8 to make the images clear.
- Set the shooting channel in Filter&Light: Filter&Light > Filter > Luminescence, Filter&Light > Light > OFF, and Filter&Light > Light Intensity > Middle.
- Set the type of image to be taken: Imaging > Type > Single-Frame, Imaging > Exposure Time > 500 ms, Imaging > HDR Mode > Low GainMode. Click on the Acquire button to acquire the image.
- Visualize the metastatic cancer growth with in vivo bioluminescence.
NOTE: At each time point of the visualization, intraperitoneally inject 200 µL of the luciferin substrate into a 20 g mouse (150 mg/kg) and wait 15 min before the anesthesia (2% isoflurane mixed with 98% oxygen in an induction chamber). Place the mouse on the bioluminescence imaging system platform. Maintain anesthesia through a nasal mask with 2% isoflurane.
3. Pathologic investigation
- Four weeks after the cell injection, sacrifice the mouse by CO2 inhalation and then cervical dislocation.
- Immobilize the mouse in a supine position. Make a vertical incision (about 6 cm) from the abdomen to the thorax with surgical scissors to expose and grossly examine all the organs in the abdomen and thorax for cancerous lesions and/or pathological changes.
NOTE: For an intra-cardiac cancer cell injection study, cancer lesions in the mediastinum adjacent to the heart suggest misinjected cancer cells (cells not injected into the left cardiac ventricle); therefore, these mice are not included in the final data collection. - Excise the metastatic tumors with scissors. Measure the long diameters (a) and short diameters (b) of the tumor using calipers to calculate the tumor volume (V) using the formula V = 1/2 × a × b2, and weigh the tumor using an electronic scale.
- Fix the tumor tissues in 10% formalin solution followed by embedding in paraffin. Alternatively, snap-freeze the tumors in liquid nitrogen.
- Excise the bones (hind limbs, vertebrae, and/or ribs) with metastatic lesions. Fix the specimen of each mouse in a 50 mL tube containing 20 mL of 10% formalin solution for 24 h after removing the skin and muscles, followed by decalcification for 14 days in 10% EDTA solution with frequent buffer change (every 3 days).
- Embed the specimen in paraffin and section the samples for the routine histological examination16.
Wyniki
Bioluminescence imaging offers tremendous advantages in monitoring the metastatic lesion development for an intra-cardiac injection model. Soon after the cancer cell injection (within 24 h), bioluminescence imaging was used to visualize the cancer cells entering the general circulation (Figure 3A). Obvious bioluminescence signaling all over the body will be seen when the cancer cells are injected into the arterial circulation properly. Data from mice showing bioluminescence signals only at the injection site (heart) should be excluded from the final data collection. Metastatic lesions in the hind limbs were observed (Figure 3B-D) 2 weeks after the cell injection. As time progressed, the metastatic lesions became larger and appeared in other sites, including the sternum, ribs, and mandible.
The X-ray images showed bone destruction representing the metastatic lesions in the bone (Figure 4A). Bone destruction was also detected by micro-CT scanning. Micro-CT scanning was performed in 3D mode using a micro-CT (µCT80) associated with 3Dcalc, cone reconstruction, and a model visualization software application. A reconstruction of the bitmap data was obtained to build the 3D model. A representative micro-CT image of the bone destruction in the proximal tibia is shown in Figure 4B. The metastatic lesions were further confirmed in paraffin-embedded tissues by H&E staining (Figure 4C).

Figure 1: Surgical apparatus. A 1 mL syringe. Please click here to view a larger version of this figure.

Figure 2: Intra-cardiac injection of prostate cancer cells. (A) The panel illustrates the jugular notch, xiphoid process, rib cage (lower margin), and midline. The injection site is equidistant from the xiphoid process and the jugular notch. (B) Surgical tape is used horizontally across the abdomen to prevent mouse movement during the injection. (C) A syringe loaded with the cell suspension and the presence of an air bubble. The air bubble helps to visualize the blood pulsation. (D) The needle is inserted vertically through the injection site. Please click here to view a larger version of this figure.
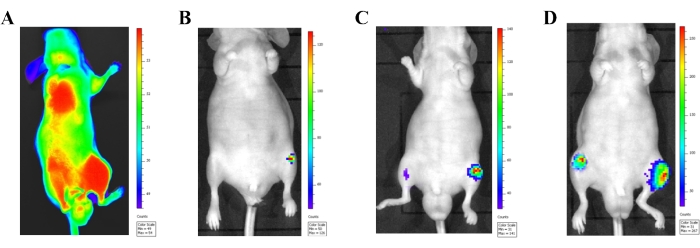
Figure 3: Confirmation of the intra-cardiac injection model with an in vivo bioluminescence imaging system. (A) In vivo bioluminescence imaging of a male athymic mouse 24 h after injecting luciferase labeled PC-3 cells (1 × 106 cells) into the left heart ventricle. Correctly injected cancer cells in the systemic circulation are seen by the bioluminescence signal released from the entire body. (B-D) Bioluminescence images of a representative mouse displaying progressive metastasis development. (B) An image showing prostate cancer cells 2 weeks after injection. (C) An image showing prostate cancer cells 3 weeks after injection. (D) An image showing prostate cancer cells 4 weeks after injection. Please click here to view a larger version of this figure.
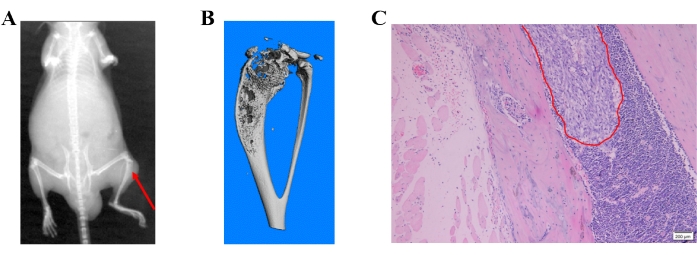
Figure 4: Different detection methods of prostate cancer cell-induced bone metastases. (A) A representative X-ray image; the red arrow shows bone destruction in the proximal tibia. (B) A representative micro-CT image of the tibia. (C) H&E image showing bone metastasis of prostate cancer cells. Please click here to view a larger version of this figure.
Dyskusje
Intra-cardiac injection of human prostate cancer cells to generate bone metastasis is an ideal mouse model for exploring the functions and mechanisms of prostate cancer bone metastasis and evaluating the therapeutic efficacy. Studies have shown that bone damage most likely occurs in the proximal tibia and the distal femur17, which may be due to their high vascularization and metabolic activity.
Since bone metastasis is a frequently observed metastatic lesion in breast cancer patients, the bone metastasis model produced by intracardiac injection of breast cancer cells is also commonly used in studies on breast cancer18,19. Therefore, the current work could help develop an intracardiac injection-produced bone metastasis model with breast and prostate cancer cells.
For consistent tumor formation, there are some key considerations. Cells should be injected as soon as possible after detachment from the culture. Mice should be randomized into experimental groups after cancer cell injection. The injection volume should be consistent and the same person should inject cancer cells to all mice using the same technique.
The whole procedure contains several critical steps. If the injection site is correctly positioned, pulsated blood should be observed during the injection. Loss of stability of the hand holding the syringe and change in the needle position while advancing the syringe plunger are potential problems. A syringe with a colored hub inside makes the blood pulsation easily seen. If air bubbles appear in the needle hub when inserting the needle (indicating misinsertion into the lungs), the needle must be removed and inserted again after the relocation. If there is no red blood pulse in the needle hub, but the person injecting is confident that the injection site is correct, pull the syringe plunger slightly to verify the injection in the cardiac ventricle. The lack of metastasis after 2-3 weeks of cancer cell injection indicates misinjection. Confirm cancer cell circulation in the whole body by bioluminescence imaging within 24 h after injection20. Bioluminescence signaling could be seen throughout the body if cancer cells wereaccurately injected into the cardiac ventricle. Furthermore, the metastatic rate, location, and number of metastatic tumors may differ in different cell lines8,21.
After the surgery, mice must be checked regularly. Due to the surgery and anesthetic exposure, mice may experience significant distress or even die. Therefore, the first week after the surgery is critical, and the mice must be carefully monitored. Throughout a bone metastasis experiment, mice should be monitored daily for changes in activity levels, mobility, and the onset of cachexia (a paraneoplastic syndrome in mice characterized by weight loss, muscle atrophy and weakness, arched appearance, and lethargy22,23). Mice should be euthanized when 10%-20% of their body weight is lost; tumor progression impairs mobility (for example, long bone fracture, head tilt, paraplegia); or the mice appears to be in respiratory distress24.
The advantage of this model is that cancer cells detected in the bone have "seeded the soil" 25, thus replicating the more natural progression of the micro-metastases formation of the disseminated cancer cells. This model also has several limitations. This is a xenograft cancer model using immunodeficient mice. This model is not beneficial for studying the interaction between cancer cells and immune cells in the bone metastasis microenvironment. It is estimated that about 30% of the mice will die during the modeling; therefore, practice will greatly improve the success rate of model development. Moreover, the metastasis lesion can also occur in the brain, lungs, and kidneys; the formation of multiple metastases interferes with the study of bone metastasis mechanisms9,26,27,28. Though the bone metastasis rate is much higher by intra-cardiac injection than by orthotopic injection, the intra-cardiac injection technique shows a bone metastasis rate of about 75% rather than 100%9,14. The lower efficiency may be because the injected cancer cells failed to enter blood circulation or the death of the recipient mouse during a cardiac injection due to the needle piercing the heart or the lung.
Despite these limitations, this established mouse model of prostate cancer bone metastasis has proven to be an excellent tool for studying bone and cancer crosstalk and evaluating potential therapeutics to prevent cancer progression and disrupt the cycle of metastasis-induced bone destruction.
Ujawnienia
All the authors declare no competing financial interests.
Podziękowania
This work is supported by grants from the National Key R&D Program of China (2018YFC1704300 and 2020YFE0201600), the National Nature Science Foundation (81973877 and 82174408), the research projects within the budget of the Shanghai University of Traditional Chinese Medicine (2021LK047), and the Shanghai Collaborative Innovation Center of Industrial Transformation of Hospital TCM Preparation.
Materiały
| Name | Company | Catalog Number | Comments |
| 1 mL syringes and needles | Shandong Weigao Group Medical Polymer Co., Ltd | 20200411 | The cells were injected into the ventricles of mice |
| Anesthesia machine | Shenzhen RWD Life Technology Co., Ltd | R500IP | Equipment for anesthetizing mice |
| Automatic cell counter | Shanghai Simo Biological Technology Co., Ltd | IC1000 | For counting cells |
| BALB/c athymic mice | Shanghai SLAC Laboratory Animal Co, Ltd. | Male | 6-8 week old, male mice |
| Bioluminescence imaging system | Shanghai Baitai Technology Co., Ltd | Vieworks | For tracking the tumor growth and pulmonary metastasis if the injected cells are labeled by luciferase |
| Centrifuge tube (15 mL, 50 mL) | Shanghai YueNian Biotechnology Co., Ltd | 430790, Corning | |
| EDTA solution | Wuhan Xavier Biotechnology Co., Ltd | G1105 | For decalcification of bone tissure |
| F-12 medium | Shanghai YueNian Biotechnology Co., Ltd | 21700075, GIBCO | Cell culture medium |
| Formalin solution | Shanghai YueNian Biotechnology Co., Ltd | BL539A | For fixing the specimen of each mouse |
| Isoflurane | Shenzhen RWD Life Technology Co., Ltd | VETEASY | For anesthesia |
| Lipofectamine 2000 | Shanghai YueNian Biotechnology Co., Ltd | 11668027, Thermo fisher | Plasmid transfection reagent |
| PC-3 cell line | Cell Bank of Chinese Academy of Sciences | TCHu 158 | Prostate cancer cell line |
| Phosphate-buffered saline | Beyotime Biotechnology | ST447 | Wash the human osteosarcoma cells |
| Trypsin (0.25%) | Shanghai YueNian Biotechnology Co., Ltd | 25200056, Gibco | For detaching the cells |
| Vector (pLV-luciferase) | Shanghai YueNian Biotechnology Co., Ltd | VL3613 | Plasmid for transfection |
| X-ray imaging system | Brook (Beijing) Technology Co., Ltd | FX PRO | For obtaining x-ray images to detect tumor growth |
| μCT80 | Shenzhen Fraun Technology Service Co., Ltd | Scanco Medical AG,Switzerland | For detection of bone destruction. The mico-CT is equipped with 3DCalc, cone reconstruction, and μCT Ray V3.4A model visualization software. |
Odniesienia
- Siegel, R. L., Miller, K. D., Fuchs, H. E., Jemal, A. Cancer Cancerstatistics, 2021. CA: A Cancer Journal for Clinicians. 71 (1), 7-33 (2021).
- Sung, H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 71 (3), 209-249 (2021).
- Coleman, R. E. Skeletal complications of malignancy. Cancer. 80, 1588-1594 (1997).
- Macedo, F., et al. Bone metastases: An overview. Oncology Reviews. 11 (1), 321 (2017).
- Rea, D., et al. Mouse models in prostate cancer translational research: From xenograft to PDX. BioMed Research International. 2016, 9750795 (2016).
- Zhang, Y., et al. Real-time GFP intravital imaging of the differences in cellular and angiogenic behavior of subcutaneous and orthotopic nude-mouse models of human PC-3 prostate cancer. Journal of Cellular Biochemistry. 117 (11), 2546-2551 (2016).
- Stephenson, R. A., et al. Metastatic model for human prostate cancer using orthotopic implantation in nude mice. Journal of the National Cancer Institute. 84 (12), 951-957 (1992).
- Simmons, J. K., et al. Animal models of bone metastasis. Veterinary Pathology. 52 (5), 827-841 (2015).
- Jenkins, D. E., Hornig, Y. S., Oei, Y., Dusich, J., Purchio, T. Bioluminescent human breast cancer cell lines that permit rapid and sensitive in vivo detection of mammary tumors and multiple metastases in immune deficient mice. Breast Cancer Research: BCR. 7 (4), 444-454 (2005).
- Corey, E., et al. Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. The Prostate. 52 (1), 20-33 (2002).
- Andersen, C., Bagi, C. M., Adams, S. W. Intra-tibial injection of human prostate cancer cell line CWR22 elicits osteoblastic response in immunodeficient rats. Journal of Musculoskeletal & Neuronal Interactions. 3 (2), 148-155 (2003).
- Sudhan, D. R., Pampo, C., Rice, L., Siemann, D. W. Cathepsin L inactivation leads to multimodal inhibition of prostate cancer cell dissemination in a preclinical bone metastasis model. International Journal of Cancer. 138 (11), 2665-2677 (2016).
- Jinnah, A. H., Zacks, B. C., Gwam, C. U., Kerr, B. A. Emerging and established models of bone metastasis. Cancers. 10 (6), 176 (2018).
- Simmons, J. K., et al. Canine prostate cancer cell line (Probasco) produces osteoblastic metastases in vivo. The Prostate. 74 (13), 1251-1265 (2014).
- Lamar, J. M., et al. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. The Journal of Biological Chemistry. 294 (7), 2302-2317 (2019).
- Chang, J., et al. Matrine inhibits prostate cancer via activation of the unfolded protein response/endoplasmic reticulum stress signaling and reversal of epithelial to mesenchymal transition. Molecular Medicine Reports. 18 (1), 945-957 (2018).
- Arguello, F., Baggs, R. B., Frantz, C. N. A murine model of experimental metastasis to bone and bone marrow. Cancer Research. 48 (23), 6876-6881 (1988).
- Brylka, L., et al. Spine Metastases in immunocompromised mice after intracardiac injection of MDA-MB-231-SCP2 breast cancer cells. Cancers. 14 (3), 556 (2022).
- Rahman, M. M., Veigas, J. M., Williams, P. J., Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Research and Treatment. 141 (3), 341-352 (2013).
- Park, S. I., Kim, S. J., McCauley, L. K., Gallick, G. E. Pre-clinical mouse models of human prostate cancer and their utility in drug discovery. Current Protocols in Pharmacology. , (2010).
- Wright, L. E., et al. Murine models of breast cancer bone metastasis. BoneKEy Reports. 5, 804 (2016).
- Fearon, K. C., Glass, D. J., Guttridge, D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabolism. 16 (2), 153-166 (2012).
- Waning, D. L., et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nature Medicine. 21 (11), 1262-1271 (2015).
- Talbot, S. R., et al. Defining body-weight reduction as a humane endpoint: a critical appraisal. Laboratory Animals. 54 (1), 99-110 (2020).
- Paget, S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Reviews. 8 (2), 98-101 (1989).
- Yin, J. J., et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. The Journal of Clinical Investigation. 103 (2), 197-206 (1999).
- Schneider, A., et al. turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 146 (4), 1727-1736 (2005).
- Padalecki, S. S., et al. Chromosome 18 suppresses prostate cancer metastases. Urologic Oncology. 21 (5), 366-373 (2003).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone