Method Article
Assessing Cell Viability and Death in 3D Spheroid Cultures of Cancer Cells
W tym Artykule
Podsumowanie
Here, we present several simple methods for evaluating viability and death in 3D cancer cell spheroids, which mimic the physico-chemical gradients of in vivo tumors much better than the 2D culture. The spheroid model, therefore, allows evaluation of the cancer drug efficacy with improved translation to in vivo conditions.
Streszczenie
Three-dimensional spheroids of cancer cells are important tools for both cancer drug screens and for gaining mechanistic insight into cancer cell biology. The power of this preparation lies in its ability to mimic many aspects of the in vivo conditions of tumors while being fast, cheap, and versatile enough to allow relatively high-throughput screening. The spheroid culture conditions can recapitulate the physico-chemical gradients in a tumor, including the increasing extracellular acidity, increased lactate, and decreasing glucose and oxygen availability, from the spheroid periphery to its core. Also, the mechanical properties and cell-cell interactions of in vivo tumors are in part mimicked by this model. The specific properties and consequently the optimal growth conditions, of 3D spheroids, differ widely between different types of cancer cells. Furthermore, the assessment of cell viability and death in 3D spheroids requires methods that differ in part from those employed for 2D cultures. Here we describe several protocols for preparing 3D spheroids of cancer cells, and for using such cultures to assess cell viability and death in the context of evaluating the efficacy of anticancer drugs.
Wprowadzenie
The use of multicellular spheroid models in cancer biology is several decades old1,2, but has gained substantial momentum in recent years. In large part, this reflects increased awareness of how strongly the phenotype of cancer cells is dependent on their microenvironment and specific growth conditions. The microenvironment in solid tumors is fundamentally different from that in corresponding normal tissues. This includes physico-chemical conditions such as pH, oxygen tension, as well as interstitial pressure, concentration gradients of soluble factors such as nutrients, waste products, and secreted signaling compounds (growth factors, cytokines). Furthermore, it includes the organization of the extracellular matrix (ECM), cell-cell interactions and intercellular signaling, and other aspects of the particular three-dimensional (3D) architecture of the tumor3,4,5,6. The specific microenvironmental conditions in which cancer cells exist, profoundly affect their gene expression profile and functional properties, and it is clear that, compared to that of cells grown in 2D, the phenotype of 3D spheroids much more closely mimics that of in vivo tumors7,8,9,10,11. 2D models, even if they employ hypoxia, acidic pH, and high lactate concentrations to mimic known aspects of the tumor microenvironment, still fail to capture the gradients of physico-chemical parameters arising within tumors, as well as their 3D tumor architecture. On the other hand, animal models are costly, slow, and ethically problematic, and generally, also have shortcomings in their ability to recapitulate human tumor conditions. Consequently, 3D spheroids have been applied as an intermediate complexity model in studies of a wide range of properties of most solid cancers9,11,12,13,14,15,16,17.
A widely employed use of 3D spheroids is in screening assays of anticancer therapy efficacy9,18,19,20. Treatment responses are particularly sensitive to the tumor microenvironment, reflecting both the impact of the tortuosity, restricted diffusion, high interstitial pressure, and acidic environmental pH on drug delivery, and the impact of hypoxia and other aspects of the microenvironment on the cell death response9,17. Because the environment within 3D spheroids inherently develops all of these properties7,8,9,10,11, employing 3D cell cultures can substantially improve the translation of results to in vivo conditions, yet allow efficient and affordable high-throughput screening of the net growth. However, the great majority of studies on the drug response of cancer cells are still carried out under 2D conditions. This likely reflects that, while some assays can relatively easily be implemented for 3D cell cultures, many, such as viability assays, western blotting, and immunofluorescence analysis, are much more conveniently done in 2D than in 3D.
The aim of the present work is to provide easily amenable assays and precise protocols for analyses of the effect of treatment with anti-cancer drugs on cancer cell viability and survival in a 3D tumor mimicking setting. Specifically, we provide and compare three different methods for spheroid formation, followed by methods for qualitative and quantitative analyses of growth, viability and drug response.
Protokół
1. Generation of Spheroids
- Preparing cell suspensions for spheroid formation
NOTE: Different cell lines have very different adhesion properties and the most suitable spheroid formation protocol must be established in each case. We have found that MCF-7 and BxPC-3 cells are suitable for spontaneous spheroid formation, while MDA-MB-231, SKBr-3, Panc-1 and MiaPaCa require the addition of reconstituted basement membrane to successfully form spheroids. Only MDA-MB-231 and BxPC-3 cells have been employed for the hanging drop protocol, however other cell lines are certainly applicable.- Grow cells as monolayer until 70-80% confluency.
- Wash cells with phosphate buffered saline (1x PBS, 5 mL for a 25 cm2 or 10 mL for a 75 cm2 flask), add the cell dissociation enzyme (0.5 mL for a 25 cm2 or 1 mL for a 75 cm2 flask) and incubate the cells for 2-5 min at 37 °C in 5% CO2 and 95% humidity.
- Check the cell detachment under a microscope and neutralize the cell dissociation enzyme by adding growth medium (6-10% serum depending on the cell line) to a total volume of 5 mL in a 25 cm2 or 10 mL for a 75 cm2 flask.
- Use a Bürker chamber to count cells and count 8 squares in the chamber per cell preparation to obtain a high reproducibility of the size of the spheroids.
NOTE: Three protocols each describing a different method for spheroid formation are presented below. Protocol 1.2 and 1.3 can be used for all the subsequent analytic protocols presented, whereas protocol 1.4 is best suited for embedding and lysate preparations. Depending on the cell line, spheroid formation takes 2-4 days, irrespective of the method used.
- Spontaneous spheroid formation
- Perform steps 1.1.1-1.1.4.
- Dilute the cell suspension in a 15 mL tube to obtain 0.5-2 x 104 cells/mL (optimal cell density needs to be determined for each cell line) (Figure 1A (ii)).
- Fill the outer ring of wells with 1x PBS or growth medium to reduce evaporation from the remaining wells. Transfer the cell suspension to a sterile reservoir and, using a multichannel pipette, dispense 200 µL/well into ultra-low attachment 96-well round bottom plates (Figure 1A (iii)).
- Incubate the plate in an incubator at 37 °C with 5% CO2, 95% humidity.
- Every 2-3 days acquire light microscopic images of the spheroids.
NOTE: The images in this paper are taken at 11.5x magnification, which is appropriate for most spheroids prepared using these protocols. - Every 2-3 days (after acquiring images) replace 100 µL of medium (remove 100 µL of the spent medium and replace with 100 µL of fresh medium.
NOTE: To avoid removing spheroids when replacing medium, it is advisable to tilt the plate a bit while slowly removing the medium and inspect the aspirated medium in the tips for visible spheroids before discarding it.
- Reconstituted basement membrane-mediated spheroid formation.
NOTE: Lactose dehydrogenase elevating virus (LDEV)-free reduced growth factor reconstituted basement membrane (rBM) was used. rBM is temperature-sensitive and should always be kept on ice, as it will clot if it reaches 15 °C. Thaw the rBM on ice either overnight at 4 °C or 2-4 h at room temperature (RT) before plating.- Thaw rBM on ice (see Table of Materials).
- Keep plates and reservoirs (if individually wrapped) on ice before use.
- Perform steps 1.1.1-1.1.4.
- Fill the outer ring of wells with 1x PBS or growth medium to reduce evaporation from the remaining wells. Dilute the cell suspension in a 15 mL tube to obtain 0.5-2 x 104 cells/mL (optimal cell density needs to be determined for each cell line) (Figure 1A (ii)).
- Place the 15 mL tube containing the diluted cell suspension on ice (e.g., in glass beaker) (Figure 1A (iia)).
- Transfer the chilled plates and reservoirs to the hood. Rinse plastic containers, fill them with ice and transfer them into the hood to allow the plates and reservoirs to be placed on ice during the entire procedure.
- Resuspend rBM gently to ensure a homogenous gel.
- Add 1-2% rBM (optimal concentration needs to be determined for each cell line) to the chilled cell suspensions (Figure 1A (iib)).
- Invert the 15 mL tube to ensure the proper mixing of rBM and cell suspension before dispensing the suspension into the plate.
- Transfer the rBM-containing cell suspension to a sterile reservoir and dispense 200 µL/well into chilled ultra-low attachment 96-well plates using a multichannel pipette (Figure 1A (iii)).
NOTE: If working with several cell suspensions (e.g., more than one cell line), it is essential to dispense each cell suspension immediately after rBM addition to prevent premature gelling. - Centrifuge the plate for 15 min at 750 x g using 'soft decent'/no braking (if possible, centrifuge at 4 °C to keep the rBM fluid longer but not a requirement for successful spheroid formation), to ensure that the cells are clustered together when the rBM hardens, facilitating the formation of one single spheroid.
- Incubate the plate in an incubator (37 °C, 5% CO2, 95% humidity).
- Every 2-3 days acquire light microscopic images for evaluation of spheroid growth.
- Every 2-3 days replace 100 µL of medium (remove 100 µL and replace with 100 µL of fresh medium).
- Hanging drop spheroids.
- Perform step 1.1.1-1.1.4.
- Dilute cells to obtain a suitable dilution. A practical dilution is 50,000 cells/mL.
- Remove the lid of a 10 cm2 cell culture dish and place it so it faces upwards. Add 6 mL of 1x PBS to the dish (Figure 1B (i)).
- Pour the cell suspension into a sterile reservoir and carefully place up to 30 drops of 40 µL of cell suspension onto the lid of the cell culture dish using a multichannel pipette (Figure 1B (ii)), resulting in a concentration of 2,000 cells/drop. Avoid placing the drops too close to the edge of the lid as these drops are more likely to lose surface tension when inverting the lid in the following step.
- Invert the lid in a quick but controlled movement and place it on top of the 1x PBS-containing cell culture dish (Figure 1B (iii)).
- Place the dish in an incubator at 37 °C with 5% CO2 and 95% humidity without disturbing the drops and leave them to grow for 4-6 days.
- If to be used for protein lysates or embedding, pool spheroids by removing the lid and tilt it, in order to wash down the drops with 1 mL of heated medium. Transfer the resulting medium containing spheroids to a 1.5 mL tube and allow them to settle to the bottom of the tube. Proceed as described in 4.4 and 6.2.2 for protein lysates and embedding, respectively.
2. Drug Treatment of Spheroids
NOTE: Long-term drug treatment can be applied to the spheroids in order to screen for effects of a drug of interest. Before initiating the drug treatment, it is advisable to perform a dose response experiment of the drug(s), in order to find an appropriate dose for the experimental treatment. The doses should be based on the determined IC50/Ki of the drug and range from around 0.2x-10x of this value.
- Set up 6-12 spheroids per the desired condition as described in 1.2 or 1.3 and place in the incubator (37 °C, 5% CO2, 95% humidity) for 2 days.
- On day 2, take light microscopic images of the spheroids.
- Prepare the first treatment doses (after acquiring images).
NOTE: The first treatment concentration must be twice the desired final concentration as the solution will be diluted 1:2 upon addition to the well containing 100 µL medium. Suggested drug treatment intervals (will depend on drug half-life): Day 2, 4 and 7. - Using a multichannel pipette, gently remove 100 µL of medium and replace it with 100 µL of drug containing medium.
- Place the 96-well plate back in the incubator at 37 °C with 5% CO2 and 95% humidity and repeat 2.3 and 2.4 on the chosen days of treatment but now without doubling the dose to obtain correct final dose.
- On the final day of the protocol/treatment schedule, one or several of the following assays can be performed.
3. Cell Viability Assay for Spheroids
- Set up 4-6 spheroids per the desired condition as described in 1.2 or 1.3 and place in the incubator at 37 °C with 5% CO2 and 95% humidity.
NOTE: In this case, the cell viability assay was performed on day 7 or 9, after having monitored spheroid growth every 2-3 days by light microscopy as described above (point 1.2.5 and 1.3.13). - Thaw the viability assay reagent (see Table of Materials) and let it equilibrate to RT prior to use.
- Mix gently by inverting to obtain a homogeneous solution.
- Before performing the assay, remove 50% of the culture medium from the spheroids (100 µL).
- Add cell viability reagent to each well at a 1:3 ratio to the amount of medium present in the well (Figure 2A (i)) For a 96-well plate, add 50 µL of reagent to 100 µL of medium.
- Mix the contents vigorously for 5 min to induce cell lysis (Figure 2A (ii)).
- Incubate for 25 min at RT to stabilize the luminescent signal (Figure 2A (iii)).
- Record the luminescent signal (Figure 2A (iv)).
4. Preparing Protein Lysates for Western Blotting from 3D Spheroid Cultures
NOTE: When collecting the spheroids, it is advisable to use a P200 pipette and cut the end of the tip to allow a bigger opening and hence an easier capture of the spheroids without disturbing their structure.
- For each condition, pool a minimum of 12, ideally 18-24 spheroids (depending on spheroid size) in a 1.5 mL tube (avoid 2 mL tubes, as the next steps will become more difficult due to their less pointy bottom).
NOTE: If the amount of medium exceeds 1.5 mL before having collected all the spheroids, allow the collected spheroids to settle at the bottom (happens very quickly, centrifugation not necessary) and discard half the volume of the tube before continuing collecting the remaining spheroids. - Place tubes on ice and allow the spheroids to settle at the bottom of the 1.5 mL tube.
- Move from the sterile cell laboratory to the regular laboratory.
- Wash spheroids twice in 1 mL of ice-cold 1x PBS. Let spheroids settle before removing 1x PBS between each washing step.
- Aspirate as much 1x PBS as possible without disturbing or removing the spheroids.
- Add 5 µL of heated lysis buffer (LB) with phosphatase- and protease inhibitors, per spheroid (e.g., 10 spheroids = 50 µL LB).
- Repeat intervals of vortex followed by spin down until spheroids are dissolved. Perform a cycle of vortexing for 30 s followed by centrifugation (a quick spin using a tabletop centrifuge is sufficient) for 10 s for approx. 5-10 min depending on the size and the compactness of the spheroids.
NOTE: The protocol can be paused here. Keep the lysates at -20 °C until proceeding with sonication, homogenization and protein determination as in a standard 2D protein lysate protocol, followed by western blotting using standard protocols.
5. Propidium Iodide (PI) Staining of Spheroids
- Set up 3-6 spheroids per desired condition as described in 1.2 or 1.3 and place in the incubator at 37 °C with 5% CO2 and 95% humidity.
- In a sterile cell culture lab, heat 1x PBS to 37 °C.
- Make a PI solution of 4 µM by diluting stock solution in 1x PBS: Dilute a 1 mg/mL aqueous stock of PI 1:350 in 1x PBS.
NOTE: This concentration will be further halved upon addition of the solution to the wells giving a final concentration of 2 µM. 100 µL of this solution is needed for each well containing a spheroid.
CAUTION: Propidium iodide (PI) must be handled in a fume hood and wearing gloves. PI is light sensitive. Protect from light when handling. - Remove 100 µL of the medium from each well in the 96-well plate without removing the spheroids.
- Wash out the remaining medium by adding 100 µL of heated 1x PBS to all wells followed by removing 100 µL of the liquid in the wells. Repeat this washing step 3 times.
- Add 100 µL of the PI solution to each well, cover the plate in aluminum foil and place it in an incubator at 37 °C with 5% CO2 and 95% humidity for 10-15 min.
- Repeat the 3 washing steps described in 4.5 to wash out PI solution, in order to diminish background signal when imaging.
- Use an epifluorescence microscope to image the spheroids. To evaluate the viability of cells in the spheroid core take z-stacks to get images with varying depths of the spheroid.
NOTE: A step size around 18-35 µm between each slice depending on spheroid size is advisable, giving approximately 11-18 stacks per spheroid. Z-stacks can be processed in ImageJ using the z-projection function, which can combine all z-stacks into one final picture, giving an overview of the staining throughout the spheroid (for further guidelines on the use of ImageJ for this purpose, see (https://imagej.net/Z-functions).
6. Embedding of 3D Spheroids
- Prepare the agarose gel into which the spheroids are embedded (only necessary first time performing the protocol).
- Mix 1 g of bactoagar in 50 mL of ddH2O.
- Heat slowly in microwave oven until the bactoagar has dissolved and a homogenous gel has formed. Do not allow the gel to boil.
- Keep the bactoagar warm in a water bath at 60 °C.
- Keep at 4 °C between experiments.
- Embedding of spheroids.
- On day 1, for each condition, pool a minimum of 12 spheroids in a 1.5 mL tube.
- Wash once with 1 mL of ice-cold 1x PBS.
- To fix the spheroids, add 1 mL of 4% paraformaldehyde.
NOTE: Handling of paraformaldehyde should be performed in a fume hood. - Let them incubate for 24 h at RT.
- On day 2, heat the agarose gel carefully by placing it in a water-filled beaker in a microwave oven. Ensure that the gel does not boil! Keep warm in a benchtop heating plate, at 60 °C until use.
- Wash spheroids twice with 1 mL of ice-cold 1x PBS.
- Aspirate most of the 1x PBS (leaving approximately 100 µL at this point is practical for handling the spheroids).
- Prepare a 20 µL pipette by cutting the pipette tip at an incline to obtain a pointier tip with a larger hole (see illustration).
NOTE: The next part has to be done quickly to ensure optimal spheroid transfer and to avoid solidification of gel drop. If no heating block is available, it is recommended to first catch the spheroids and then make the agarose drop (i.e., switching the order of points 6.2.9 and 6.2.10). - Make an agarose gel drop on a microscope slide. Place the slide on a warm heating block to prevent the agarose from solidifying.
- Using the modified pipette tip (see 6.2.8), catch as many spheroids as possible in a volume of 15-20 µL.
- Carefully inject the 15-20 µL spheroid-containing 1x PBS into the center of the agarose gel drop without touching the microscope slide.
NOTE: This is a slightly difficult point. The spheroids will be lost if the pipette tip touches the microscope slide when injecting the spheroids into the gel drop. It is advisable to practice the whole process of making the agarose drop and injecting the spheroids by injecting a colored liquid into the drop. This will allow visualization of a potential penetration through the drop, as the colored liquid will be leaking out onto the slide. - Let the agarose gel drop harden by incubating for 5-10 min at RT or at 4 °C. Once the gel drop has solidified somewhat (but is still rather soft), carefully push the gel drop from the microscope slide into a plastic tissue cassette with a scalpel.
- Cover the plastic tissue cassettes in 70% ethanol.
NOTE: At this point the spheroids can be used directly or stored for months. - Embed the agarose-embedded spheroid in paraffin, section into 2-3 µm thick layer slides and stain with hematoxylin and eosin or subject to immuno-histological staining.
Wyniki
Spheroid growth assays based on the spheroid formation protocol schematically illustrated in Figure 1A and Figure 1B, were used as a starting point for analysis of the effects of anti-cancer drug treatments in a 3D tumor mimicking setting. The ease with which spheroids are formed is cell line specific, and some cell lines require supplementation with rBM in order to form coherent spheroids22. The concentration of rBM added can profoundly affect the morphology of the spheroids. As shown in Figure 1C and Figure 1D, varying the concentration of rBM between 0 and 4% alters the compactness and morphology of the spheroids in a cell type dependent manner. Figure 1C demonstrates how the addition of up to 2.5% rBM allows spheroid formation in SKBr-3 breast cancer cells, with no further effect at concentrations above 2.5% rBM. In contrast, BxPC3 pancreatic ductal adenocarcinoma (PDAC) cells, which exhibit an epithelial morphology, spontaneously form small, compact spheroids (Figure 1D, upper, left panel). In this cell type, increasing rBM concentration to 1.5% or above elicits a distinct morphological change from spheroid to more convoluted structures with protrusions and invaginations, reminiscent of ductal tubular structure formation. Conversely, the addition of rBM to two other PDAC cell lines, MiaPaCa and Panc-1, which have a more mesenchymal phenotype, allows the loose cellular aggregates to become tighter and form more compact spheroids (Figure 1D, middle and lower panels). These results show that the precise amount of rBM resulting in optimal spheroid formation must be titrated for each cell line and condition.
A quantitative assessment of cell viability within the spheroids upon drug treatment was necessary to evaluate the effect of anti-cancer drug treatments. The assay described here is a luciferin-luciferase-based assay, which measures ATP released from live cells within spheroids. The principle of the assay is illustrated in Figure 2A. The luminescent signal generated in this assay is easily recorded by a plate reader (Figure 2A) and correlates well with viability measured by other methods23. The linear relation between ATP concentration and luminescence in the relevant concentration range is shown in Figure 2B, while Figure 2C shows the ability of the assay to assess cell death in 3D spheroids treated with anti-cancer therapy. In order to further evaluate the linearity of the assay in the relevant range, experiments to establish standard curves of the luminescent signal as a function of the number of cells were carried out (Figure 2D and Figure 2E). These results indicate that the assay is suitable for estimating cell viability in 3D spheroid cultures and that it is applicable for investigating drug-induced loss of cell viability.
A combination of light microscopic images acquired every two to three days, during the treatment period and a final quantitative assessment of cell viability allows close supervision of spheroid growth and morphology as well as the assessment of optimal treatment dose. The latter is exemplified in Figure 3A and Figure 3B, where a dose-response experiment was performed to determine the dose necessary for 50% reduced cell viability in MDA-MB-231 breast cancer spheroids. Treatment effects on spheroid morphology are visualized in Figure 3C and Figure 3D for MDA-MB-231 and MCF-7 spheroids, respectively. During treatment with the chosen chemotherapeutic cocktail, the compactness of MDA-MB-231 spheroids increases, while during treatment with tamoxifen, MCF-7 spheroids become increasingly frayed and uneven. In both cases, a clear drop in cell viability is visible after 7 (MDA-MB-231) or 9 (MCF-7) days of treatment (Figure 3E and Figure 3F). This demonstrates the need for both a visual and a quantitative assessment of treatment-mediated effects on spheroid cell viability and morphology as well as that these parameters are highly cell- and treatment-type specific.
As a supplement to the cell viability assay, staining of dead cells with PI, which cannot cross the membrane and therefore only stains necrotic or late apoptotic cells with compromised membrane integrity, allows for a quick spatial evaluation of dead cells in response to treatment, without the time-consuming protocol of embedding, sectioning and IHC. As illustrated in Figure 4A the spatial arrangement of dead cells upon an increasing concentration of an inhibitor, in this case, the Na+/H+ exchanger 1 (NHE1) inhibitor 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), can be visualized. As seen, control spheroids show a limited necrotic/late apoptotic core, whereas the dead cells are distributed throughout the spheroid as the concentration of EIPA is increased.
In order to quantify the relative induction of apoptotic stress following different treatments, spheroids were lysed and subjected to SDS-PAGE gel electrophoresis and western blotting for full-length and cleaved poly (ADP-ribose) polymerase (PARP). Representative results are shown in Figure 4B and Figure 4C. In this experiment, spheroids were prepared from MDA-MB-231 cells in which the lactate-proton cotransporter MCT4 or the Na+, HCO3- cotransporter NBCn1 were knocked down using siRNA. The knockdown was evaluated by western blotting for MCT4 and NBCn1 (unpublished data). As seen, the knockdown of MCT4, but not of NBCn1, robustly increases PARP cleavage, consistent with our previous demonstration that stable knockdown of MCT4 in MDA-MB-231 cells decreases tumor growth in vivo24.
To further analyze the effects of treatment and obtain information on the specific signaling-, growth arrest, and death pathways activated, the spheroids can in addition to western blot analysis be embedded and subjected to immunohistochemistry (IHC) analysis. IHC analysis of the spheroid sections allows the use of specific antibodies or markers of cell proliferation, cell cycle and programmed cell death, and facilitates a visualization of the spatial arrangement of proliferative and apoptotic cells in the spheroid.
A schematic figure of the embedding protocol for IHC analysis of spheroids is presented in Figure 5A. A representative light microscopic image of an approx. 3 µm thick microtome section of an embedded spheroid is shown in Figure 5B, and an immunofluorescence image of a spheroid stained for the tumor suppressor protein p53 (nuclei stained using DAPI), is shown as Figure 5C. Examples of DMSO and chemotherapy-treated spheroids stained for the cell proliferation marker Ki-67 or for p53 are shown in Figure 5D and Figure 5E, respectively. Consistent with the antiproliferative effect of the chemotherapy treatment, the number of Ki-67 positive cells are greater in the DMSO control than in the chemotherapy-treated spheroid (Figure 5D). In contrast, p53 expression is increased during conditions of cell stress, apoptosis and growth arrest, and consequently, the number of p53-stained cells is substantially higher in the chemotherapy-treated spheroids compared to DMSO controls (Figure 5E).
These results illustrate examples of how spatially resolved (PI staining, IHC) or quantitative (western blotting) information on drug treatment effects in 3D spheroids can be obtained.
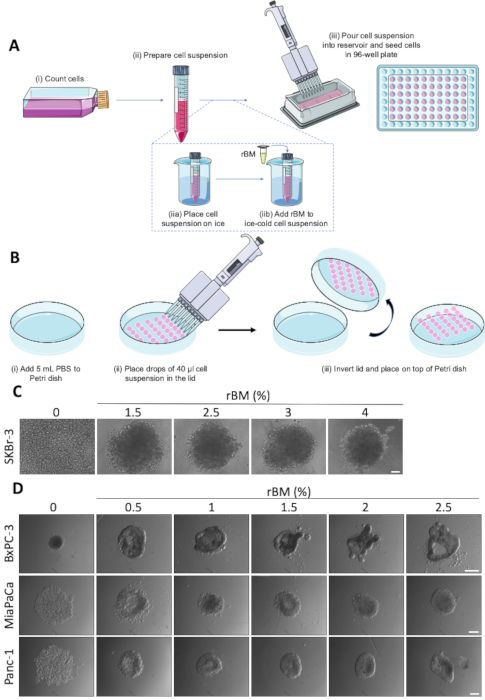
Figure 1: Spontaneous and rBM-mediated spheroid formation. (A) Schematic representation of spheroid formation using ultra-low attachment 96-well round bottom plates, with optional use of rBM. Individual steps marked by (i-iii). (B) Schematic representation of spheroid formation using the hanging drop method. Individual steps are marked by (i-iii) (C) Representative images of rBM-mediated spheroid formation of SKBr-3 cells. Cells were seeded in ultra-low attachment 96-well round bottom plates with increasing concentrations of rBM and grown for 9 days. Scale bar 100 µm. (n=3). (D) Representative images of BxPC-3, MiaPaCa and Panc-1 cells seeded for spheroid formation in ultra-low attachment 96-well round bottom plates with concentrations of rBM from 0.5-2.5 %. Spheroids were grown for 4 days. Scale bar = 250 µm. (n=3). Please click here to view a larger version of this figure.
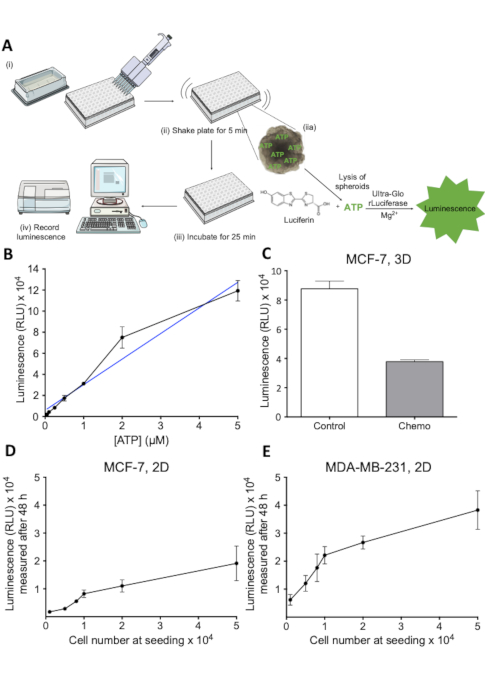
Figure 2: Principle and evaluation of the cell viability assay. (A) Schematic representation of the 3D cell viability assay. Individual steps denoted by (i-iv). (B) Luminescent signal as a function of ATP concentration. Dilutions of ATP were plated in a 96-well plate and cell viability reagent added to each well. Luminescence was recorded after 30 min at 405 nm. 1 n. (C) Viability, measured as luminescence, of control and chemotherapy-treated MCF-7 spheroids. MCF-7 cells were seeded in ultra-low attachment round-bottom plates and were grown for 7 days. Chemotherapy treatment (5 μM Cisplatin, 5 μM Doxorubicin and 30 nM 5-FU) was applied on day 2 and 4. Bars represent mean values with SD. 1 n. (D) Luminescent signal as function of the number of MCF-7 cells seeded. MCF-7 cells were seeded in 96-well plates at the indicated cell number and allowed to grow for 48 h, after which cell viability was measured. Error bars represent SD. 1 n. (E) As described in D for MDA-MB-231 cells. Please click here to view a larger version of this figure.
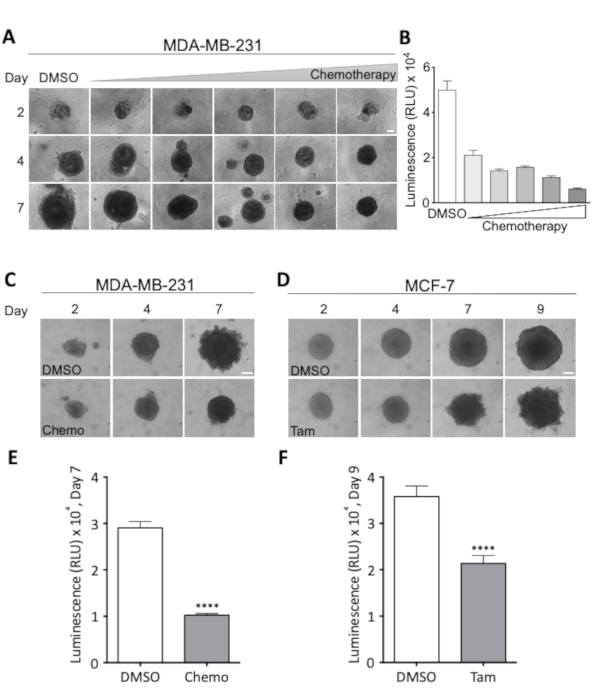
Figure 3: Effects of treatment regimens on spheroid morphology and cell viability. (A) Representative images of MDA-MB-231 spheroids on day 2, 4 and 7. MDA-MB-231 cells were seeded in ultra-low attachment round bottom 96-well plates. Treatment with increasing doses of chemotherapy was started on day 2, at which time all spheroids were of similar size. Rows show spheroids at increasing doses of chemotherapy, and columns show spheroids representative of size at day 2, 4, and 7 at the indicated dose. The lowest dose was 18.75 nM Cisplatin, 18.75 nM Doxorubicin, 0.0625 nM 5-Fluorouracil (5-FU) and this dose was doubled for each image shown, resulting in a maximal dose of 0.3 µM Cisplatin, 0.3 µM Doxorubicin and 2 nM 5-FU. Scale bar = 100 µm. (2 n). (B) Viability of MDA-MB-231 spheroids, measured as luminescence, after 7 days of chemotherapeutic treatment. The bars represent mean values with SEM. 2 n. (C,D) Representative images of MDA-MB-231 (C) and MCF-7 spheroids (D) on day 2, 4, 7 and for MCF-7 spheroids 9. Cells seeded as in (A) and treated with either chemotherapy (Chemo, 18.75 nM Cisplatin, 18.75 nM Doxorubicin, 0.0625 nM 5-FU) on day 2 and 4 (C) or with 2 µM Tamoxifen (Tam) on day 2, 4 and 7 (D). Scale bar = 100 µm. (4 n and 3 n), respectively. (E,F) Viability, measured as luminescence, on day 7 and 9 for (C) and (D), respectively. To test for statistically significant difference between conditions an unpaired Student’s t-test was performed. **** denotes p < 0.0001. Please click here to view a larger version of this figure.

Figure 4: Propidium iodide staining and western blot analysis of spheroids. (A) Representative images of PI-stained MCF-7 spheroids after 9 days of treatment. MCF-7 cells were seeded in ultra-low attachment 96-well plates grown for 9 days and treated with increasing concentrations of EIPA on day 2, 4 and 7. On day 9, the spheroids were stained with PI and images were acquired on an epifluorescence microscope. Scale bar = 200 µm. (1 n.) (B) Representative western blots of MDA-MB-231 cells after knockout/knockdown of acid-base transporters. NHE1 was knocked out by CRISPR/Cas9 in MDA-MB-231 cells 12 and the cells were subsequently transiently transfected with siRNA against MCT4 or NBCn1, and grown as spheroids for 9 days before being lysed and subjected to western blotting using an antibody recognizing total and cleaved (c)PARP. (C) Quantification of the ratio of cPARP to PARP protein level, normalized to loading control (β-actin). (1 n). Please click here to view a larger version of this figure.
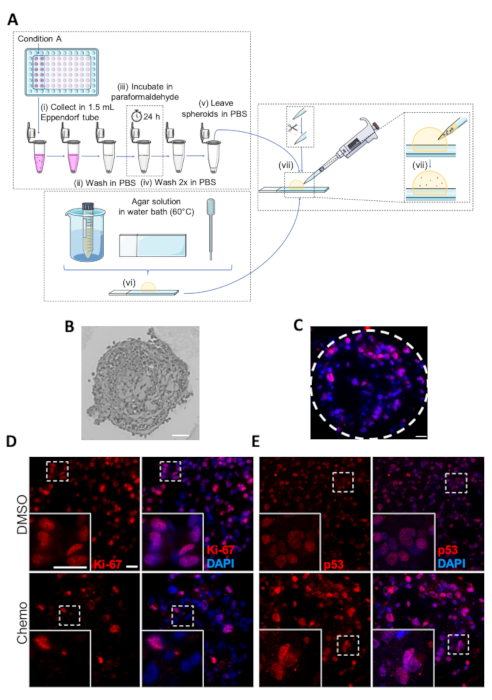
Figure 5: Fixing, embedding and immunohistochemistry analysis of spheroids. (A) Schematic representation of the protocol for embedding of spheroids. Individual steps are marked as (i-vii). (B) Image of embedded MDA-MB-231 spheroid. Scale bar: 50 µm. (C) Representative image of chemotherapy-treated MDA-MB-231 spheroid subjected to IHC analysis with antibodies against p-53. Dashed lines show the circumference of the spheroid. Scale bar = 20 µm. (D,E) Representative images of DMSO- or chemotherapy-treated (upper and lower panels, respectively) MDA-MB-231 spheroids. MDA-MB-231 cells were seeded in ultra-low attachment 96-well plates, grown for 7 days and treated with chemotherapy on day 2 and 4. On day 7, the spheroids were embedded followed by analysis by IHC with primary antibodies against Ki-67 (D) and p53 (E). White boxes represent zoom images. Scale bar = 20 µm in both magnifications, (n=3). Please click here to view a larger version of this figure.
Dyskusje
The use of 3D cancer cell spheroids has proven a valuable and versatile tool not only for anticancer drug screening, but also for gaining mechanistic insight into the regulation of cancer cell death and viability under conditions mimicking those in the tumor microenvironment. This is particularly crucial as the accessibility, cellular uptake, and intracellular effects of chemotherapeutic drugs are profoundly impacted by the physico-chemical conditions in the tumor, including pH, oxygen tension, tortuosity, and physical and chemical cell-cell interactions9,17. For example, the acidity of extracellular pH, which can reach values as low as 6-6.5 in many solid tumors25,26,27,28,29, causes weakly basic chemotherapeutic compounds, such as doxorubicin, mitoxantrone and the zwitterion paclitaxel, to be charged. This reduces their uptake into the tumor cells and can influence the activity of multidrug resistance proteins such as p-glycoprotein30,31,32. Also the cell proliferation, which is pivotal to the effect of most chemotherapeutic compounds, is generally reduced in 3D compared to 2D conditions and hence is likely better mimicked in tumor spheroids than in 2D cell culture8,33,34. Finally, the dense tumor microenvironment is the origin of numerous physical and soluble signaling cues directing intracellular signaling pathways regulating cell growth, survival and death. Thus, when analyzing drug efficacy, 3D culture systems are a pivotal step before embarking on in vivo models. A major drawback of 3D culture is, however, the increased complexity of analysis compared to that of 2D culture. We have described here simple and relatively inexpensive techniques for spheroid formation using a variety of cancer cell types. We have shown examples of how spheroid formation must be optimized for each cell type studied and have described how to obtain quantitative data on cell viability, cell death, and associated signaling pathways, in such spheroids. There is no obvious growth- or morphological differences between the three models described here. In our hands, the variation in morphology may be slightly greater using the hanging drop method, yet an advantage of this method is that rBM is not needed. We have focused here on spheroids produced from a single cancer cell type. The spheroid model is, however, also amenable to co-culture, for instance of cancer cells with fibroblasts, monocytes/macrophages, endothelial cells, and/or adipocytes35,36,37. Other advanced applications of this model include the combination with 3D printed fluidic devices allowing dosing through a semipermeable membrane, followed by harvesting for quantitative proteomic profiling38.
While, as noted above, the phenotype of cells grown in 3D spheroids generally mimics that of in vivo tumors much better than do cells grown in 2D, the extent to which such spheroids are in fact relevant models of the corresponding in vivo tumors is dependent on numerous factors and has to be carefully evaluated. Parameters which will impact how well such spheroids mimic the in vivo condition include the cellular composition of the tumor and its relative ECM composition. For instance, the rBM which we have employed as ECM in the protocols provided here is a good choice for mimicking early stages of epithelial cancers, around the time of breaching the basement membrane, other ECM compositions will be more relevant for certain tumor types and -stages. Furthermore, the capacity for cell-cell adhesion differs widely between cancer cell lines, depending on their expression of cell-cell and cell-matrix adhesion proteins such as cadherins and integrins22.
As described here, spheroid growth and morphology can easily and non-invasively be monitored every 2-3 days using a light microscope with low magnification optics and a large field of view. However, because the cytotoxic stress, such as chemotherapy treatment, affects spheroid morphology very differently and, in a manner, depending on the cell type and treatment scheme, it is not enough to rely on the morphology and circumference alone for evaluating treatment effect. For instance, spheroids may become looser with treatment and emerging cell death, or all death may occur in the necrotic core, while the surface is not detectably affected. In both cases, the result may be an erroneous impression that the number of live cells in the spheroid is not reduced by the treatment. Quantitative- and whole-spheroid techniques are therefore essential for evaluating treatment effect. For quantitative evaluation of cell death, the acid phosphatase assay, which as the name implies measures the activity of cytosolic acid phosphatase has been employed21. However, in our hands, while this assay generally nicely reflects the number of cells seeded, it does not adequately capture rapid treatment-induced cell death (data not shown), likely because the acid phosphatase remains active for some time after cell death. Furthermore, this assay requires complete removal of the medium, which increases error especially with fragile, chemotherapy-treated spheroids. The cell viability assay described here, which is based on cellular ATP content, was chosen based on its simple and time efficient protocol and high reproducibility. Furthermore, this assay does not require complete removal of culture medium which is an advantage when working with spheroids. As shown in representative results, this assay captures well both cell number and expected chemotherapy treatment effects. However, a pitfall of this technique is, obviously, that metabolic changes reducing intracellular ATP content may erroneously be recorded as a lower cell number. Hence, parallel assessment of spheroid volume and morphology, or PI staining, is advisable to validate results.
Spheroid lysis followed by western blotting can provide semi-quantitative insight into the state of signaling processes, cell death-, growth- and viability pathways. The use of western blotting is complicated when rBM is used to prepare the spheroids, since this will comprise a substantial fraction of the lysate protein content, and more importantly, its fractional contribution will increase with decreasing cellular content during chemotherapeutic cell death. It is in principle possible to remove the rBM by centrifugation; however, this is a critical step, as it is difficult to completely remove all rBM, and this will preclude quantitative comparison between conditions. For such spheroids, and in general for spatially resolved assessment of death pathways and relevant signaling parameters, embedding and IHC are strong tools. Other approaches may be considered: live confocal imaging of (relatively small) intact spheroids39. Another interesting property of spheroids is that given their rather regular "ball" shape, they lend themselves well to iteration between mathematical modeling and wet lab experiments, to increase the understanding of the importance of the above-mentioned gradients of oxygen, pH, and nutrients within spheroids, and, by extrapolation, tumors40,41. Thus, although important 3D tumor models of much greater complexity are emerging, including a wide range of organotypic and organoid cultures based on complex biological as well as inert scaffolds, and, not least, patient-derived xenografts42, spheroids remain an important tool because of their superior biological relevance compared to 2D culture, combined with relative ease of handling.
In summary, we present here a series of simple methods for analysis of anti-cancer treatment-induced changes in cancer cell viability and death in 3D culture. The composition of the spheroids can be modified depending on the properties and biology of the cells employed, and the quantitative and qualitative analyses presented are useful both for assessing dose-response relationships and for gaining insight into the signaling- and death pathways involved.
Ujawnienia
The authors declare no conflict of interest.
Podziękowania
We are grateful to Katrine Franklin Mark and Annette Bartels for excellent technical assistance and to Asbjørn Nøhr-Nielsen for performing the experiments in Figure 1D. This work was funded by the Einar Willumsen Foundation, the Novo Nordisk Foundation, and Fondation Juchum (all to SFP).
Materiały
| Name | Company | Catalog Number | Comments |
| 2-(4-amidinophenyl)-1H-indole-6-carboxamidine (DAPI) | Invitrogen | # C10595 | For staining nuclei |
| 5-Fluorouracil (5-FU) | Sigma-Aldrich | #F6627 | Component in chemotherapeutic treatment |
| 5-(N-ethyl-isopropyl) amiloride (EIPA) | Life Technologies | #E3111 | Inhibitor of NHE1 |
| Antibody against PARP and cPARP | Cell signaling | #9542 | Used in western blotting |
| Antibody against Ki-67 | Cell signaling | #9449 | Used for IHC |
| Antibody against p53 | Cell Signaling | #2524 | Used for IHC |
| Antibody against β-actin | Sigma | A5441 | Used in western blotting |
| Bactoagar | BD Bioscience | #214010 | Used for agarose gel preparation |
| Benchmark protein ladder | Invitrogen | #10747-012 | Used for SDS-PAGE |
| Bio-Rad DC Protein Assay kit | Bio-Rad Laboratories | #500-0113, #500-0114, #500-0115 | Used for protein determination from lysates |
| Bürker chamber | Marienfeld | 610311 | For cell counting |
| BX63 epifluoresence microscope | Olympus | Used for fluorescent imaging | |
| CellTiter-Glo 3D Cell Viability Assay | Promega | #G9681 | Used for the cell viability assay |
| Cisplatin | Sigma-Aldrich | #P4394 | Component in chemotherapeutic treatment |
| Corning Spheroid Microplate, 96 well, Black with clear round bottom, Ultra-low attachment, With lid, Sterile | Corning | #4520 | Used for growing spheroids with luminescence measurements as end point |
| Corning 96 well, clear round bottom, Ultra-low attachment microplate, With lid, Sterile | Corning | #7007 | Sufficient for spheroid growth without luminescence measurements as end point |
| Criterion TGX Precast Gels | Bio-Rad | 5671025 | Used for SDS-PAGE |
| Doxorubicin | Abcam | #120629 | Component in chemotherapeutic treatment |
| FLUOStar Optima Microplate reader | BMG Labtech | Used for recording luminescence | |
| Formaldehyde | VWR Chemicals | #9713.1000 | Used for cell fixation |
| Geltrex LDEV-Free Reduced Growth Factor Basement Membrane Matrix | Gibco | #A1413202 | Keep at 4 °C to prevent solidification. Referred to as rBM in the protocol. |
| Heat-inactivated FBS | Sigma | #F9665 | Serum for growth media |
| ImageJ | NIH | Scientific Image analysis | |
| Medim Uni-safe casette | Medim Histotechnologie | 10-0114 | Used for storage of embedded spheroids |
| Mini protease inhibitor cocktail tablets | Roche Diagnostics GmBH | # 11836153001 | Used for lysis buffer preparation |
| MZ16 microscope | Leica | Used for light microscopic images | |
| NuPAGE LDS 4x Sample Buffer | Invitrogen | #NP0007 | Used for western blotting |
| Pierce ECL Western blotting substrate | Thermo scientific | #32106 | Used for western blotting |
| Ponceau S | Sigma-Aldrich | #P7170-1L | Used for protein band staining |
| Prism 6.0 | Graphpad | Scientific graphing and statistical software | |
| Propidium iodide (1mg/ml solution in water) | Invitrogen | P3566 | Light sensitive |
| Sterile reservoirs, multichannel | SPL lifesciences | 21002 | Used for seeding cells for spheroid formation |
| Superfrost Ultra-Plus Adhesion slide | Menzel-Gläser | #J3800AMNZ | Microscope glass slide used for embedding |
| Tamoxifen | Sigma-Aldrich | #T5648 | Used as chemotherapeutic treatment |
| Trans-blot Turbo 0.2 µm nitrocellulose membranes | Bio-Rad | #170-4159 | Used for western blotting |
| Tris/Glycine/SDS running buffer | Bio-Rad | #161 0732 | Used for SDS-PAGE |
| Trypsin-EDTA solution | Sigma | #T4174 | Cell dissociation enzyme |
Odniesienia
- Sutherland, R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 240 (4849), 177-184 (1988).
- Mueller-Klieser, W., Freyer, J. P., Sutherland, R. M. Influence of glucose and oxygen supply conditions on the oxygenation of multicellular spheroids. British Journal of Cancer. 53 (3), 345-353 (1986).
- Gaedtke, L., Thoenes, L., Culmsee, C., Mayer, B., Wagner, E. Proteomic analysis reveals differences in protein expression in spheroid versus monolayer cultures of low-passage colon carcinoma cells. Journal of Proteome Research. 6 (11), 4111-4118 (2007).
- Chen, J. L., et al. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genetics. 4 (12), 1000293 (2008).
- Cukierman, E., Pankov, R., Stevens, D. R., Yamada, K. M. Taking cell-matrix adhesions to the third dimension. Science. 294 (5547), 1708-1712 (2001).
- Gudjonsson, T., Ronnov-Jessen, L., Villadsen, R., Bissell, M. J., Petersen, O. W. To create the correct microenvironment: three-dimensional heterotypic collagen assays for human breast epithelial morphogenesis and neoplasia. Methods. 30 (3), 247-255 (2003).
- Pampaloni, F., Reynaud, E. G., Stelzer, E. H. The third dimension bridges the gap between cell culture and live tissue. Nature Reviews in Molecular and Cell Biology. 8 (10), 839-845 (2007).
- Hirschhaeuser, F., et al. Multicellular tumor spheroids: an underestimated tool is catching up again. Journal of Biotechnology. 148 (1), 3-15 (2010).
- Jacobi, N., et al. Organotypic three-dimensional cancer cell cultures mirror drug responses in vivo: lessons learned from the inhibition of EGFR signaling. Oncotarget. 8 (64), 107423-107440 (2017).
- Rodriguez-Enriquez, S., et al. Energy metabolism transition in multi-cellular human tumor spheroids. Journal of Cell Physiology. 216 (1), 189-197 (2008).
- Kunz-Schughart, L. A. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biology International. 23 (3), 157-161 (1999).
- Andersen, A. P., et al. Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Molecular Cancer. 15 (1), 45 (2016).
- Swietach, P., Patiar, S., Supuran, C. T., Harris, A. L., Vaughan-Jones, R. D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. Journal of Biological Chemistry. 284 (30), 20299-20310 (2009).
- Walenta, S., Doetsch, J., Mueller-Klieser, W., Kunz-Schughart, L. A. Metabolic imaging in multicellular spheroids of oncogene-transfected fibroblasts. Journal of Histochemistry and Cytochemistry. 48 (4), 509-522 (2000).
- Kunz-Schughart, L. A., Groebe, K., Mueller-Klieser, W. Three-dimensional cell culture induces novel proliferative and metabolic alterations associated with oncogenic transformation. International Journal of Cancer. 66 (4), 578-586 (1996).
- Feng, H., et al. Homogeneous pancreatic cancer spheroids mimic growth pattern of circulating tumor cell clusters and macrometastases: displaying heterogeneity and crater-like structure on inner layer. Journal of Cancer Research and Clinical Oncology. 143 (9), 1771-1786 (2017).
- Santini, M. T., Rainaldi, G., Indovina, P. L. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Critical Reviews in Oncology/Hematology. 36 (2-3), 75-87 (2000).
- Vinci, M., et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biology. 10, 29 (2012).
- Pickl, M., Ries, C. H. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 28 (3), 461-468 (2009).
- Wong, C., Vosburgh, E., Levine, A. J., Cong, L., Xu, E. Y. Human neuroendocrine tumor cell lines as a three-dimensional model for the study of human neuroendocrine tumor therapy. Journal of Visual Experiments. (66), e4218 (2012).
- Friedrich, J., et al. A reliable tool to determine cell viability in complex 3-d culture: the acid phosphatase assay. Journal of Biomolecular Screening. 12 (7), 925-937 (2007).
- Ivascu, A., Kubbies, M. Diversity of cell-mediated adhesions in breast cancer spheroids. International Journal of Oncology. 31 (6), 1403-1413 (2007).
- Crouch, S. P., Kozlowski, R., Slater, K. J., Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. Journal of Immunological Methods. 160 (1), 81-88 (1993).
- Andersen, A. P., et al. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. International Journal of Cancer. , (2018).
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Seminars in Radiation Oncology. 14 (3), 198-206 (2004).
- Vaupel, P. W., Frinak, S., Bicher, H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Research. 41 (5), 2008-2013 (1981).
- Helmlinger, G., Yuan, F., Dellian, M., Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Medicine. 3 (2), 177-182 (1997).
- Zhang, X., Lin, Y., Gillies, R. J. Tumor pH and its measurement. Journal of Nuclear Medicine. 51 (8), 1167-1170 (2010).
- Gillies, R. J., Raghunand, N., Karczmar, G. S., Bhujwalla, Z. M. MRI of the tumor microenvironment. Journal of Magnetic Resonance Imaging. 16 (4), 430-450 (2002).
- Vukovic, V., Tannock, I. F. Influence of low pH on cytotoxicity of paclitaxel, mitoxantrone and topotecan. British Journal of Cancer. 75 (8), 1167-1172 (1997).
- Song, C. W., Griffin, R., Park, H. J., Teicher, B. A. . Cancer Drug Resistance. , 21-42 (2006).
- Lotz, C., et al. Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncology Reports. 17 (1), 239-244 (2007).
- Sant, S., Johnston, P. A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discovery Today: Technologies. 23, 27-36 (2017).
- Stratmann, A. T., et al. Establishment of a human 3D lung cancer model based on a biological tissue matrix combined with a Boolean in silico model. Molecular Oncology. 8 (2), 351-365 (2014).
- Kuen, J., Darowski, D., Kluge, T., Majety, M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS One. 12 (7), 0182039 (2017).
- Bochet, L., et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Research. 73 (18), 5657-5668 (2013).
- Amann, A., et al. Development of a 3D angiogenesis model to study tumour - endothelial cell interactions and the effects of anti-angiogenic drugs. Scientific Reports. 7 (1), 2963 (2017).
- LaBonia, G. J., Ludwig, K. R., Mousseau, C. B., Hummon, A. B. iTRAQ Quantitative Proteomic Profiling and MALDI-MSI of Colon Cancer Spheroids Treated with Combination Chemotherapies in a 3D Printed Fluidic Device. Analytical Chemistry. 90 (2), 1423-1430 (2018).
- Hulikova, A., Vaughan-Jones, R. D., Swietach, P. Dual role of CO2/HCO3(-) formula buffer in the regulation of intracellular pH of three-dimensional tumor growths. Journal of Biological Chemistry. 286 (16), 13815-13826 (2011).
- Wallace, D. I., Guo, X. Properties of tumor spheroid growth exhibited by simple mathematical models. Frontiers in Oncology. 3, 51 (2013).
- Michel, T., et al. Mathematical modeling of the proliferation gradient in multicellular tumor spheroids. Journal of Theoretical Biology. 458, 133-147 (2018).
- Meijer, T. G., Naipal, K. A., Jager, A., van Gent, D. C. Ex vivo tumor culture systems for functional drug testing and therapy response prediction. Future Science OA. 3 (2), (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone