Method Article
High-speed Video Microscopy Analysis for First-line Diagnosis of Primary Ciliary Dyskinesia
W tym Artykule
Podsumowanie
High-speed video microscopy analysis is a relatively easy-to-perform, fast, cost-effective, and, in experienced hands, a considerably reliable tool for first-line diagnostics of primary ciliary dyskinesia, which should be available in every center involved in diagnostics and the treatment of severe lung diseases.
Streszczenie
Primary ciliary dyskinesia (PCD) is a congenital disorder predominantly inherited in an autosomal recessive trait. The disorder causes disturbance in the motion of cilia, leading to severe impairment of mucociliary clearance (MCC). If undiagnosed or diagnosed too late, the condition leads to the development of bronchiectasis and serious damage to the lungs in later life. Most of the methods for diagnosing PCD are time-consuming and demand extensive economic resources to establish them. High-speed video microscopy analysis (HSVMA) is the only diagnostic tool to visualize and analyze living respiratory cells with beating cilia in vitro. It is fast, cost-effective, and, in experienced hands, very reliable as a diagnostic tool for PCD. In addition, classical diagnostic measures such as transmission electron microscopy (TEM) are not applicable for some mutations as morphological changes are absent.
This paper describes the process of collecting respiratory epithelial cells, the further preparation of the specimen, and the process of HSVMA. We also describe how brushed cells can be successfully kept unharmed and beating by keeping them in a nourishing medium for storage and transport to the investigation site in cases where a clinic does not possess the equipment to perform HSVMA. Also shown are videos with pathologic beating patterns from patients with a mutation in the dynein arm heavy chain 11 gene (DNAH11), which cannot be diagnosed with TEM; the result of an inconclusive HSVMA due to infection of the upper airways, as well as an unsuccessful brushing with superimposition of red blood cells. With this article, we would like to encourage every unit dealing with pulmonology patients and rare lung diseases to perform HSVMA as part of their daily routine diagnostics for PCD or send the specimens over to a center specializing in performing HSVMA.
Wprowadzenie
Primary ciliary dyskinesia (PCD) is a rare, hereditary genetic disorder, which causes disturbances in the movement of beating cilia. If undiagnosed, it leads to severe lung damage in later life due to severe impairment of MCC. In the past, its prevalence has been estimated to be in the range of 1:4,000 to 50,000. Due to steadily improving diagnostics and a growing awareness of the condition, updates on the prevalence of PCD suggest that it might be much more common and probably in the range of 1:4,000 to 20,000 instead1,2. However, patients with PCD are still underdiagnosed or diagnosed too late1,3. Therefore, infants with either congenital situs inversus and/or heterotaxy or perinatal rhinorrhea, neonatal respiratory distress, a blocked nose, and feeding difficulties should be suspects for PCD. In later life, chronic otitis, recurrent pneumonia, rhinosinusitis, and a chronic, typical wet cough due to impaired MCC are the hallmark symptoms of PCD, which in combination with bronchiectasis and impaired lung function, continue into adulthood2.
Patients suspected of having PCD can be diagnosed by using different diagnostic tools. TEM has been considered the gold standard for first-line diagnostics in the past. However, up to 30% of PCD cases do not show abnormal ultrastructure1,3,4,5,6, demanding a different diagnostic approach. Therefore, a growing number of centers and the guidelines of the European Respiratory Society (ERS) suggest a combination of nasal nitric oxide (nNO) and HSVMA as first-line diagnostics1,7,9,10. HSVMA and nNO are also the most cost-effective options in identifying a patient with PCD11. However, even if genetic testing were included in the diagnostics, it must be kept in mind that there is currently no stand-alone test or combination of tests that can exclude PCD with 100% certainty8,9,10.
Out of the available diagnostic options, HSVMA is the only test that focuses on living, cilia-coated respiratory cells and evaluates ciliary beat pattern (CBP) and ciliary beat frequency (CBF). In contrast to TEM, the results of HSVMA are available quickly, usually on the day of testing, whereas results of TEM might arrive months after the specimen has been taken. HSVMA can be applied for all age groups, whereas nNO demands a high degree of compliance; attempts to use it under the age of 5 years are usually unsuccessful10. In experienced hands, HSVMA has excellent sensitivity and specificity to diagnose PCD at 100% and 96%, respectively12.
This paper describes the step-by-step procedure to perform HSVMA, including the harvesting of cilia-coated respiratory cells from the inferior turbinate of the nose, the preservation of harvested cells in a cell-nourishing medium for transport to the site of investigation, and the process of microscopic video analysis to determine CBF and CBP. Additionally, some video clips from patients are shown, comparing normal CBPs and CBFs with abnormal cilia function (Video 3, Video 4, Video 5, Video 6, Video 7, and Video 8).
Protokół
Ethics Statement:
This study was approved by the local ethics committee (69/2017) and was conducted in compliance with the declaration of Helsinki.
1. Collection and transport of respiratory epithelial cells
- Brushing
- Before brushing, administer a two-week course of oral amoxicillin-clavulanic acid to the patient to eradicate biofilms interfering with cilia function. Ensure the antibiotic is terminated 2 days before the procedure.
- To diminish an overlay of mucous material on the brushed epithelial cell strips, ask the patient to blow their nose thoroughly. Ask parents to help their small children blow their noses.
- For brushing, use an interdental brush of 0.6 mm size (see the Table of Materials). Keep the head of the patient fixed with one hand, and with the other hand, brush the inferior turbinate of both nostrils to collect sufficient epithelial cell strips.
NOTE: A slight resistance is usually experienced when inserting the interdental brush deeply under the inferior turbinate. Nasal brushing is carried out by quick turning and picking for approximately 2 s. Longer and intense brushing might otherwise cause severe epithelial injury and consecutive epistaxis. - If bronchoscopy is planned for reasons other than suspected PCD, collect samples during bronchoscopy by brushing the carina or bronchial epithelium or by biopsy13.
- Drop the harvested cell strips into a 1.5 mL microcentrifuge tube containing the cell-nourishing Dulbecco´s Modified Eagle Medium (DMEM).
NOTE: Epithelial cell strips usually drop off the brush more easily by tossing and turning the brush against the tube wall. - Close the lid of the microcentrifuge tube and hold the tube against a light source. Shake the tube to discover harvested cell strips and conglomerates.
- Transport of the specimen
- Place the microcentrifuge tubes in a tightly closed polystyrene box and ensure that the lid of the tubes is properly closed and the tubes are fixed inside the box.
- Add a cold pack; however, avoid freezing the specimen.
NOTE: The optimal temperature for preserving the specimen before investigation is approximately 4-8 °C (39.2-46.4 °F). - Ensure that the preserved samples are analyzed during the next 24 h.
NOTE: In these experiments, the mean time between brushings and HSVMA was 3 h.
2. High-speed video microscopy analysis (HSVMA)
- Video microscopy of the samples
- After receiving the microcentrifuge tubes containing the samples, warm them up to 37 °C (98.6 °F) to mimic an optimal, in vivo-like environment.
- If the microscope is equipped with a heating unit, place the tubes under the hood and warm them up there. Alternatively, use an incubator to warm up the samples.
- Start the camera and the software of the video unit on the PC.
- Using a pipette, take a small amount of the sample and place two drops into a cuvette or glass-bottom dish (see the Table of Materials). Cover the cuvette or dish with a lid and place it under the microscope.
- For evaluation of the samples, use a differential-interference microscope equipped with a cold-light source and a video camera able to record at high speed (at least 200 frames/s). Use an oil immersion lens with a 100x magnification and put a drop of immersion oil onto the surface of the optic.
NOTE: Microscopes with a lens approaching from below are recommended. - Approach the bottom of the dish with the microscope lens and search for cell clusters without red blood cells and with low mucus content. After finding a representative region of interest (ROI), focus on one specific group of beating cilia with the largest cilia movements and record a video sequence. Record cells with beating cilia sidewise and from above. After that, search for another representative cluster of cells and repeat the recording.
NOTE: The cilia coated cells must be investigated under a 1,000x magnification, and beating cilia should be recorded with a digital high-speed video (DHSV) camera set on a frame rate of 200/s or more (256/s in this protocol). Because the data obtained from the recorded video clips requires a lot of space on the hard drive, an external SSD hard drive is recommended.
- Analysis of video sequences
- To determine CBF and CBP, play the video clips back frame by frame.
- To determine CBF, set the frame rate into slow motion with 15 frames/s and count 10 consecutive beats.
- Record the number of frames passing by during a single cycle of 10 beats and insert the result into Eq (1). Determine the frequency by calculating the mean of all recorded cilia beat cycles and compare the result with the reference values for age (see Table 1)14.
 (1)
(1)
Where X is the number of frames passing by during a cycle of 10 beats. - For evaluating CBP, watch if the movement of the beating cilia is in full range (see Figure 1) and synchronized. Have two independent operators evaluate the CBP to prevent selection bias.
- Report the results of the HSVMA analysis to be either compatible, unlikely, or inconclusive with PCD.
Wyniki
Video 1 and Video 2 show a normal control where CBF and CBP are in the normal range (see Figure 1). Video 3, Video 4, Video 5, and Video 6 represent two cases of PCD patients with a homozygous mutation in the DNAH11 gene (c.2341G > A; p. Glu781Lys)3. These representative videos were chosen because phenotypes of mutations in the DNAH11 gene are noteworthy because they cannot be diagnosed by TEM due to the absence of morphological changes3,4,5.
Video 3 shows the classic stiff, minimally moving pattern of ciliary beat compatible with PCD. The normal, full-range pattern shown in Video 1 and Video 2 is absent (see Figure 1). Video 4 shows a sequence of the same patient (Video 3) but recorded from above. Video 5 and Video 6 show a hyperkinetic, ineffective phenotype of beating cilia also compatible with PCD in patients carrying a mutation in the DNAH11 gene. The CBF was so high that it could not be determined. Video 6 is from the same patient (Video 5) but recorded from above. The CBP is abnormal and does not show the full-range movement of healthy cilia (see Figure 1, Video 1, and Video 2).
Video 7 and Video 8 show a pathological CBF and CBP sidewise (Video 7) and from above (Video 8) from a patient who has been suffering from recurrent, upper-airway infections; however, PCD could not be established. At 10 Hz, the CBF remains slightly under the normal range for age (see Table 1), and the CBP is abnormal compared to the normal CBP of the control video sequence (Video 1, Video 2, and Figure 1), showing a rotatory ciliary movement. The case is inconclusive, and further diagnostic measures, including nNO, TEM, and genetic testing, must be considered, although the clinical picture of the patient is suggestive of PCD.
If the brushing procedure is carried out rigorously, the epithelium of the nose might be injured, and epistaxis could occur. If too many blood cells are in the specimen, HSVMA analysis cannot be performed because ciliated epithelial cells are covered with a coating of red blood cells, as can be seen in Video 9.
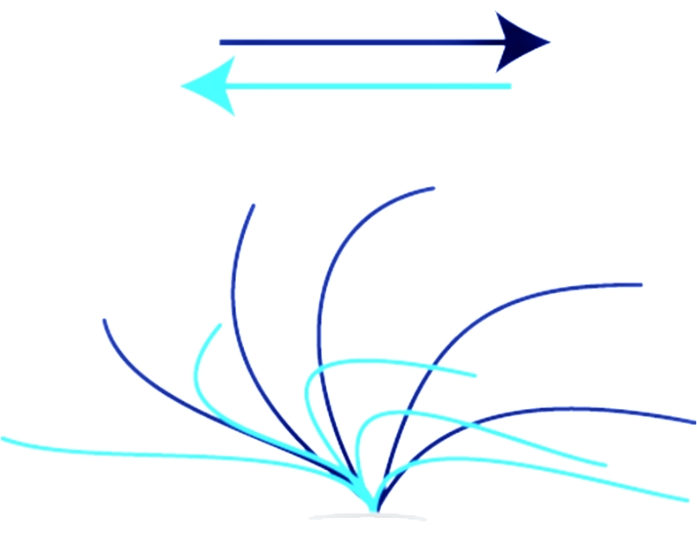
Figure 1: Normal ciliary stroke. Ciliary stroke of a healthy individual showing the entire range of a normal forward- and recovery stroke. The effective stroke (dark blue) moves from the left to the right in a whiplash manner. The recovery stroke (light blue) moves the cilia back from right to left into the starting position. Please click here to view a larger version of this figure.
Video 1: Normal cilia motion of a patient without PCD, seen sidewise. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 2: Normal cilia motion of a patient without PCD, from above. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 3: Video sequence sidewise of a PCD patient with a mutation in the DNAH11 gene (c.2341G > A p. Glu781Lys)3, showing stiff, almost immotile cilia. Abbreviations: PCD = Primary Ciliary Dyskinesia; DNAH11 = dynein arm heavy chain 11 gene. Please click here to download this Video.
Video 4: Video sequence from the same PCD patient (Video 3) recorded from above. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 5: Video sequence sidewise of a PCD patient showing a totally different, hyperkinetic but inefficient pattern of beating cilia. The patient was a member of the same family and with the same mutation as the patient in Video 3 and Video 4. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 6: Video sequence from the same PCD patient (Video 5), recorded from above. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 7: Video sequence of abnormal, uncoordinated, and slow cilia motion in a PCD patient suffering from recurrent upper-airway infection. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 8: Video sequence from the same PCD patient (Video 7) from above. Abbreviation: PCD = Primary Ciliary Dyskinesia. Please click here to download this Video.
Video 9: Video sequence of a specimen, which could not be analyzed due to careless brushing, leading to epistaxis and superimposition of red blood cells. Please click here to download this Video.
| Ciliary beat frequency (Hz)* | ||||
| Age (years) | Mean | SD | 5th, 95th centiles | Dyskinetically beating edges (%) † |
| 0-6 | 12.9 | 2.3 | 10.0, 18.1 | 10.4 (0.0, 36.8) |
| 7-12 | 12.9 | 1.4 | 10.9, 15.0 | 9.1 (0.0, 40.3) |
| 13-18 | 12.6 | 1.7 | 10.9, 15.3 | 24.8 (0.0, 56.9) |
| ≥19 | 11.5 | 2.8 | 7.7, 15.5 | 5.8 (0.0, 24.3) |
| *Mean ciliary beat frequency, standard deviation (SD), and 5th and 95th percentiles | ||||
| †Mean (5th, 95th percentiles) percentage of edges exhibiting areas of ciliary dyskinesia | ||||
Table 1: Normal beat frequencies. Normal, age-related, ciliary beat frequency. This table was modified from Chilvers et al.14. *Mean ciliary beat frequency, SD, and 5th and 95th percentiles. †Mean (5th, 95th percentiles) percentage of edges exhibiting areas of ciliary dyskinesia. Abbreviation: SD = standard deviation.
Dyskusje
Here, the diagnostic process for PCD using HSVMA is described and discussed in the light of first-line diagnostics. Despite being relatively easy to establish, cost-effective11, and a reliable method in experienced hands12, HSVMA is not a diagnostic measure without pitfalls. Abnormal CBF and CBP may be due to secondary infection, leading to inflammation of the broncho-respiratory epithelia15, and for the same reason, smoking individuals may have abnormal beat frequencies16,17. In addition, cystic fibrosis should be excluded before establishing the diagnosis of PCD. Before the analysis of a patient sample is judged to be compatible with PCD, abnormal results should be confirmed with two independently collected samples, requiring new brushings and a reanalysis of CBF and CBP. This might be challenging, especially with children. Therefore, some investigators recommend culturing epithelial cells obtained from the first brushing session to avoid repeat brushings9,15.
Although bronchoscopy is not the preferred choice for diagnosing PCD, if it is carried out for reasons other than PCD, samples can be alternatively obtained under anesthesia by brushing the bronchial epithelium or taking a biopsy13. Secondary infection may alter ciliary movement and beat frequency. To diminish these unwanted effects, it is recommended to administer a two-week course with a broad-spectrum oral antibiotic such as amoxicillin plus clavulanic acid or a cephalosporin to target common respiratory pathogens. Despite having substantial benefits in the treatment of PCD18, macrolides probably should be avoided for this purpose because they might alter CBF due to a prokinetic effect on beating cilia19. Further, regardless of the lack of evidence in the literature, antibiotics should be stopped at least 48 h before brushing and analysis to avoid any impact on CBF. No substantial impact of antibiotics could be observed on CBF or CBP in experiments where samples obtained by brushing or biopsy were cultivated in liquids containing antibiotics20,21,22.
Although HSVMA is considered the most accurate and reproducible technique in Europe, it bears the risk of operator error due to selection bias and the problems mentioned above15. Therefore, several groups have recently developed software solutions to automate analysis from digital images to overcome this problem23,24,25. Because this automation is still under development, the authors use two independent, expert operators to analyze CBF and CBP manually, achieving excellent and reliable results.
The representative results of this paper show video clips from patients with a mutation in the DNAH11 gene. Mutations in this gene show a normal ultrastructure, and patients with this mutation can therefore not be diagnosed by TEM. The normal ultrastructure of cilia demonstrated by TEM can be seen in up to 30% of all PCD cases6. Additionally, nNO might be normal with the hyperkinetic phenotype of this mutation (Video 5 and Video 6), making HSVMA, together with genetic testing, the only reliable diagnostic tool3,8. In addition, pediatric patients constitute the primary target for PCD diagnostics. In many cases, symptoms suggestive of PCD can be observed in the neonatal period26, rendering HSVMA a quick, first-line diagnostic measure preferable to other alternatives.
In a North American study, nNO has been examined and proposed as a first-line diagnostic screening test for PCD27. Although specificity and sensitivity were reported to be close to those of HSVMA (0.98/0.79), it must be noted that the youngest patient was 5.1 years old, and the mean age was even much higher. It must also be mentioned that several investigators have reported normal nNO values associated with PCD15. Therefore, while an improvement in the technical equipment to perform nNO in preschool-age subjects is still in progress, HSVMA remains the only reliable first-line diagnostic for PCD, and normal results rule out PCD with almost 100% certainty in all age groups.
However, to become an expert for diagnosing PCD using HSVMA, a high throughput of normal and pathologic samples is needed, which demands proper training and specialist equipment. This should be mandatory and will be rewarding for a clinic dealing with diagnostics and treatment of rare lung diseases. For any reason, if the processing of the samples constitutes an obstacle, a center with specialized personnel in PCD diagnostics using HSVMA can be used instead. In such cases, the treating physician can perform the brushing, and the sample can be sent over to the diagnosing center. In general, the samples should be analyzed as soon as possible after brushing. However, in our experience, if the samples are processed properly (see protocol step 1.2), epithelial cells remain vital and ready for analysis for at least 24 h after brushing20,22. Most centers that perform HSVMA themselves usually carry out the analysis within 4 h of sampling15. In any case, and before final analysis, the samples should be warmed up to body temperature to mimic optimal, in vivo conditions.
As described earlier in this paper, there are pitfalls in the diagnostic process of PCD using HSVMA, especially with inconclusive cases such as those that have been described in the representative results section (Video 7 and Video 8). For the final diagnosis of PCD, guidelines are available that show that sometimes a battery of different diagnostic measures is necessary9,10,15, and most importantly, that there is no single test or combination of tests that diagnose PCD with 100% certainty. Nevertheless, every unit involved in the diagnosis and treatment of PCD should be encouraged to use HSVMA as a tool in first-line diagnostics.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We wish to express special thanks to the pediatric nurse Mrs. Johanna Juvankoski for her excellent help with the brushings. We would also like to express special gratitude to Professor Heymut Omran (University Clinic Münster, UKM) for granting permission to use the schematic figure of normal ciliary motion from their website. Finally, we would like to thank Mr. Alan Brown BA (Hons), PGCE, for proofreading the manuscript.
Materiały
| Name | Company | Catalog Number | Comments |
| Amoxiciline-clavulanic acid | Orion Oyj | 40 mg/kg divided in 2 doses/day, for adults 875/125 mg 1 tablet x2/day | |
| Camera Software | Hamamatsu | HCI Image | |
| Cold pack | any | for preservation and transport | |
| Differential interference microscope | Carl Zeiss | Inverted, cell observer microscope | |
| Digitial High Speed Video Camera | Hamamatsu | Orca Flash 4.0, digital camera type C11440 | |
| Dulbecco´s Modified Eagle Medium | Thermo Fisher | 10565018 | basal cell culture medium |
| Eppendorf tube | Eppendorf | 30120086 | 1.5 mL tube |
| Glass-bottom microwell dish | MatTek | P35G-1.5-14-C | cuvette for microscopy |
| Heating Unit | Carl Zeiss/PeCon | 810-450001 | Carl Zeiss incubation elements with PeCon TempModule S1 temperature control |
| Interdental brush 0.6 mm | Doft | 872267 | Interdental brush on a long wire with a reusable handle and cap in zipbag |
| Objective | Carl Zeiss | 100x/1.46, α Plan-Apochromat DIC objective | |
| Small polystyrene box with lid | any | for transport |
Odniesienia
- Werner, C., Onnebrink, J. G., Omran, H. Diagnosis and management of primary ciliary dyskinesia. Cilia. 4 (1), 2 (2015).
- Mirra, V., Werner, C., Santamaria, F. Primary ciliary dyskinesia: An update on clinical aspects, genetics, diagnosis and future treatment strategies. Frontiers in Pediatrics. 5, 135 (2017).
- Schultz, R., Elenius, V., Lukkarinen, H., Saarela, T. Two novel mutations in the DNAH11 gene in primary ciliary dyskinesia (CILD7) with considerable variety in the clinical and beating cilia phenotype. BMC Medical Genetics. 21 (1), 237 (2020).
- Knowles, M. R., et al. Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax. 67 (5), 433-441 (2012).
- Boon, M., et al. Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet Journal of Rare Diseases. 9, 11 (2014).
- Kouis, P., et al. Prevalence of primary ciliary dyskinesia in consecutive referrals of suspected cases and the transmission electron microscopy detection rate: a systematic review and meta-analysis. Pediatric Research. 81 (3), 398-405 (2017).
- Marthin, J. K., Nielsen, K. G. Choice of nasal nitric oxide technique as first-line test for primary ciliary dyskinesia. European Respiratory Journal. 37 (3), 559-565 (2011).
- Jackson, C. L., Behan, L., Collins, S. A. Accuracy of diagnostic testing in primary ciliary dyskinesia. European Respiratory Journal. 47 (3), 837-848 (2016).
- Lucas, J. S., et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. European Respiratory Journal. 49 (1), 1601090 (2017).
- Shoemark, A., Dell, S., Shapiro, A., Lucas, J. S. ERS and ATS diagnostic guidelines for primary ciliary dyskinesia: similarities and differences in approach to diagnosis. European Respiratory Journal. 54 (3), 1901066 (2019).
- Kouis, P. Cost-effectiveness analysis of three algorithms for diagnosing primary ciliary dyskinesia: a simulation study. Orphanet Journal of Rare Diseases. 14 (1), 142 (2019).
- Rubbo, B., et al. Accuracy of high-speed video analysis to diagnose primary ciliary dyskinesia. Chest. 155 (5), 1008-1017 (2019).
- Friedman, N. R., Pachigolla, R., Deskin, R. W., Hawkins, H. K. Optimal technique to diagnose primary ciliary dyskinesia. The Laryngoscope. 110 (9), 1548-1551 (2000).
- Chilvers, M. A., Rutman, A., O´Callaghan, C. Functional analysis of cilia and ciliated epithelial ultrastructure in healthy children and young adults. Thorax. 58 (4), 333-338 (2003).
- Lucas, J. S., Paff, T., Goggin, P., Haarman, E. Diagnostic methods in primary ciliary dyskinesia. Paediatric Respiratory Reviews. 18, 8-17 (2016).
- Ballenger, J. J. Experimental effect of cigarette smoke on human respiratory cilia. New England Journal of Medicine. 263, 832-835 (1960).
- Stanley, P. J., Wilson, R., Greenstone, M. A., Mac William, L., Cole, P. J. Effect of cigarette smoking on nasal mucociliary clearance and ciliary beat frequency. Thorax. 41 (7), 519-523 (1986).
- Kobbernagel, H. E., et al. Efficacy and safety of azithromycin maintenance therapy in primary cilia dyskinesia (BESTCILIA): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respiratory Medicine. 8 (5), 493-505 (2020).
- Takeyama, K., Tamaoki, J., Chiyotani, A., Tagaya, E., Konno, K. Effect of macrolide antibiotics on ciliary motility in rabbit airway epithelium in-vitro. Journal of Pharmacy and Pharmacology. 45 (8), 756-758 (1993).
- Toskala, E., Haataja, J., Shirasaki, H., Rautliainen, M. Culture of cells harvested with nasal brushing: a method for evaluating ciliary function. Rhinology. 43 (2), 121-124 (2005).
- Pifferi, M., et al. Simplified cell culture method for the diagnosis of atypical primary ciliary dyskinesia. Thorax. 64 (12), 1077-1081 (2009).
- Hirst, R. A., et al. Culture of primary ciliary dyskinesia epithelial cells at air liquid interface can alter ciliary phenotype but remains a robust and informative, diagnostic aid. PLoS One. 9 (2), 89675 (2014).
- Papon, J. F., et al. Quantitative analysis of ciliary beating in primary ciliary dyskinesia: a pilot study. Orphanet Journal of Rare Diseases. 7 (10), 78 (2012).
- Smith, C. M., et al. Cilia FA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia. 1 (8), 14 (2012).
- Sampaio, P., et al. Ciliar Move: new software for evaluating ciliary beat frequency helps find novel mutations by a Portuguese multidisciplinary team on primary ciliary dyskinesia. European Respiratory Journal Open Research. 7 (1), 00792 (2021).
- Mullowney, T., et al. Primary ciliary dyskinesia and neonatal respiratory distress. Pediatrics. 134 (6), 1160-1166 (2014).
- Leigh, M. W., et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Annals of the American Thoracic Society. 10 (6), 574-581 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone