Method Article
Genome-Wide CRISPR Screen for Unveiling Radiosensitive and Radioresistant Genes
* These authors contributed equally
In This Article
Summary
A meticulous and structured approach is given to select resistant and sensitive genes of radiation through the application of a genome-wide CRISPR/Cas9 screen method. This protocol also has the potential to serve as a versatile framework for other research endeavors investigating the mechanisms of resistance to clinically administered chemical drugs.
Abstract
The CRISPR-Cas9 system has been harnessed and repurposed into a powerful genome editing tool. By leveraging this technology, researchers can precisely cut, paste, and even rewrite DNA sequences within living cells. Nevertheless, the application of CRISPR screen technology goes far beyond mere experimentation. It serves as a pivotal tool in the fight against genetic diseases, systematically dissecting complex genetic landscapes, empowering researchers to unravel the molecular mechanisms underlying biological phenomena, and enabling scientists to identify and target the root causes of illnesses such as cancer, cystic fibrosis, and sickle cell anemia. Among all, cancer poses a formidable challenge for medicine, spurring eradication efforts. Radiotherapy, as a traditional treatment, yields results but has limitations. It eradicates cancer cells but also damages healthy tissues, causing adverse effects that reduce quality of life. Additionally, not all cancer cells respond to radiotherapy, and some may develop resistance, worsening the condition. To address this, a comprehensive whole-genome CRISPR screen technology is introduced, as it enables the efficient identification of radiosensitive and radioresistant genes, thereby advancing the field of cancer research and treatment. A genome-wide CRISPR screen was conducted in lung adenocarcinoma cells exposed to irradiation following the described protocol, through which both radioresistance- and radiosensitivity-associated genes were identified.
Introduction
The investigation of biological phenomena is inherently intertwined with the study of cellular behaviors, and in turn, the examination of cellular behaviors is fundamentally connected to the exploration of its genome. As modern technology continues to evolve, medical researchers are progressively redirecting their attention toward altering cellular behaviors via gene editing in order to enhance the treatment outcomes of various diseases. In this regard, clustered regularly interspaced short palindromic repeats (CRISPR) technology has emerged as a revolutionary tool for genome editing due to its relatively simple application1. The CRISPR-Cas9 system consists of Cas9 nuclease and single-guide RNA (sgRNA), which specifically recognizes and binds to the target DNA sequence, guiding Cas9 nuclease to cut at that location, resulting in a double-strand break (DSB) in the genome DNA2,3,4. Additionally, the introduction of other substances can lead to specific insertions, deletions, or mutations in the genome, enabling targeted gene editing.
In functional genomics research, RNA interference (RNAi) screen was once a widely used method for conducting large-scale loss-of-function experiments to investigate gene roles in cancer. The RNAi technology studies gene function by specifically silencing target genes, helping researchers identify critical oncogenic factors. However, it is limited by off-target effects and incomplete gene knockdown efficiency. Off-target effects may lead to silencing of other non-target genes, thereby compromising the accuracy and reliability of experimental results1,2. Additionally, RNAi exhibits low knockdown efficiency for certain genes, potentially failing to fully suppress target gene expression. In contrast to traditional RNAi screens, the CRISPR screen demonstrates higher specificity and efficiency3. This technology not only enables precise editing of specific genes but also allows for genome-wide large-scale screening, providing robust support for gene function research. CRISPR screen technology, a powerful gene editing tool based on the CRISPR-Cas9 system, is used for efficiently screening and unveiling unknown functions of specific genes in cells5,6,7,8. Researchers design sgRNAs in batches for specific genes or gene regions, and prepare corresponding sgRNA libraries with precision and rigor, ensuring their integrity and functionality9. These sgRNA libraries are then encapsulated into lentiviral particles, which are utilized to efficiently infect host cells. Following successful infection, the infected cells are cultivated under personally defined screening conditions. Upon screening, the genomic DNA of the screened cells is extracted, maintaining high standards of purity and quantity. Subsequently, targeted regions of sgRNA interest are subjected to PCR amplification, a process that accurately replicates the desired segments of nucleic acids3,9. Finally, high-throughput sequencing is performed on the amplified DNA fragments, enabling comprehensive and efficient analysis of the targeted regions, thus providing valuable insights into the function and behavior of the genes under study4.
Cancer poses a formidable threat to human health as a complex disease. Worldwide, researchers and clinicians are making concerted efforts to unravel the molecular mechanisms of carcinogenesis and develop novel therapeutic strategies. International collaborations have been established to accelerate the translation of basic research findings into clinical applications, with the ultimate goal of improving patient outcomes. Sasmal et al. proposed a bioorthogonal assembly strategy based on a synthetic host-guest system for precise targeting of metastatic cancer cells, which has significantly assisted dozens of scientists in advancing medical technologies. Their outstanding research work has high innovation and unique insights, making meaningful contributions to the scientific community10. Cancer is characterized by the tumultuous state of genomic instability, arising from the erratic regulation of the DNA damage responses11-14. DNA damage includes single-nucleotide defects, single-strand breaks, and DSBs. Homologous recombination (HR) and non-homologous end joining (NHEJ) participate in the repair of DSBs at different stages15,16,17. On this basis, radiotherapy has emerged as a viable treatment option, which utilizes high-energy rays (such as X-rays and γ-rays) to irradiate tumor tissue, causing DNA damage in tumor cells, thereby disrupting their growth and proliferation18. However, radiotherapy does not always yield the desired effects in a significant proportion of cancer patients, potentially resulting from damages to paracancerous tissues and limitations imposed by the tumor's inherent characteristics, such as low sensitivity to radiotherapy19,20,21.
Theoretically, any cell type can be used for a CRISPR screen. However, maintaining sufficient representation in mutated populations requires a large number of starting cells. Cell types with low abundance are not particularly suitable for genome-wide screen. As for the choice of library, most libraries contain 3-6 gRNAs per target gene, and maintaining the distribution of each gRNA within the population is critical18. Loss of representation due to enrichment or depletion of specific gRNAs may lead to uneven result distribution. To address this issue, opting for commercially available CRISPR libraries that have been market-tested may be a preferable choice20. In vitro CRISPR screen using homogeneous cancer cell lines may not fully capture the genetic and epigenetic heterogeneity of in vivo tumors. While in vitro screen revealed key genes involved in DNA damage repair and radiation-induced autocrine signaling, it did not fully replicate the tumor microenvironment, including hypoxia-induced radioresistance (via ROS, metabolic adaptation, and autophagy), immune-mediated paracrine effects, and ECM-dependent cytokine modulation. Before employing the CRISPR screen to explore genes associated with radiation sensitivity or resistance, these factors must be carefully considered. In light of the current treatment landscape, it is urgent to identify and deeply study factors associated with radioresistance and radiosensitivity to effectively enhance radiotherapy efficacy22. Given the CRISPR screen's key advantage in studying the functions of unknown genes, a systematically detailed whole-genome CRISPR screen technology is provided to efficiently identify radiosensitive and radioresistant genes.
Protocol
The reagents and the equipment used in this study are listed in the Table of Materials.
1. Selecting an appropriate dose of radiation
- Preparation and plating of adherent cells
- Adjust the cell density to 5 x 105 cells per mL with RPMI 1640 complete cell culture medium containing 10% FBS for passage and cell growth. Distribute the cells into 3.5-cm culture dishes for radiation at different doses. Add 2 mL (1 x 106 cells) to each dish and incubate overnight at 37 °C with 5% CO2.
- Application of different radiation doses
- Number the 3.5-cm culture dishes from 1 to 5. Utilize Group #1 as the control group, with the remaining 4 groups designated as the treatment groups.
- Seal the edges of the 6-well plates with a sealing membrane for treatment groups and administer radiation doses of 2, 4, 6, and 8 Gy, respectively. After treatment, return the dishes to the incubator.
- Plating of cell lines after radiation
- Prepare 6-well and 96-well plates. Wash the cells in the 3.5-cm culture dishes once with PBS, digest the logarithmically growing cells with 0.25% trypsin, and adjust the cell density to 1 x 105 cells per mL with RPMI 1640 complete medium containing 10% FBS.
- Seed 10 µL/well (1,000 cells/100 µL) into the 6-well plates at 3 replicates for each radiation dose. Add 2 mL of complete medium to each well and count after 14 days (when each clone group has approximately 50 cells).
- Seed 30 µL/well (3,000 cells/100 µL) into the 96-well plates at 5 replicates for each radiation dose. Add 70 µL of complete medium to each well and measure cell viability after 72 h.
- Measurement of cell viability
NOTE: Clonogenic inhibition rate = 1 - (number of clones in the treatment group/number of clones in the control group) x 100%. The CCK-8 assay utilizes a water-soluble tetrazolium salt (WST-8) that can be reduced by cellular dehydrogenases to generate a highly water-soluble yellow formazan product11. The amount of formazan produced is directly proportional to the number of viable cells, and its optical density (measured at 450 nm wavelength) accurately reflects cellular metabolic activity and proliferative status11,13. Based on this well-established principle, the CCK-8 assay has been widely adopted for various applications, including cell proliferation assays and tumor drug sensitivity tests. In this protocol, the CCK-8 assay is employed to systematically evaluate cellular viability under different radiation doses.- Mix CCK8 reagent with RPMI 1640 medium without FBS in a 1:9 ratio. Discard the medium in the 96-well plates and add 100 µL of the CCK8-containing medium to each well.
- Incubate in the dark for 1 h and measure the OD value at 450 nm using a microplate reader.
NOTE: Cell survival rate = [(OD value of the treatment group - OD value of the blank well) / (OD value of the control group - OD value of the blank well)] x 100%
- Selection of radiation dose
- Integrate the clonogenic inhibition rate with the cell survival rate in order to select an appropriate radiation dose based on research needs. Select a radiation dose with an inhibition rate of 50% for screening both radioresistant and radiosensitive genes.
2. Selecting the appropriate MOI and puromycin concentration
- Plating of adherent cells
- Adjust the cell density to 3 x 105 cells per mL and inoculate 1 mL (3 x 105 cells) per well into a 12-well plate. Incubate the plate overnight at 37 °C with 5% CO2.
- Lentiviral infection
- Use a pipette to aspirate the medium from the 12-well plate and replace it with 1 mL of RPMI 1640 complete medium containing 10% FBS. Prepare a quality-checked, whole-genome CRISPR lentiviral library (commercially available libraries are recommended) and set up a logarithmic concentration gradient (e.g., 0-10-50-100-200-400-800)4,5,6,7,8.
- Add the corresponding amount of lentivirus to 2 µL/dish of polybrene and equilibrate at room temperature for 5 min.Slowly drip the lentivirus and polybrene mixture into each well, mix well, and incubate overnight at 37 °C with 5% CO2.
- Determination of the minimum puromycin concentration for cell killing
- Adjust the parental cell density to 3 x 105 cells/mL and inoculate 1 mL (3 x 105 cells) per well into a 12-well plate. Incubate the plate overnight at 37 °C with 5% CO2.
- Add puromycin to each well of the 12-well plate in a concentration gradient of 0-0.1-0.2-0.5-1-2-4-8 µM. Count the cells after 72 h. The minimum concentration that kills all cells in the well is the minimum puromycin concentration for cell killing, which will be used for subsequent screening of virally infected cells.
- Puromycin selection post-infection
- On the second day after infection, aspirate the medium from each well and replace it with 1 mL of RPMI 1640 complete medium containing 10% FBS. Continue culturing for 48 h.
- After 72 h of infection, replace the existing medium with complete medium containing the minimum puromycin concentration for cell killing. Set up 2 wells in the 12-well plate without lentiviral infection as negative and positive controls. Treat the negative control with puromycin, and leave the positive control untreated. Continue culturing for 72 h.
- After 72 h of puromycin selection, all parental cells in the negative control will be dead, while the parental cells in the positive control show minimal death. Calculate the MOI for each well.
NOTE: MOI = (Number of cells in virus-infected group / Number of cells in the positive control group) x 100%. Use the virus concentration with an MOI of ~0.3 for subsequent screening.
3. Genome-wide CRISPR lentiviral library infection
- Seeding of adherent cell lines
- Adjust the cell density to 1 x 107 cells per mL in RPMI 1640 complete medium containing 10% FBS for passaging and cell growth. Inoculate 1 mL of cells (1 x 107 cells) into each 15-cm culture dish at 37 °C with 5% CO2 for 8 h. Once the cells adhered and reached a confluence of 70%-80%, they were ready for viral infection (making a copy number of approximately 500).
- Lentiviral infection
- Aspirate the medium from the 15-cm culture dishes and replace it with 15 mL of RPMI 1640 complete medium containing 10% FBS. Prepare a quality-checked whole-genome CRISPR lentiviral library.
- Add the corresponding amount of lentivirus with the MOI = 0.3 to 30 µL/dish of polybrene and equilibrate at room temperature for 5 min, slowly drip the lentivirus and polybrene mixture into the 15-cm culture dishes, mix well, and incubate at 37 °C with 5% CO2 overnight. Simultaneously, prepare a 15-cm culture dish with the parental cell as a contrast.
- Puromycin selection post-infection
- On the second day after viral infection, aspirate the medium from the 15-cm culture dishes and replace it with 15 mL of RPMI 1640 complete medium containing 10% FBS. Continue culturing for 48 h.
- At 72 h post-infection, replace the medium with complete medium containing the minimum lethal concentration of puromycin. Treat the uninfected parental cells as a negative control in the same manner and continue culturing for 72 h. After 72 h of puromycin selection, the parental cells in the negative control group will all be killed, and the surviving cells from the lentiviral infection are considered successfully infected.
- Extraction of Day 0 genome
- Digest the cells from one 15-cm culture dish using 0.25% trypsin, resuspend in RPMI 1640 complete medium containing 10% FBS, and count the cell number. Label this sample as Day 0.
- Centrifuge at 300 x g for 5 min (at room temperature) and discard the supernatant. Resuspend in 1 mL of PBS, centrifuge at 300 x g for 5 min, and discard the supernatant. Extract Day 0 genomic DNA, measure the DNA concentration and purity using a nanodrop UV spectrophotometer.
- For sgRNA integrity detection, use the agarose gel electrophoresis to analyze the amplified sgRNA library, ensuring that the bands were clear and no significant degradation, thereby preserving the integrity of the library23. For lentiviral library coverage evaluation, employ deep sequencing technology to conduct sequencing analysis on the sgRNAs within the library24,25.
- In the CRISPR screen, PCR amplification serves as a preliminary verification of sgRNA fragment integrity and library quality6. To more comprehensively analyze library coverage and sgRNA distribution patterns, and to ensure adequate representation of sgRNAs for each target gene during screening, perform NGS for in-depth insights into the robustness of screen9.
NOTE: By detecting changes in pre- and post-screening sgRNA abundance, and identifying potential sgRNA off-target integration or library contamination, the accuracy and reliability of the CRISPR screen could be further improved. These Day 0 samples serve as crucial negative controls and undergo comprehensive quality assessment through PCR amplification and NGS to achieve 2 key objectives: (1) confirmation of essential gene depletion (indicating adequate library representation), and (2) demonstration of stable expression in non-essential genes (establishing experimental baseline conditions)9. - Ensure that the coverage reaches the expected level to ensure the diversity and representativeness of the library and avoid biases in the screening process. For infection efficiency measurement, use fluorescence imaging to assess infection efficiency, ensuring that it meets the experiment requirements.
4. Application of radiation as a screening condition
- Grouping
- Adjust the density of CRISPR-infected cells to 1 x 107 cells per mL and inoculate 1 mL into each 15-cm Petri dishes. Incubate overnight at 37 °C with 5% CO2.
- Radiation treatment
- Randomly divide the cells into 2 groups: a treatment group and a control group, with 6 dishes per group. Administer an appropriate dose of radiation to the cells in the treatment group, while leave the control group cells untreated to propagated normally. After radiation, continue to incubate at 37 °C with 5% CO2 for 7 days.
- In the second week, repeat administering an appropriate dose of radiation to the treatment group cells, leaving the control group cells untreated and allowing them to propagate normally. After radiotherapy, continue to incubate at 37 °C with 5% CO2 for another 7 days.
5. Genome extraction and sequencing
- Extraction of Day 14
- After 14 days of treatment, digest the cells in the treatment and control groups with 0.25% trypsin, resuspend them using complete RPMI 1640 medium containing 10% FBS, and label them as Day 14-RT or Day 14-NC.
- Centrifuge the cells at 300 x g for 5 min and discard the supernatant. Resuspend in 1 mL of PBS, centrifuge at 300 x g for 5 min, and discard the supernatant again. Extract the genomic DNA of Day 14, and detect the concentration, and purity of DNA using nanodrop UV absorbance spectroscopy.
- PCR amplification
- Prepare corresponding primers of the sequence (see Table of Materials) and dilute them to 10 µM.Set up a 20 µL reaction system by adding the reagents into a sterile microcentrifuge tube, centrifuge for 5 s at 300 x g, and mix well. Set the PCR instrument parameters according to the amplification condition to obtain PCR products.
- Agarose gel electrophoresis detection
- Prepare a gel casting plate, seal the edges of the mold with agarose, insert the comb, and prepare an agarose gel of appropriate concentration according to the length of the sample DNA.
- Accurately weigh a certain amount of agarose powder, add an appropriate amount of electrophoresis buffer, mix well, and heat in a microwave oven to melt. After cooling slightly, add an appropriate amount of nucleic acid dye, mix gently, and pour slowly into the gel casting mold. Allow the gel to solidify for 30 min.
- Remove the comb and add an appropriate amount of electrophoresis buffer to the electrophoresis tank until it covers the gel. Add an appropriate amount of loading buffer to the DNA sample, mix well, and use a pipette to slowly add the mixture to the sample well.
- Set the appropriate voltage based on the size of the DNA fragment and the concentration of the agarose gel. After electrophoresis, carefully remove the gel and place it in a gel imager to observe the results and check if the PCR amplification was successful.
- Illumina sequencing
- Collect the genomic DNA from the three groups (Day 0 group, control group, and treatment group), and send it to a company for library construction and Illumina sequencing. Perform bioinformatic analysis and visualization of the sequencing results to obtain radiotherapy-sensitive and radiotherapy-resistant genes26.
- Set up multiple repeated experiments for data analysis, and conduct statistical analysis on the experimental results to ensure the consistency and reproducibility of the data27,28.
- Quality assessment of sequencing data
- Subject the raw data to rigorous filtering and quality control mechanisms to guarantee the precision of sequencing information5. Compute the Phred score (Qphred) for each nucleotide based on the sequencing error rate, using a specified conversion algorithm and a model that evaluates the likelihood of an error occurring during base calling28.
- Maintain the sequencing error rate for each base position below 1% (equivalent to a Q30 threshold), and ensure that at least 80% of the sequencing data achieves this Q30 standard to support subsequent analytical procedures.
- Data processing and sample-level quality control
NOTE: For details, please refer to previously published reports29,30.- Align the filtered reads from sequencing data with sgRNA library sequences. Report statistics, including the count of sgRNAs in the library with perfect alignment, the average abundance of sgRNAs, the number of sgRNAs not detected, and the proportion of reads from individual samples successfully mapped to the sgRNA library. Use a higher mapping ratio to indicate greater coverage.
- Count the enrichment of reads for each gene (targeted by different sgRNAs) in each sample. Normalize the supporting reads for each sgRNA across samples using MAGeCK's "median" normalization method. Perform sample-level quality control by evaluating the distribution of sgRNA read counts, generating box plots, conducting principal component analysis (PCA), and constructing correlation heatmaps.
- Assume that sgRNA read counts follow a Poisson distribution. Depict normalized sgRNA counts from different groups in box plots to visualize the overall data distribution within samples and compare distributions between groups.
- Use PCA to simplify data analysis by reflecting differences between principal components and the rate of variation within each component, facilitating the observation of intra- and inter-group variations. Use a correlation heatmap to illustrate the relationships among samples.
- Basic analysis
- Perform differential sgRNA analysis between groups and identify essential genes after quality control and preliminary data processing. Conduct functional enrichment analysis of the relevant genes30. Rank essential genes based on the robust rank aggregation (RRA) score calculated by MAGeCK or other statistical methods such as MLE, with a lower RRA score indicating higher importance.
- Use R language or other programming languages to perform bioinformatics analysis on the ranked results, and visualize the findings using GO and KEGG analysis diagrams30. Link genes or proteins to corresponding GO terms (molecular function, biological process, and cellular component) through ID mapping or sequence annotation.
- Use KEGG as a database to understand high-level functions and biological systems, connecting the identified gene catalog to system functions at cellular, species, and ecosystem levels. Select the top 10 or 20 pathways from GO and KEGG analyses for an intuitive display of pathway directions.
Results
Lung cancer, with the leading mortality rate, represents a highly aggressive and prevalent medical disease. Using the lung cancer cell line A549 as an example to conduct a genome-wide CRISPR screen with radiation as the screening condition, the schematic workflow is shown in Figure 1. First, explore the sensitivity of A549 cells to different doses of radiation through clone formation and CCK8 experiments (Figure 2). In the clonogenic assay, the colony count was 140 ± 5.35 at 2 Gy versus 303 ± 31.63 at 0 Gy, while in the CCK-8 assay, the optical density (OD) value was 0.65 ± 0.05 at 2 Gy versus 1.35 ± 0.08 at 0 Gy. To investigate candidate genes for radiosensitivity and radioresistance, select 2 Gy (IC50) as the subsequent radiation dose. A549 cells were treated with different doses of puromycin and counted after 72 h. The minimum killing concentration of puromycin for A549 cells was detected as 1 µM (Figure 3). A549 cells were infected with lentiviruses at different gradient MOIs. After 72 h of infection, the infection efficiency was observed by fluorescence microscope and counted before and after 72 h of puromycin treatment. The MOI with an infection efficiency of ~0.3 was obtained (Table 1). The human genome contains 19,050 genes, with 6 sgRNAs corresponding to each gene and a copy number of 500 (19,050 x 6 x 500 = 5.7 x 107 cells). Using lentiviruses with MOI = 0.3 and, accordingly, infect 5.7 x 107 A549 cells. After 72 h, the cells were treated with 1 µM puromycin for an additional 72 h. The Day 0 genome was harvested, and PCR amplification was performed. The sgRNA sequences within the CRISPR whole-genome library exhibit a length of 231 base pairs, which is corroborated by the agarose gel electrophoresis results (Figure 4).
The PCR products were subsequently sent to Novogene for data quality control and sample-level quality control. The mapping ratio yielded a comprehensive coverage rate of approximately 60%, a metric deemed adequate for whole-genome screening procedures, given the sheer complexity and inevitable attrition during the processing of an entire genome (Figure 5). The sgRNA read counts adhered to a Poisson distribution, conforming to theoretical expectations. The subsequent analysis through PCA plotting and correlation heatmapping vividly portrayed the discernible disparity between distinct groups, with inter-group variation markedly outweighing intra-group discrepancies. Moreover, the variation rate within the grouped samples fell within acceptable boundaries, substantiating the success of the sample-level quality control measures. Subsequently, the RRA rankings devised by MAGeCK will be harnessed to embark on a foundational bioinformatics assessment of the ranking outcomes utilizing the R language. Notably, the top 15 pathways in GO terms prominently featured mechanisms linked to DNA damage, which seamlessly aligns with the experiment's underlying screening criteria.

Figure 1: Schematic of genome-wide radiotherapy CRISPR/Cas9 screen in A549 lung cancer cells. A schematic representation of the screening workflow in A549 cells using genome-wide CRISPR/Cas9 and radiotherapy. Please click here to view a larger version of this figure.
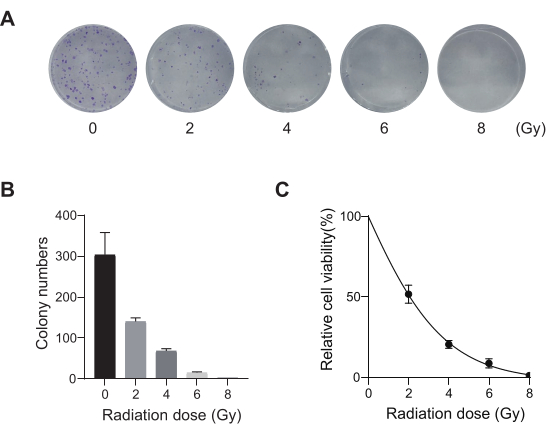
Figure 2: Radiation dose optimization for A549 cells. (A) A549 cells were exposed to increasing doses of radiation (0 Gy, 2 Gy, 4 Gy, 6 Gy, and 8 Gy) and allowed to grow for 14 days. Colony formation assay images are shown. Results are representative of two independent biological experiments. (B) Quantification results of cloned formation assay images. Error bars represent standard deviation (n = 3). One-way ANOVA yielded P < 0.01. Results are representative of two independent biological experiments. (C) Dose-response survival curves of A549 cells following exposure to different radiation doses. Error bars represent standard deviation (n = 3). One-way ANOVA yielded P < 0.01. Results are representative of two independent biological experiments. Please click here to view a larger version of this figure.
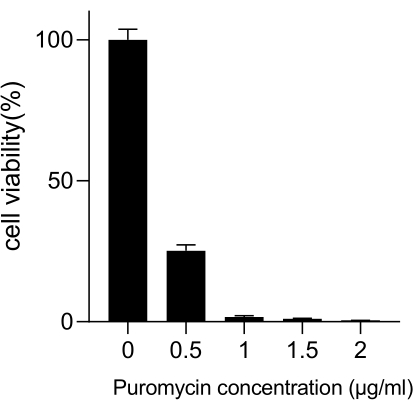
Figure 3: Determination of the minimum puromycin concentration required for A549 cell selection. A549 cells were treated with increasing concentrations of puromycin and incubated for 72 h. Error bars represent standard deviation (n = 3). One-way ANOVA yielded P < 0.01. Results are representative of two independent biological experiments. Please click here to view a larger version of this figure.
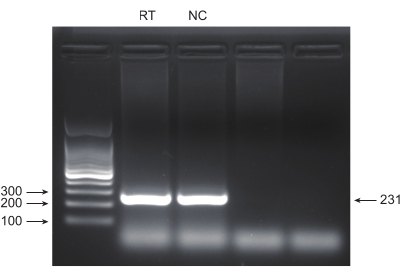
Figure 4: PCR product validation by agarose gel electrophoresis. Agarose gel electrophoresis was used to examine the presence and quality of PCR product bands. Please click here to view a larger version of this figure.

Figure 5: Quality control and basic analysis of PCR-amplified products. (A) The mapping ratio indicated approximately 60% coverage of the reference genome. (B) Read count distribution of sgRNAs followed a Poisson distribution. (C) Most of the top 10 Gene Ontology (GO) terms were associated with DNA damage response. (D) Volcano plot showing gene enrichment patterns, with negatively enriched genes in red, positively enriched genes in blue, and non-significant genes in gray. Please click here to view a larger version of this figure.
| Virus particle number (vp) | Count number (before puromycin treatment) | Count number (after puromycin treatment) | Infection efficiency |
| 1.23 × 104 | 1.23 × 105 | 1.04 × 104 | 0.084552846 |
| 3.72 × 104 | 1.24 × 106 | 3.61 × 104 | 0.291129032 |
| 6.15 × 104 | 1.23 × 107 | 5.4 × 104 | 0.43902439 |
Table 1: A549 cell counts before and after puromycin selection under different virus particles. Counts of A549 cells recorded prior to and following puromycin treatment under varying virus particles.
Discussion
As a cutting-edge gene editing technology, the CRISPR screen has sparked profound changes in the field of scientific research5. Arising from the CRISPR-Cas9 system, this technology has become an essential tool for studying gene functions due to its high efficiency and precision9. The CRISPR/Cas9 engineering principle involves designing and introducing specific sgRNAs with approximately 20 nucleotides to guide the Cas9 nuclease to precisely locate and cut target DNA sequences, enabling gene editing such as knockout, activation, or repression6. This principle underlies the development of high-throughput and customizable CRISPR screen. By constructing libraries containing a vast number of sgRNAs, researchers can simultaneously edit and screen multiple genes, rapidly identifying those associated with specific phenotypes or disease states. Furthermore, the CRISPR screen allows for experimentation in various cell types, tissues, and even entire organisms, providing a more comprehensive understanding of gene function and mechanisms of action.
With the speeding technology development, CRISPR screen has shown tremendous potential for application in various fields. For instance, CRISPR screen can assist scientists in discovering novel drug targets, thereby accelerating the process of drug discovery and development. In disease research, this technology reveals the mechanisms of disease pathogenesis, offering fresh insights and approaches for diagnosis and treatment. CRISPR screen differs fundamentally from traditional screening methods (such as RNAi and small molecule inhibitors) in several key aspects: Mechanistically, CRISPR achieves permanent genetic modifications through gene editing, while RNAi only temporarily suppresses gene expression and small molecule inhibitors reversibly block protein function28,29. In terms of specificity, CRISPR demonstrates superior precision through accurate DNA targeting, whereas RNAi is prone to off-target effects3,5. Regarding duration of effect, CRISPR creates permanent genetic alterations, compared to the transient interventions of conventional methods. Furthermore, CRISPR enables genome-wide screening capabilities and can simultaneously investigate multiple genes/pathways, while maintaining high efficiency across diverse cell types9. Traditional approaches are typically limited to specific pathway analysis and often show reduced efficacy in certain cell lines3. These distinctive characteristics make CRISPR screen particularly valuable for functional genomics research and target discovery, especially in studies requiring long-term observation or precise genetic regulation, where it demonstrates unique advantages over conventional screening methodologies.
However, the specificity of this process is not absolute, and it is possible for the Cas9 protein to erroneously recognize and cleave DNA regions that are similar but not entirely identical to the intended target sequence5. This occurrence is commonly referred to as off-target effects. This unintended DNA cleavage can lead to genomic instability and unpredictable genetic consequences, especially when applied in human therapy. CRISPR technology also struggles when dealing with complex genomic editing tasks. When confronted with editing demands that encompass numerous genes, gene clusters, or intricate genetic settings, the CRISPR system may experience limitations in precisely anticipating and directing the outcomes of the editing process1.
The primary technical hurdle in conducting genome-wide screening using CRISPR technology stems from the vast complexity of the genome, which encompasses tens of thousands of genes, thereby significantly increasing the risk of off-target effects9. To ensure the integrity and reliability of subsequent sequencing outcomes, it is imperative that each cell is transfected with a singular sgRNA23. This necessitates the meticulous control of the MOI by the experimenter, ensuring that it remains below 0.5 while simultaneously maintaining an adequate copy number threshold, which must be at least 500 or greater24. Given the fact that each of the tens of thousands of genes in the entire genome necessitates the utilization of 4-6 sgRNAs, the entire screening process necessitates an extensive quantity of cells25. The prolonged duration of the experiment, the substantial number of cells involved, and the elevated risk necessitate that the experimenter devise strategies to alleviate these concerns26. The potential genes are required to be further validated with RNA-seq, as well as both gain- and loss-of-function assays. In the following experiments, after obtaining the overexpression and knockout cells of identified genes using CRISPR editing, genome-wide genomics, and transcriptomics were performed to access potential off-target alterations, particularly at loci with partial sgRNA complementarity that may undergo nonspecific Cas9 cleavage. Bioinformatics prediction tools such as COSMID might help identify high-risk off-target sites. Rescue assays could also be used to ensure the specificity of the involvement of identified genes.
In the subsequent analysis, the criteria for differentially expressed sgRNAs relied on the robust rank aggregation (RRA) score calculated by MAGeCK, which involved statistical significance, fold change, and replicate consistency31,32. For a dataset, each gene is ranked according to comprehensive criteria combining gene expression levels and statistical significance. RRA utilizes Spearman's rank correlation coefficient for rank to evaluate the situation of the comprehensive rank of each gene in all datasets and integrates the sorting results of multiple gene expression datasets. A gene with a relatively high rank in most of the datasets might be regarded as a genuine differentially expressed gene33. A p-value of RRA less than 0.05 was considered statistically significant. A fold change of at least a 1.5-fold increase or 33% decrease was regarded as a differentially expression. In addition, the consistency of 3 independent replicates was required to be considered as a statistically different expression. After the RRA ranking for essential genes, a lower RRA score signifies higher importance.
As a potent tool for genome editing, CRISPR-Cas9 technology demonstrates unparalleled potential and accuracy in investigating the area of radioresistance26,27. However, its application still has limitations. Apart from the risk of off-target effects, the intricacies associated with radiosensitivity introduce complexities into the screening process27. This sensitivity is not merely governed by a solitary gene, but rather emerges as a consequence of the intricate interplay between multiple genes and their regulatory networks26. Hence, despite the successful identification of specific genes pertaining to radiosensitivity, they might only represent a fraction of the broader picture, posing significant challenges to comprehensively elucidate the underlying mechanisms34,35,36. Here are solutions to common issues in CRISPR screen, such as low infection efficiency, sgRNA degradation, and sequencing failure: since CRISPR screen requires an MOI value <0.5, cell state adjustment is critical for ensuring that cells are in a logarithmic growth phase or evaluate infection efficiency via fluorescence assays; potential causes of sgRNA degradation include nuclease contamination or vector design flaws, so repeated freeze-thaw cycles of sgRNA libraries should be avoided (store aliquots at -80 °C), and NGS sequencing should be used to verify sgRNA library integrity; sequencing failure may result from insufficient sequencing depth or sgRNA primer design issues, paired-end sequencing is recommended to improve sgRNA sequence identification accuracy, with a suggested sequencing depth of >500× coverage37,38,39,40,41,42.
Radiotherapy remains a cornerstone in cancer treatment, yet exhibits remarkable variability in therapeutic efficacy across different tumor types and even among patients with the same cancer9. This inter-individual heterogeneity frequently necessitates combination therapies to achieve optimal tumor eradication11,12. In clinical practice, the integration of radiotherapy with immunotherapy - particularly immune checkpoint inhibitors and antibody-drug conjugates - has emerged as a prominent therapeutic paradigm19. This protocol presents an in vitro CRISPR screen using a relatively homogeneous cancer cell line, which could not totally reflect the genetic and epigenetic heterogeneity of tumors in vivo. After determining the appropriate dose of radiation for screening, the results provide potential radiosensitive or radioresistant genes, which need to be further confirmed in both in vitro cellular assays and in vivo animal models. Moreover, in vivo CRISPR screen plus single-cell transcriptomics might be helpful in deciphering the complexity of tumor heterogeneity. The in vitro CRISPR screen identified important genes in DNA damage repair within cells, as well as in autocrine mechanisms in response to radiation, but indeed did not fully replicate TME heterogeneous. For instance, hypoxia increases ROS production, triggering a feedback loop that stimulates metabolic adaptation, antioxidant generation, and autophagy activation, ultimately promoting radioresistance18. Immune cells might be activated in the paracrine mechanism after radiation, inducing radiosensitivity14. The ECM serves as a reservoir for growth factors, and cytokines have both positive and negative effects on radiosensitivity21. The in vivo CRISPR screen should be performed to reflect the heterogeneity of TME. Besides, the in vitro CRISPR screen could only identify important genes in DNA damage repair within cells, as well as in autocrine mechanisms in response to radiation, but did not fully capture in vivo influence on radiosensitivity, including tumor vasculature and systemic effects20,21. The in vivo CRISPR screen should be performed to identify potential factors involved in radiosensitivity in vivo.
Here, a comprehensive protocol aimed at utilizing CRISPR genome-wide screening is devised to pinpoint genes that are implicated in either the sensitivity or resistance to radiotherapy. By unraveling novel pathways, this methodology seeks to pave the way for advancements in tumor radiotherapy research. This protocol could also serve as a reference for exploring mechanisms of clinical constraints in chemotherapy and the progression of alternative therapeutic agents. In summary, CRISPR technology embodies significant potential and worth, yet its inherent constraints underscore the importance of sober recognition and a judicious approach. As future research and applications are delved into, it is imperative to relentlessly endeavor to explore and optimize CRISPR technology to overcome its constraints and harness its fullest potential.
Disclosures
None.
Acknowledgements
This study was supported by Regional Science and Technology Innovation Project of Hubei Province (2024EIA001), and Medical Science and Technology Innovation Platform Construction Support Project of Zhongnan Hospital of Wuhan University (PTXM2025001). Figure 1 was created using Figdraw.
Materials
| Name | Company | Catalog Number | Comments |
| 150 mm cell and tissue culture dish | Wuxi NEST Biotechnology Co., Ltd. | 715011 | Cell culture |
| 35 mm cell and tissue culture dish | Wuxi NEST Biotechnology Co., Ltd. | 706011 | Cell culture |
| A549 cell line | ATCC | - | - |
| Cell counting kit-8 | Shanghai Beyotime Biotech Inc | C0041 | For cell viability assay |
| CO2-independent medium | PHCbi | MCO-50AIC | Cell culture |
| Countess 3 FL automated cell counter | Themo Scientific | AMQAF2001 | For cell counting |
| EDTA | Gibco | 25200056 | Cell culture |
| Fetal bovine serum | Gibco | 10099141 | Cell culture |
| Fluorescence microscopy | LEICA | ebq 100-04 | For fluorescence microscope |
| Genome-wide CRISPR lentiviral library | Shanghai OBiO Technology Co., Ltd. | H5070 | For lentiviral infection |
| MiniAmp plus PCR | Themo Scientific | C37835 | For PCR amplification |
| NEST cell culture plates, 12-well | Wuxi NEST Biotechnology Co., Ltd. | 712002 | Cell culture |
| NEST cell culture plates, 6-well | Wuxi NEST Biotechnology Co., Ltd. | 703002 | Cell culture |
| NEST cell culture plates, 96-well | Wuxi NEST Biotechnology Co., Ltd. | 701001 | Cell culture |
| PBS | Gibco | C10010500BT | Cell culture |
| PCR forward primer (AATGGACTATCATATGCTTACCGTAACTTGAAAGTATTTCG) | Beijing AuGCT Biotech Co., Ltd. | - | For PCR amplification |
| PCR reverse primer (GATGTGCGCTCTGCCCACTGACGGGCA) | Beijing AuGCT Biotech Co., Ltd. | - | For PCR amplification |
| Polybrene | Shanghai Beyotime Biotech Inc | C0351 | For lentiviral infection |
| Puromycin | MCE | HY-K1057 | For seletion post lentiviral infection |
| RPMI 1640 cell culture medium | Gibco | 23400-021 | Cell culture |
| TIANGENamp genomic DNA kit | Beijing TIANGEN Biotech Co.,Ltd. | DP304 | For genomic DNA extraction |
| X-ray powder diffractometer | PerkinElmer | For radiotherapy |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved