Method Article
Real-time Cytotoxicity Assays in Human Whole Blood
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
The whole blood cytotoxicity assay (WCA) is a cytotoxicity assay developed by incorporating high-throughput cell positioning technology with fluorescence microscopy and automated image processing. Here, we describe how lymphoma cells treated with an anti-CD20 antibody can be analyzed real-time in human whole blood to provide quantitative cellular cytotoxicity analysis.
Streszczenie
A live cell-based whole blood cytotoxicity assay (WCA) that allows access to temporal information of the overall cell cytotoxicity is developed with high-throughput cell positioning technology. The targeted tumor cell populations are first preprogrammed to immobilization into an array format, and labeled with green fluorescent cytosolic dyes. Following the cell array formation, antibody drugs are added in combination with human whole blood. Propidium iodide (PI) is then added to assess cell death. The cell array is analyzed with an automatic imaging system. While cytosolic dye labels the targeted tumor cell populations, PI labels the dead tumor cell populations. Thus, the percentage of target cancer cell killing can be quantified by calculating the number of surviving targeted cells to the number of dead targeted cells. With this method, researchers are able to access time-dependent and dose-dependent cell cytotoxicity information. Remarkably, no hazardous radiochemicals are used. The WCA presented here has been tested with lymphoma, leukemia, and solid tumor cell lines. Therefore, WCA allows researchers to assess drug efficacy in a highly relevant ex vivo condition.
Wprowadzenie
Recent advances in the pharmaceutical industry have led to an increased interest in realizing the specific identifications of tumor cell antibodies and personalized cancer treatments; however, several obstacles are encountered in the process. Only 5% of agents that have anticancer activity in preclinical development are licensed after showing sufficient efficacy in phase II-III testing1,2. The preclinical strategies (both in vitro and in vivo) are suboptimal as many examples have shown that antitumor drugs behave differently in human and in laboratory animals mainly due to their different blood components3-5.
To address the necessity of an antitumor drug screening platform and to provide a check-point before costly animal experiments and clinical trials, a human whole blood cytotoxicity assay (WCA) is proposed for evaluating antitumor drug efficacy in a more relevant biological environment. The whole blood cytotoxicity assay can be used to evaluate the response of individual cells to antibodies and other drug candidates in human whole blood.
The WCA is developed by incorporating high-throughput cell positioning technology with high-throughput and high-content imaging6. By utilizing an automated imaging system, the number of both living and dead cells can be determined with a high degree of precision. Due to the fact that target cells are immobilized on the same focal plane, WCA is able to provide quantitative cytotoxicity analysis in real time without the removal of red blood cells. Moreover, the automatic imaging system provides several advantages such as that only target cells that have reached the specified criteria (e.g., fluorescently labeled cells, and cell morphology) are gated and processed. Also, it allows the production of 144 plates per day. Consequently, this imaging capability and throughput allows running of high-content and high-throughput experiments simultaneously. By combining WCA and the automated imaging system, high throughput quantitative cell cytotoxicity analysis can be achieved within a more biological relevant environment.
Protokół
1. Target Cell Preparation
- Maintain target cells (e.g. Raji lymphoma cells) in growth media (RPMI 1640 culture medium, with 10% heat-inactivated fetal bovine serum (FBS), 4nM L-glutamine, and 500 IU/ml penicillin/ streptomycin) at 37oC in a 5% CO2 incubator.
- Centrifuge the sample to pellet the target cells in a 15 ml tube. Centrifugation time varies with different cell types; centrifuge for 3 min at 468 x g for Raji cells.
- First aspirate any bubbles formed on surface of the supernatant, and remove the entire supernatant.
- Add 10 ml of PBS to the tube. Re-suspend the cells by pipetting the solution up and down several times to break up the cell clump.
- Centrifuge the sample for 3 min at 468 x g.
- Aspirate any bubbles formed on the surface of the supernatant, and remove the entire supernatant.
2. Preparation of Cell Microarrays
- Obtain cell attachment 96 well plate kits or single cell array 8 well chamber slides (see table of materials/reagents).
- Apply 1.2 μl of the Activator to the DNA reagent from the kit. Cap the vial, invert it, and gently shake to mix the solution.
- Allow the solution to react for 20 min at RT.
- Apply the mixed solution to the target cells pellet (in the 15 ml tube).
- Add green fluorescence cytosolic dyes at a final concentration of 500 nM.
- Incubate the target cells with the mixed solution and the dye for 30 min at RT on an orbital shaker.
- Wash the cells twice with 5 ml PBS, and resuspend the cells in 10 ml growth media.
- Apply 100 µl of the solution from step 2.7 to each well. Count the cell number by using a hemacytometer. Make sure cell numbers range between 5×104 to 1×106 cells per well.
- After 10-15 min of incubation at RT, gently wash the samples twice with 200-300 µl growth media to remove any unbound cells.
NOTE: About 5-10 µl growth media remains in each well after washing.
3. Whole Blood Cytotoxicity Assays
- Add 180 μl of human whole blood to each sample well.
- Obtain Anti-CD20 antibody solution at 5 mg/ml, and perform serial dilution to make 50, 20, 10, 5, 2, 1, 0.5 μg/ml of Anti-CD20 antibody solution in 1.5 ml tubes. Add 20 µl of each concentration of Anti-CD20 antibody to each sample well making triplicates.
- Add Propidium iodide at a final concentration of 500 nM to each well.
- Incubate the resulting plate at 37°C with 5% CO2 for 16 hr.
- Image the cells on the slides or 96 well plates with an automatic imaging system with 8X magnification.
- Use the cell counting program of the automatic imaging system to quantify the results.
NOTE: The software of the automatic imaging system provides simple step-by-step operations for cell counting. The protocol of using this software can be found at provider’s website. - Select [Review Results] from the main page of the software.
- Select [The File Saved for The Samples].
- First gate FITC mean intensity >50, then gate TxRed mean intensity >20.
- Select [Plate Overview].
- Select [Export Tables].
NOTE: The percentage cell killing formula of the program is shown below:
% target cell killing = (# of cells stained both green and red/ # of cells stained green)*100%
Wyniki
Anti-CD20 antibodies and lymphoma cells (Raji cells and MC/CAR) were chosen as a model system to demonstrate the whole blood cytotoxicity assay (WCA)7,8. Raji cells had high copy number of CD20 on the cell surface, while MC/CAR cells had low copy number of CD20 on their membrane. Targeted cells were first stained green with green fluorescence cytosolic dyes and arrayed on the 96-well plate. 10,000 - 50,000 target lymphoma cells were immobilized in each well. 180 µl of freshly drawn human whole blood was added into each well. Anti-CD20 antibody was then added into each well of a 96 well plate with gradient concentrations. After 16 h of incubation at 37°C, cell numbers were assessed quantitatively through the automatic imager by analyzing the number of green fluorescent cells and red fluorescent cells. Since the target lymphoma cells were all arrayed on the same focal plane, the fluorescence of each cell was detected directly even without removing the blood cells in the well.
As shown in Figure 1, the detection of the targeted Raji lymphoma cells (both alive and dead) was performed. Green dots in Figure 1 indicated the number of target cells presented. Dead cells (red dots) in Figure 1 were analyzed quantitatively through the intensity of the red fluorescence because they were stained fluorescent red with PI. Antibody cytotoxicity was analyzed through the ratio of dead cells (stained both green and red) to the cells presented (green dots). The number of fluorescent red dots decreased as the concentration of Anti-CD20 antibody decreased in concentration.
Each antibody concentration was tested independently in triplicates. For each antibody concentration, more than 400 target cells per well were imaged to provide the mean value and the standard deviation of cytotoxicity results. Based on our data, with 400 data points per antibody concentration, we obtained statistical results with p value <0.01. The dose-dependent drug efficiency was obtained in Figure 2. The MC/CAR cells with low copy number of CD20 antigen were used as a control cell line. An EC50 value of 0.12 μg/ml was obtained for the WCA result against Raji lymphoma cells, which was in the range with the value obtained in the regular antibody cytotoxicity assays including complement dependent cytotoxicity assay (CDC) or antibody-dependent cellular cytotoxicity (ADCC) assay.9-11
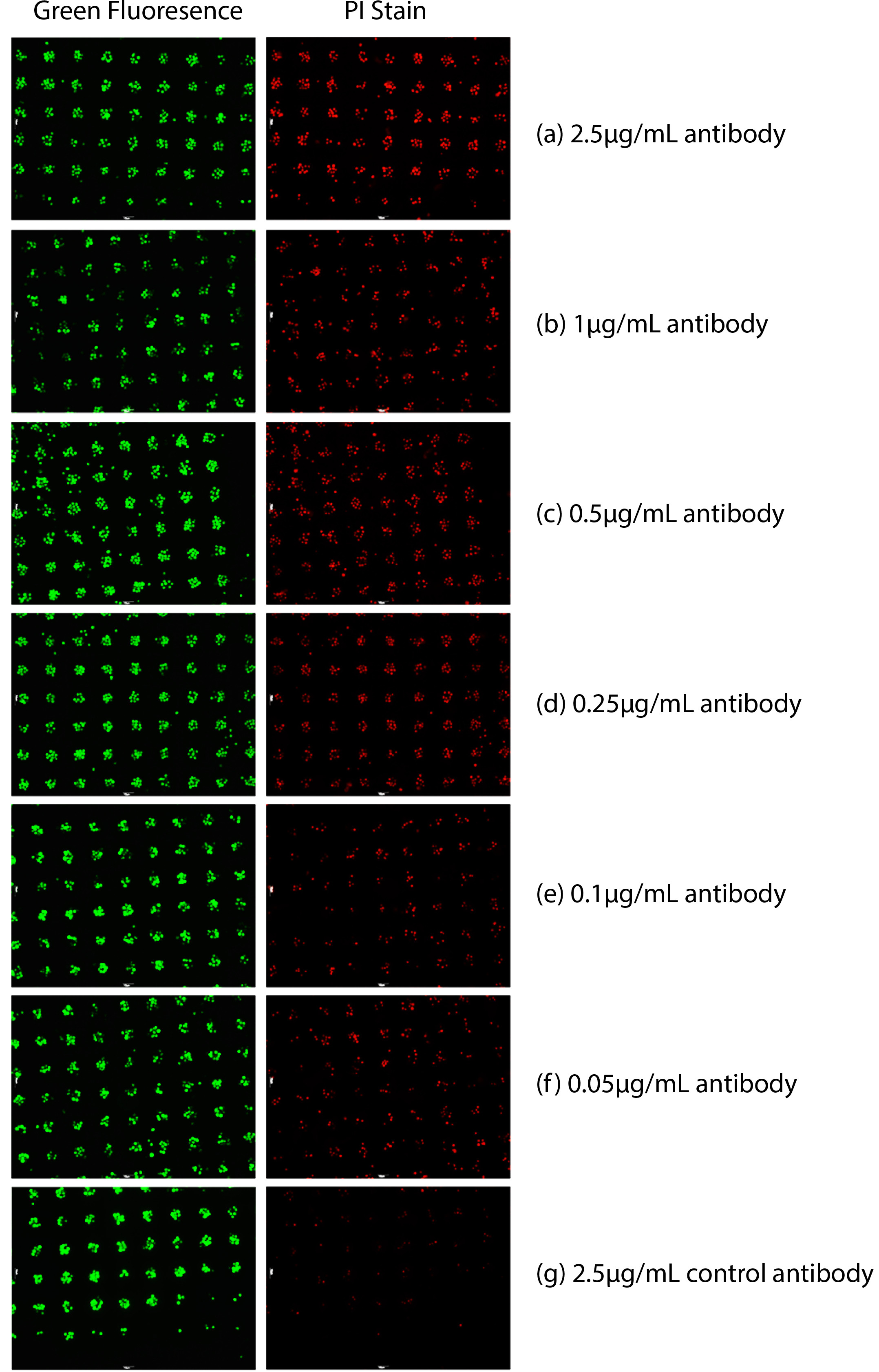
Figure 1. Antibody-based cytotoxicity measured in whole blood. Raji cells were labeled with a green cytosolic dye and then immobilized. PI was also added to access cell death. Dead cells were stained red by PI. Varying concentrations of Anti-CD20 antibody were added with the addition of whole blood. (a) 2.5 µg/ml Anti-CD20 antibody 16 hr after, (b) 1 µg/ml Anti-CD20 antibody 16 hr after, (c) 0.5 µg/ml Anti-CD20 antibody 16 hr after, (d) 0.25 µg/ml Anti-CD20 antibody 16 hr after , (e) 0.1 µg/ml Anti-CD20 antibody 16 hr after, (f) 0.05 µg/ml Anti-CD20 antibody 16 hr after, (g) 2.5 µg/ml control antibody 16 hr after.
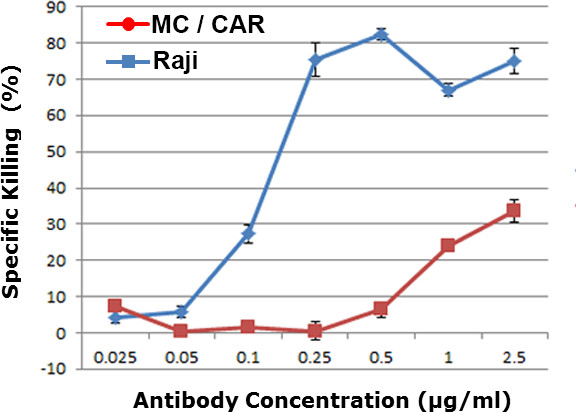
Figure 2. Dose-dependent cytotoxicity against different cell lines in human whole blood. Raji was a CD20 positive lymphoma cells, and MC/CAR was a CD20 negative cell lines. The level of whole blood cytotoxicity was shown for different concentration of α-CD20 antibody (Anti-CD20 antibody).
Dyskusje
WCA is a critical in vitro anti-cancer screening tool with single cell resolution12-16, ideally utilized after traditional target screenings such as CDC and ADCC assays9-11, and before preclinical animal tests. Currently, primary target screening assays such as CDC or ADCC assays are all performed in a simplified media or a buffer system. However, drug candidates that show efficacy in these simplified buffer system are not always effective in the more complex whole blood system. Therefore, WCA can bridge the gap between traditional target screenings and costly animal studies, reduce false positives, and thus prevent the preventable failures in animal tests or in human clinical trials. The WCA will be beneficial for the researchers working on the preclinical studies in order to test the drug efficacy in the content of human whole blood. Counting the dead and live cells using flow cytometry requires the complete lysis of red blood cells in order to detect target cells. The advantage of WCA technique over flow cytometry is that it can identify target cells without lysis of red blood cells. It is difficult to completely lyse all the red blood cells in the blood sample, and target cells are also partially lysed during the lysis procedure.
Even more exciting opportunities arise if screening panels can be generated using the live primary cells obtained from individual patients, paving the way to personalized cancer treatments, the evaluation of cell heterogeneity within a given tumor, and the identification of cells that are resistant to a given drug treatment. Moving the healthcare system to an approach that is “personalized, predictive, preventive and centered on the needs of the patient” is the future of medicine17,18. Numerous initiatives within the US Department of Health and Human Services, including the FDA, the Centers for Disease Control, the NIH, the Centers for Medicare and Medicaid Services, and the Health Resources and Services Administration exist to support initiatives to promote personalized care. The realization of personalized medicine depends on reliable technologies and products that can capture and hold any human cells while maintaining them in a relevant biological state. We can foresee applying WCA to screen drugs against cancer patient’s tumor cells in the matrix of his/her own blood for personalized medicine applications.
The critical steps in the protocol are the preparations of the cell array. The users need to remove all of the supernatant without losing the cells during the cell washing step. In addition, users need to do a short centrifugation if the liquid cannot be seen in the vials. Once DNA reagent solution has been prepared, it must be used with the cells within 30 min.
The cell array formation efficiency is cell type dependent. There have been more than 100 types of cells tested with this protocol; however, it is still possible that some specific cell types will not form the cell array efficiently. If no cell array forms, a higher concentration of DNA reagent is recommended to perform the same protocol to get better cell array formation.
Ujawnienia
Authors have no competing financial interests.
Podziękowania
We thank National Cancer Institute IMAT program from NIH for funding this work [R33 CA174616-01A1].
Materiały
| Name | Company | Catalog Number | Comments |
| Cell attachment 96 well plate kits | Adheren | AP9601 | |
| Suspension Single cell array 8 well chamber slide | Adheren | SS0801 | |
| Adherent Single cell array 8 well chamber slide | Adheren | SS0802 | |
| Lymphoma cell line CD20+ | ATCC | CCL-86 | Raji cells |
| Lymphoma cell line CD20- | ATCC | CRL-8083 | MC/CAR |
| RPMI 1640 with L-glutamine | Life Technologies | 11875-119 | |
| Fetal Bovine Serum | Thermo | SH30070.01HI | |
| Peni/Strep | Life Technologies | 15070063 | |
| Cytosolic dye | Life Technologies | C7025 | Cell Tracker Green |
| Rituxan (Biosimilar) | Eureka Therapeutics | ||
| Human whole blood | Allcells | WB001 | |
| Propidium Iodide | Sigma | P4170-10MG | |
| Automatic imaging system | Molecular Devices | Contact Vendor | Cell Reporter |
| Cell counting program | Molecular Devices | Contact Vendor | Cell Reporter |
Odniesienia
- Hutchinson, L., Kirk, R. High drug attrition rates--where are we going wrong. Nat Rev Clin Oncol. 8, 189-1890 (2011).
- Moreno, L., Pearson, A. D. Attrition rates be reduced in cancer drug discovery. Informa healthcare. 8, 363-368 (2013).
- Seok, J., et al. Inflammation and Host Response to Injury, Large Scale Collaborative Research Program. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 110, 3507-3512 (2013).
- Suntharalingam, G., et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 355, 1018-1028 (2006).
- Eastwood, D., et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 161, 512-526 (2010).
- Hsiao, S. C., Liu, H., Holstlaw, T. A., Liu, C., Francis, C. Y., Francis, M. B. Real time assays for quantifying cytotoxicity with single cell resolution. PLoS One. 8, 10-1371 (2013).
- Wang, S. Y., Weiner, G. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Expert Opin Biol Ther. 8, (2008).
- Dalle, S., Thieblemont, C., Thomas, L., Dumontet, C. Monoclonal antibodies in clinical oncology. Anticancer Agents Med Chem. 8, 523-532 (2008).
- Brunner, K. T., Mauel, J., Cerottini, J. C., Chapuis, B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 14, 181-196 (1968).
- Korzeniewski, C., Callewaert, D. M. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 64, (1983).
- Gerlier, D., Thomasset, N. J. Use of MTT colorimetric assay to measure cell activation. Immunol Methods. 94, 57-63 (1986).
- Toriello, N. M., et al. Integrated microfluidic bioprocessor for single-cell gene expression analysis. Proc Natl Acad Sci U S A. 105, (2008).
- Douglas, E. S., Hsiao, S. C., Onoe, H., Bertozzi, C. R., Francis, M. B., Mathies, R. A. DNA-barcode directed capture and electrochemical metabolic analysis of single mammalian cells on a microelectrode array. Lab on a Chip. 9, 2010-2015 (2008).
- Mayr, L. M., Bojanic, D. Novel trends in high-throughput screening. Current opinion in pharmacology. 9, 580 (2009).
- Zanella, F., Lorens, J. B., Link, W. High content screening: seeing is believing. Trends in Biotechnology. 28, 237-245 (2010).
- Coelho, J., P, L., Quinn, S., Murphy, R. F. Automated image analysis for high-content screening and analysis. J Biomol Screen. 15, 726-734 (2010).
- Sundberg, S. A. High-throughput and ultra-high-throughput screening: solution- and cell-based approaches. Current Opinion in Biotechnology. 11, 47 (2000).
- Simmons, L. A. Personalized medicine is more than genomic medicine: confusion over terminology impedes progress towards personalized healthcare. PERS MED. 9, 85 (2012).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone