Method Article
A Simplified Model for Heterotopic Heart Valve Transplantation in Rodents
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This protocol describes a simple and efficient method for the transplantation of aortic valve leaflets under the renal capsule to allow for the study of alloreactivity of heart valves.
Streszczenie
There is an urgent clinical need for heart valve replacements that can grow in children. Heart valve transplantation is proposed as a new type of transplant with the potential to deliver durable heart valves capable of somatic growth with no requirement for anticoagulation. However, the immunobiology of heart valve transplants remains unexplored, highlighting the need for animal models to study this new type of transplant. Previous rat models for heterotopic aortic valve transplantation into the abdominal aorta have been described, though they are technically challenging and costly. For addressing this challenge, a renal subcapsular transplant model was developed in rodents as a practical and more straightforward method for studying heart valve transplant immunobiology. In this model, a single aortic valve leaflet is harvested and inserted into the renal subcapsular space. The kidney is easily accessible, and the transplanted tissue is securely contained in a subcapsular space that is well vascularized and can accommodate a variety of tissue sizes. Furthermore, because a single rat can provide three donor aortic leaflets and a single kidney can provide multiple sites for transplanted tissue, fewer rats are required for a given study. Here, the transplantation technique is described, providing a significant step forward in studying the transplant immunology of heart valve transplantation.
Wprowadzenie
Congenital heart defects are the most common congenital disability in humans, affecting 7 in 1,000 live-born children each year1. Unlike adult patients in which various mechanical and bioprosthetic valves are routinely implanted, pediatric patients currently have no good options for valve replacement. These conventional implants do not have the potential to grow in recipient children. As a result, morbid re-operations are required to exchange the heart valve implants for successively larger versions as the children grow, with affected kids often requiring up to five or more open-heart surgeries in their lifetime2,3. Studies have shown that freedom from intervention or death is significantly poor for infants than older children, with 60% of infants with prosthetic heart valves facing re-operation or death within 3 years of their initial operation4. Therefore, there is an urgent need to deliver a heart valve that can grow and maintain function in pediatric patients.
For decades, attempts to deliver growing heart valve replacements have been centered on tissue engineering and stem cells. However, attempts to translate these valves to the clinic have been unsuccessful thus far5,6,7,8. For addressing this, a heart valve transplantation is proposed as a more creative operation for delivering growing heart valve replacements having the ability to self-repair and avoid thrombogenesis. Instead of transplanting the whole heart, only the heart valve is transplanted and will then grow with the recipient child, similar to conventional heart transplants or a Ross pulmonary autograph9,10,11. Post-operatively, recipient children will receive immunosuppression until the transplanted valve can be exchanged for an adult-sized mechanical prosthetic when the growth of the valve is no longer required. However, the transplant biology of heart valve transplant grafts remains unexplored. Therefore, animal models are needed to study this new type of transplant.
Several rat models have been previously described for heterotopic transplantation of the aortic valve into the abdominal aorta12,13,14,15,16,17,18. However, these models are prohibitively tricky, often requiring trained surgeons to operate successfully. Additionally, they are costly and time-consuming19. A novel rat model was developed to create a simpler animal model for studying the immunobiology of heart valve transplants. Single aortic valve leaflets are excised and inserted into the renal subcapsular space. The kidney is especially suited to study transplant rejection as it is highly vascularized with access to circulating immune cells20,21. While several others have utilized a renal subcapsular model to study the transplant biology of other allograft transplants such as pancreas, liver, kidney, and cornea22,23,24,25,26,27, this is the first description of transplantation of cardiac tissue in this position. Here, the transplantation technique is described, providing a significant step forward in studying the transplant immunology of heart valve transplantation.
Protokół
The study was approved by the Committee of Animal Research following the National Institutes of Health Guide for Care and Use of Laboratory Animals.
1. Information on the animal model (Rats)
- Use an operating microscope (see Table of Materials) with up to 20x magnification for all surgical procedures.
- Use syngeneic (such as Lewis-Lewis) or allogeneic (such as Lewis-Brown Norway) strains for the transplants as needed for the experiment.
- Use rats of age between 5-7 weeks and bodyweight of 100-200 g that are appropriate for the experimental question.
2. Removal of fur, preparation of the skin, and anesthesia
- Perform all operations under sterile conditions.
NOTE: The step is performed in a dedicated surgical space and under sterile conditions. - Place the rats into an anesthetic induction chamber and induce anesthesia with 5% isoflurane in oxygen. Maintain anesthesia with 3.5% isoflurane in oxygen throughout the procedure.
- For the donor operation, remove the rat's fur from the umbilicus to the sternal notch using fur clippers. For the recipient operation, clip the hair over the surgical field at the posterior axillary line from the ribs to the pelvis. Next, prepare the skin with a surgical disinfectant.
- Obtain a surgical plane of anesthesia before starting the procedure. Confirm adequate depth of anesthesia by firmly compressing the toes of the rat with forceps. If the rat withdraws to pain, titrate the anesthetic as needed.
- Monitor the respiratory rate and the depth of anesthesia clinically throughout the procedure; the level of isoflurane is adjusted as needed to maintain a breathing rate of 55-65 breaths/min.
3. Donor operation
- Prepare and anesthetize the rat as stated in step 2. Incise the skin from the xiphoid to the sternal notch using dissecting scissors. Perform a sternectomy by cutting the ribs on each side lateral to the sternum until optimal access to the heart is achieved.
- Heparinize the rat with a 100 U/100 g of injection into the left atrium.
- Sacrifice the donor via exsanguination.
- Excise the thymus to improve the visualization of the great vessels. Then, remove the heart en bloc with the ascending aorta until the level of the innominate artery.
4. Preparation of aortic valve leaflets
- Place the donor heart in a sterile Petri dish immediately following the cardiectomy. Dissect the donor heart in an ice-cold cold storage buffer (see Table of Materials).
- Using forceps and Vannas spring scissors, dissect the donor heart until only the aortic root remains with a 1 mm ventricular cuff proximal to the aortic valve.
- Open the aortic valve by making a longitudinal cut to open the Sinus of Valsalva between the left and non-coronary sinuses to visualize all three leaflets.
NOTE: The cut should be the entire length of the Sinus of Valsalva. The actual dimensions depend on the size of the rat. - Excise each aortic valve leaflet individually. Specifically, use blunt forceps to grasp the edge of the leaflet and use Vannas spring scissors to excise the leaflet by cutting from one commissure down to the annulus, and then toward the next commissure.
NOTE: Take special care to only grasp the edge of the leaflet to minimize disruption of the valvular endothelial cells. - Store the samples following leaflet excision in ice-cold storage buffer solution until they are ready to be implanted in the recipient rat. Implant all the leaflets within 4 h of cold storage.
5. Recipient operation
- Prepare and anesthetize the rat as stated in step 2. Use a heating pad maintained at 36-38 °C to perform the surgery.
- Administer buprenorphine (0.03 mg/kg subcutaneously) to all recipient rats before surgery and every 6-12 h post-operatively as needed to alleviate the pain.
- Place the rat in a right lateral recumbent position to access the left kidney.
NOTE: The left kidney is preferred due to its more caudal position relative to the right kidney. - Incise the skin over the flank longitudinally over 1-inch using scissors.
NOTE: The incision must remain smaller than the size of the kidney to provide enough tension to prevent the kidney from retracting back into the abdominal cavity during the procedure. - Similarly, incise the underlying abdominal wall.
- Externalize the kidney
- Using the thumb and forefinger, apply light pressure dorsally and ventrally while using curved forceps to lift the caudal pole of the kidney through the abdominal and skin incision. Externalize the cranial end of the kidney similarly.
- Alternatively, the kidney may be externalized by grasping the perirenal fat and pulling upward with light tension.
NOTE: Take care not to grasp the kidney or the renal vessels directly. - Once the kidney is externalized, keep it moist with warm saline trickled onto the kidney.
- Create a subcapsular pocket.
- Lightly apply pressure to the renal capsule using one set of blunt forceps so that the renal capsule can be clearly distinguished from the underlying parenchyma. Simultaneously using another set of blunt forceps, carefully grasp the capsule and gently pull upward to create a hole in the capsule.
NOTE: Due to the delicate nature of the capsule, minimal force is required to establish this incision. - Continue using blunt forceps to extend the incision until a ~2mm space has been created to accommodate the aortic valve leaflet.
- Develop a shallow subcapsular pocket that is slightly larger than the valve leaflet while lifting the edge of the incision with one set of forceps and advancing a blunt probe under the renal capsule.
- Lightly apply pressure to the renal capsule using one set of blunt forceps so that the renal capsule can be clearly distinguished from the underlying parenchyma. Simultaneously using another set of blunt forceps, carefully grasp the capsule and gently pull upward to create a hole in the capsule.
- Transplant the aortic valve into the subcapsular pocket.
- Retrieve the aortic leaflet from cold storage and place it in the surgical field.
- While lifting the edge fibrous capsule, advance the aortic leaflet into the subcapsular pocket with blunt forceps.
NOTE: Ensure the tissue is far enough away from the incision so that it is firmly secured under the capsule. Care should be taken to avoid damage to the underlying parenchyma or further ripping of the fibrous capsule. - The incision in the renal capsule can be left open.
- Push the kidney gently back to its anatomical position using counter traction applied to the incision edges.
- Close the abdominal incision with a running sterile surgical suture. Close the skin with staples.
- Post-operative care
- Following the operation, place the rat in a clean cage on a heating pad with access to food and water.
- Monitor the animal daily to assess for routine wound healing and signs of pain or distress. Remove the staples after 7-10 days.
6. Collection of tissue for analysis
- At selected endpoints after transplantation, euthanize the animal by exsanguination. Specifically, perform a median laparotomy and transect the abdominal aorta under 5% isoflurane in oxygen.
- Mobilize the kidney and excise it by cutting the renal artery, vein, and ureter with scissors.
NOTE: Take care not to grasp the area containing the transplanted leaflet. - Place the kidney in formalin overnight, embed it in paraffin, and section it for the desired staining. Orient the specimen with the kidney capsule facing anteriorly and the kidney parenchyma facing posteriorly.
Wyniki
A graphical depiction of the experimental design is provided for the rat model (Figure 1). Additionally, an aortic root dissected from the donor's heart and an individual aortic valve leaflet prepared for implantation is also shown in Figure 2. Next, a representative image of the position of the aortic valve leaflet under the renal capsule for implantation is shown in Figure 3A and after 3, 7, and 28 days within the recipient rat (Figures 3B-D), demonstrating the ease of locating and recovering the transplanted tissue.
The aortic valve leaflets retain their native architecture after heterotopic transplantation in syngeneic animals, demonstrating the utility of this model as a baseline to compare the immune response in allogeneic transplants. Specifically, histology with hematoxylin and eosin (H&E) staining revealed that valve leaflets in syngeneic transplants after 7 days were structurally intact with no signs of edematous swelling (Figure 4A). The structural integrity of the valve leaflet was further confirmed by immunohistochemistry for Alpha Smooth Muscle Actin (aSMA) and CD31 (Figure 4B).
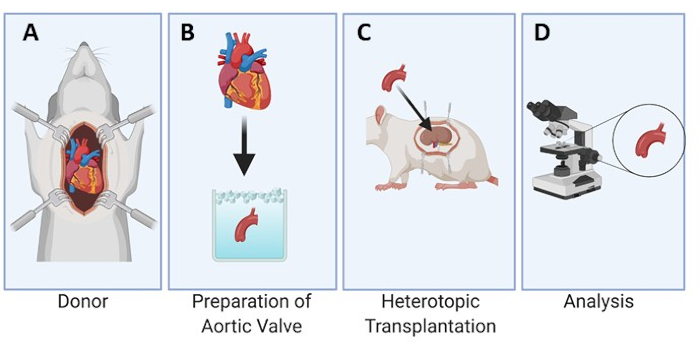
Figure 1: Experimental design of the heterotopic transplantation of the aortic valve under the renal capsule in rats. The heart is collected from the donor rat (A). The aortic valve leaflets are dissected and kept in cold storage (B) until the implantation process under the renal capsule in the recipient rat (C). The leaflets are then explanted at set time points and analyzed microscopically (D). Please click here to view a larger version of this figure.

Figure 2: Preparation of aortic valve leaflet for implantation. Example of an aortic root dissected from the donor heart (A) and further dissection of an aortic valve leaflet for implantation (B). Please click here to view a larger version of this figure.

Figure 3: Visualization of aortic valve leaflet under the renal capsule. The aortic valve leaflet is visualized under the renal capsule at implantation (A), after 3 (B), 7 (C), and 28 days (D) in syngeneic animals and after 3 (E), 7 (F), and 28 days (G) in allogeneic animals. Please click here to view a larger version of this figure.

Figure 4: Aortic valve leaflets remain structurally intact after transplantation under the renal capsule for 7 days in syngeneic animals. The top row shows H&E staining and immunostaining for DAPI, aSMA, and CD31 for control heart valves that were procured but not transplanted. The bottom row shows H&E staining and immunostaining for DAPI, aSMA, and CD31 in a syngeneic valve leaflet explanted after 7 days. Please click here to view a larger version of this figure.
Dyskusje
Importance and potential applications
While mechanical and bioprosthetic heart valves are routinely used in adult patients requiring valve replacement, these valves lack the potential to grow and, therefore, are suboptimal for pediatric patients. Heart valve transplantation is an experimental operation designed to deliver growing heart valve replacements for neonates and infants with congenital heart disease. However, unlike the transplant immunobiology of conventional heart transplants, the transplant immunobiology of this new type of transplant remains poorly explored. Here, a unique rat model for subcapsular renal transplantation of aortic valve leaflets is described, providing a significant step forward in studying the transplant immunobiology of heart valve transplantation.
The renal subcapsular space provides an optimal environment to study transplant immunobiology of heart valves. The transplanted tissue is securely contained in a well-vascularized location with access to circulating immune cells20. Additionally, subcapsular models have previously been successfully utilized to test allograft rejection in many tissues such as the pancreas, liver, kidney, and the other cell types22,23,24,25,26,27, indicating this model is justified in studying the immunogenicity of aortic valve leaflets.
This model has several protentional applications for studying the transplant immunology of aortic valves. First, the model may be used to determine the level of systemic immune suppression required for heart valve transplantation to prevent graft rejection, such as tacrolimus, mycophenolate, and steroids. Furthermore, several studies have indicated that valve tissue may be immunologically distinct from other heart tissue, as the valves are relatively spared during fulminant rejection of conventional heart transplants28,29,30. This model allows for exploration of this concept as the subcapsular space can accommodate various tissue types, such as valve leaflet and myocardium, to compare the immunogenicity of these tissues.
This model is advantageous because it is technically straightforward, quick, and has a high survival rate with a low risk of complications. Because each donor can provide three aortic valve leaflets, one rat can serve as the donor for three different recipients. On average, the length of the donor operation was 27.2 min (n = 12), and the duration of the recipient operation was 29.7 min (n = 36). The survival rate of the recipient operation was 97.2% (n = 35/36), with one intraoperative death due to respiratory depression. Minimal bleeding due to trauma to the renal parenchyma while creating the subcapsular pocket was noted in 11.1% of recipient operations. However, the bleeding was easily controlled in all cases with compression from a cotton tip applicator. One sample was dislodged from the subcapsular space and not recovered upon explanation even after 7 days.
Previously, the valve leaflets were explanted by removing them from the subcapsular space and embedded, sectioned, and stained without any attached aortic tissue. However, this method is suboptimal as the leaflets themselves are extremely small, thin, and transparent, resulting in the loss of several samples in processing. Instead, it is recommended to remove the kidney en bloc and embed and section the tissue while still secured under the renal capsule to ensure no samples are lost. Additionally, this approach minimizes the trauma and manipulation of the leaflet.
Critical steps
The critical steps of the procedure are to establish a surgical plane of anesthesia, incising the abdominal wall over the kidneys, eviscerating the kidney, raising the subcapsular flap, insertion of the heterotopic transplant tissue, obtaining hemostasis, returning the kidney to the anatomical position, and closing the skin.
Modifications and troubleshooting
While this is the first description of transplantation of heart tissue under the renal capsule, several others have described transplantation of other tissue types in the renal subcapsular space20,22,23,24,25,26,27. In this protocol, minor adjustments were made to previous subcapsular models to optimize the technique and minimize complications. Specifically, while others have recommended using Vannas spring scissors to make the initial incision into the renal capsule20,26, this method is more likely to cause trauma to the underlying parenchyma and result in subcapsular hematoma formation. Too much bleeding will result in distention of the capsule and compromise the security of the transplanted26. Therefore, blunt forceps should be used to open the capsule. Additionally, while some protocols advocate for the placement of commercial products with homostatic property over the capsular incision26,31, this step is unnecessary as long as the tissue is advanced far enough into the subcapsular pocket.
In larger rats, the kidney may be covered in perirenal fat, and externalizing the kidney via lifting with curved forceps may not be feasible. In these cases, it is best to externalize the kidney by gently tugging the perirenal fat with forceps and pulling the kidney out of the abdominal cavity without causing damage or bleeding.
Comparison with existing heterotopic transplant models
While several other animal models for heterotopic aortic valve transplantation have been previously described12,13,14,15,16,17,18, the current protocol provides a straightforward and more practical alternative that improves previous models in several ways. First, due to the technically simple nature of the procedure, very little training is required to operate successfully. This is in stark contrast to previously described heterotopic aortic valve transplants into the abdominal aorta. Therefore, this model provides a more practical and cost-effective alternative to study aortic valve transplantation while minimizing the morbidity, pain, and mortality of the rats. Additionally, because only one aortic valve leaflet is needed for the recipient operation and each donor rat provides three leaflets, fewer donor rats are required for any given experiment. Furthermore, implanting tissue into the contralateral kidney or a separate subcapsular pocket may allow for internal control or comparison of immune responses to varying tissues within a single rat. In this case, the best approach is via a midline laparotomy incision.
In addition to the animal models describing heterotopic aortic valve transplantation into the abdominal aorta, other studies have utilized a subcutaneous model to study the immunogenicity of aortic valves32. While this approach is undoubtedly more straightforward than transplantation into the abdominal aorta, existing evidence suggests that subcutaneous implantation is a less effective method of antigen presentation33,34. The implanted specimen is also challenging to find and analyze. Therefore, the renal subcapsular space is proposed as a site of implantation that is both simplified yet optimal for studying aortic valve transplant biology.
In summary, the newly proposed model serves as an addition to scientists' armamentarium to study heart valve transplantation and supplements the previously described models.
Limitations
Although the transplantation of aortic valve leaflets under the renal capsule is an efficient method for studying alloimmunity in vivo, some limitations of this model exist. While the subcapsular space is well-vascularized, it does not offer the same hemodynamic environment as the sub-coronary position. This may affect the immune response to transplanted tissue. Some have hypothesized that the distinct immune properties observed in valve tissue may result from the high-pressure blood flow over the aortic valve in the sub-coronary position, nullifying the chemotactic response28,35. Furthermore, this model is insufficient to study the effect of alloreactivity on the valve function as the leaflets are not performing their physiologic function under the renal capsule. However, similar limitations exist for the heterotopic abdominal aorta transplant models as the success of these models relies on rendering the valve leaflets incompetent to avoid graft thrombosis15,36.
Limitations to the protocol include the possibility of tissue becoming dislodged from the renal subcapsular space and un-recoverable (1 in 36 animals). Another limitation is the death of the animal during surgery (1 in 36 animals); however, the death was caused by the overdose of buprenorphine, and other methods for dosing of analgesia may be employed.
Ujawnienia
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Podziękowania
Figure 1 was created with biorender.com. This work was supported in part by the AATS Foundation Surgical Investigator Program to TKR, the Children's Excellence Fund held by the Department of Pediatrics at the Medical University of South Carolina to TKR, an Emerson Rose Heart Foundation grant to TKR, Philanthropy by Senator Paul Campbell to TKR, NIH-NHLBI Institutional Postdoctoral Training Grants (T32 HL-007260) to JHK and BG, and the Medical University of South Carolina College of Medicine Pre-clerkship FLEX Research Fund to MAH.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.9% Sodium Chlordie, USP | Baxter | NDC 0338-0048-04 | |
| 4-0 Polyglactin 910 | Ethicon | J415H | |
| 7.5% Povidone-Iodine | CareFusion | 29904-004 | |
| 70% ETOH | Fisher Scientific | BP82031GAL | |
| Anesthesia induction chamber | Harvard Apparatus | 75-2030 | Air-tight inducton chamber for rats |
| Anesthesia machine | Harvard Apparatus | 75-0238 | Mobile Anesthesia System with Passive Scavenging |
| Anesthesia Mask | Harvard Apparatus | 59-8255 | Rat anesthesia mask |
| Brown Norway Rats (BN/Crl) | Charles River | Strain Code 091 | Male, 5-7 weeks, 100-200 g |
| Buprenorphine Hydrochloride, 0.3 mg/mL | PAR Pharmaceutical | NDC 42023-179-05 | 0.03 mg/kg, administered subcutaneously |
| Electric hair clippers | WAHL | 79434 | |
| Electric Heating Pad | Harvard Apparatus | 72-0492 | Maintained at 36-38 °C |
| Heparin | Sagent Pharmaceuticals | NDC 25021-400-10 | 100U/100g injection into the left atrium |
| Insulin Syringe, 1 mL | Fisher Scientific | 14-841-33 | |
| Iris forceps curved | World Precision Instruments | 15917 | |
| Iris forceps straight | World Precision Instruments | 15916 | |
| Isoflurane, USP | Piramal Critical Care | NDC 66794-017-25 | Induced at 5% isoflurance in oxygen and maintained with 3.5% isoflurane in oxygen |
| Lewis Rats (LEW/ Crl) | Charles River | Strain Code 004 | Male, 5-7 weeks, 100-200 g |
| Micro forceps | World Precision Instruments | 500233 | Dumont #5 |
| Micro scissors | World Precision Instruments | 501930 | Spring-loaded Vannas Scissors |
| Needle Driver | World Precision Instruments | 500226 | Ryder Needle Driver |
| Operating microscope | AmScope | SM-3BZ-80S | 3.5x - 90x Stereo Microscope |
| Petri Dish | Fisher Scientific | FB0875714 | |
| Petrolatum ophthalmic ointment | Dechra | NDC 17033-211-38 | |
| Skin staples | Ethicon | PXR35 | Proximate 35 |
| Sterile cotton swabs | Puritan | 25-806 1WC | |
| Sterile gauze sponges | Fisher Scientific | 22-037-902 | |
| Surgical Scissors | World Precision Instruments | 1962C | Metzenbaum Scissors |
| University of Wisconsin Buffer (Servator B) | S.A.L.F S.p.A. | 6484A1 | Stored at 4 °C |
Odniesienia
- Van Der Linde, D., et al. Birth prevalence of congenital heart disease worldwide: A systematic review and meta-analysis. Journal of the American College of Cardiology. 58 (21), 2241-2247 (2011).
- Jacobs, J. P., et al. Reoperations for pediatric and congenital heart disease: An analysis of the Society of Thoracic Surgeons (STS) congenital heart surgery database. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 17 (1), 2-8 (2014).
- Syedain, Z. H., et al. Pediatric tri-tube valved conduits made from fibroblast-produced extracellular matrix evaluated over 52 weeks in growing lambs. Science Translational Medicine. 13 (585), 1-16 (2021).
- Khan, M. S., Samayoa, A. X., Chen, D. W., Petit, C. J., Fraser, C. D. Contemporary experience with surgical treatment of aortic valve disease in children. Journal of Thoracic and Cardiovascular Surgery. 146 (3), 512-521 (2013).
- Boyd, R., Parisi, F., Kalfa, D. State of the art: Tissue engineering in congenital heart surgery. Seminars in Thoracic and Cardiovascular Surgery. 31 (4), 807-817 (2019).
- Feins, E. N., Emani, S. M. Expandable valves, annuloplasty rings, shunts, and bands for growing children. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 23, 17-23 (2020).
- Lintas, V., et al. TCT-795 Human cell derived off-the-shelf tissue engineered heart valves for next generation transcatheter aortic valve replacement: a proof-of-concept study in adult sheep. Journal of the American College of Cardiology. 70 (18), 271 (2017).
- Blum, K. M., Drews, J. D., Breuer, C. K. Tissue-engineered heart valves: A call for mechanistic studies. Tissue Engineering Part B: Reviews. 24 (3), 240-253 (2018).
- Bernstein, D., et al. Cardiac growth after pediatric heart transplantation. Circulation. 85 (4), 1433-1439 (1992).
- Delmo Walter, E. M., et al. Adaptive growth and remodeling of transplanted hearts in children. Europeon Journal of Cardiothoracic Surgery. 40 (6), 1374-1383 (2011).
- Simon, P., et al. Growth of the pulmonary autograft after the Ross operation in childhood. Europeon Journal of Cardiothoracic Surgery. 19 (2), 118-121 (2001).
- Oei, F. B. S., et al. A size-matching heterotopic aortic valve implantation model in the rat. Journal of Surgical Research. 87 (2), 239-244 (1999).
- Oei, F. B. S., et al. Heart valve dysfunction resulting from cellular rejection in a novel heterotopic transplantation rat model. Transplant International. 13, (2000).
- El Khatib, H., Lupinetti, F. M. Antigenicity of fresh and cryopreserved rat valve allografts. Transplantation. 49 (4), 765-767 (1990).
- Yankah, A. C., Wottge, H. U. Allograft conduit wall calcification in a model of chronic arterial graft rejection. Journal of Cardiac Surgery. 12 (2), 86-92 (1997).
- Moustapha, A., et al. Aortic valve grafts in the rat: Evidence for rejection. Journal of Thoracic and Cardiovascualr Surgery. 114 (6), 891-902 (1997).
- Légaré, J. F., et al. Prevention of allograft heart valve failure in a rat model. Journal of Thoracic and Cardiovascualr Surgery. 122 (2), 310-317 (2001).
- Legare, J. F., Lee, T. D. G., Creaser, K., Ross, D. B., Green, M. T lymphocytes mediate leaflet destruction and allograft aortic valve failure in rats. The Annals of Thoracic Surgery. 70 (4), 1238-1245 (2000).
- Niimi, M. The technique for heterotopic cardiac transplantation in mice: Experience of 3000 operations by one surgeon. Journal of Heart and Lung Transplantation. 20 (10), 1123-1128 (2001).
- Burgin, M., et al. Kidney Subcapsular Allograft Transplants as a Model to Test Virus-Derived Chemokine-Modulating Proteins as Therapeutics. Methods in molecular biology. 2225, 257-273 (2021).
- Foglia, R. P., DiPreta, J., Donahoe, P. K., Statter, M. B. Fetal allograft survival in immunocompetent recipients is age dependent and organ specific. Annals of Surgery. 204 (4), 402-410 (1986).
- Cunha, G. R., Baskin, L. Use of sub-renal capsule transplantation in developmental biology. Differentiation. 91 (4-5), 4-9 (2016).
- Hori, J., Joyce, N., Streilein, J. W. Epithelium-deficient corneal allografts display immune privilege beneath the kidney capsule. Investigative Opthalmology & Visual Science. 41 (2), 443-452 (2000).
- Mandel, T., et al. transplantation of organ cultured fetal pig pancreas in non-obese diabetic (NOD) mice and primates (Macaca fascicularis). Xenotransplantation. 2 (3), 128-132 (1995).
- Ricordi, C., Flye, M. W., Lacy, P. E. Renal subcapsular transplantation of clusters of hepatocytes in conjunction with pancreatic islets. Transplantation. 45 (6), 1148-1150 (1988).
- Shultz, L. D., et al. Subcapsular transplantation of tissue in the kidney. Cold Spring Harbor Protocols. 2014 (7), 737-740 (2014).
- Vanden Berg, C. W., et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 10 (3), 751-765 (2018).
- Mitchell, R. N., Jonas, R. A., Schoen, F. J. Pathology of explanted cryopreserved allograft heart valves: Comparison with aortic valves from orthotopic heart transplants. Journal of Thoracic and Cardiovasular Surgery. 115 (1), 118-127 (1998).
- Valante, M., et al. The aortic valve after heart transplantation. Annals of Thoracic Surgery. 60, (1995).
- O'Brien, M. F., Stafford, E. G., Gardner, M. A. H., Pohlner, P. G., McGiffin, D. C. A comparison of aortic valve replacement with viable cryopreserved and fresh allograft valves, with a note on chromosomal studies. Journal of Thoracic and Cardiovascular Surgery. 94 (6), 812-823 (1987).
- Ng, T. F., Osawa, H., Hori, J., Young, M. J., Streilein, J. W. Allogeneic neonatal neuronal retina grafts display partial immune privilege in the subcapsular space of the kidney. The Journal of Immunology. 169 (10), 5601-5606 (2002).
- Heslop, B. F., Wilson, S. E., Hardy, B. E. Antigenicity of aortic valve allografts. Annals of Surgery. 177 (3), 301-306 (1973).
- Steinmuller, D., Weiner, L. J. Evocation and persistence of transplantation immunity in rats. Transplantation. 1 (1), 97-106 (1963).
- Billingham, R. E., Brent, L., Brown, J. B., Medawar, P. B. Time of onset and duration of transplantation immunity. Plastic and Reconstructive Surgery. 24 (1), 410-413 (1959).
- Tector, A. J., Boyd, W. C., Korns, M. E. Aortic valve allograft rejection. Journal of Thoracic and Cardiovascular Surgery. 62 (4), 592-601 (1971).
- Sugimura, Y., Schmidt, A. K., Lichtenberg, A., Akhyari, P., Assmann, A. A rat model for the in vivo assessment of biological and tissue-engineered valvular and vascular grafts. Tissue Engineering Methods (Part C). 23 (12), 982-994 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone