Method Article
Isolation of Splenic Microvesicles in a Murine Model of Intraperitoneal Bacterial Infection
In This Article
Summary
Microvesicles shed from the plasma membrane act as cellular effectors. Spleen microvesicles (SMVs) are surrogate markers of pathophysiological conditions. In rats and mice, SMV content and properties characterize aging or inflammation and are altered by cytoprotective treatments, making them a valuable yet abundant monitoring tool for preclinical research.
Abstract
Microvesicles (MVs) are submicron fragments released from the plasma membrane of activated cells that act as proinflammatory and procoagulant cellular effectors. In rats, spleen MVs (SMVs) are surrogate markers of pathophysiological conditions. Previous in vitro studies demonstrated that Porphyromonas gingivalis (P. gingivalis), a major periodontal pathogen, enables the endothelial shedding and apoptosis while lipopolysaccharide (LPS) favors the shedding of splenocyte-derived microvesicles (SMVs). In vivo studies showed the feasibility of pharmacological control of SMV shedding. The present protocol establishes a standardized procedure for isolating splenic SMVs from the P. gingivalis acute murine infection model. P. gingivalis infection was induced in young C57BL/6 mice by intraperitoneal injection (three injections of 5 x 107 bacteria/week). After two weeks, the spleens were collected, weighed, and the splenocytes were counted. SMVs were isolated and quantified by protein, RNA, and prothrombinase assays. Cell viability was assessed by either propidium iodide or trypan blue exclusion dyes. Following splenocyte extraction, neutrophil counts were obtained by flow cytometry after 24 h of splenocyte culture. In P. gingivalis-injected mice, a 2.5-fold increase in spleen weight and a 2.3-fold rise in the splenocyte count were observed, while the neutrophils count was enhanced by 40-folds. The cell viability of splenocytes from P. gingivalis-injected mice ranged from 75%-96% and was decreased by 50% after 24 h of culture without any significant difference compared to unexposed controls. However, splenocytes from injected mice shed higher amounts of MVs by prothrombinase assay or protein measurements. The data demonstrate that the procoagulant SMVs are reliable tools to assess an early spleen response to intraperitoneal P. gingivalis infection.
Introduction
Microvesicles (MVs), also termed microparticles or ectosomes, are plasma membrane fragments with a diameter of 0.1-1.0 µm released in body fluids and the extracellular space in response to various cell stimuli. First identified as platelet dust exposing procoagulant phospholipids, mostly phosphatidylserine (PSer), MVs constitute an additional surface for the assembly of the blood coagulation complexes1,2. The key role of circulating MVs as procoagulant effectors has been demonstrated in patients with Scott syndrome2, a rare genetic disease that leads to dysfunctional PSer exposure and bleeding (Supplementary Figure 1). MVs have been extensively studied as circulating biomarkers of thrombotic risk in chronic diseases associated with cardiovascular disorders such as diabetes, chronic kidney disease, preeclampsia, and hypertension3,4. They are currently recognized as true cellular effectors in fluids or organ tissues like the myocardium1. Because they convey active proteins, lipids, and miRNA, they remotely modulate vascular responses such as hemostasis, inflammation, vascular angiogenesis, and vascular growth or tissue remodeling5.
While clinical studies examine the prognosis value of MVs concerning risk factors, MVs isolated from the patient's fluids or tissue enable the ex vivo assessment of their effector properties6. The deciphering of the mechanisms governing MV biogenesis and cross-talk abilities is generally achieved in cell culture models to identify active molecules exposed by or encapsulated within the MVs and their downstream signaling. The MV interactions with target cells will depend on membrane protein/protein binding, when appropriate counter-ligands are available, and/or lipid fusion7.
Under physiological conditions, MVs circulating in the plasma mostly originate from vascular cells, as identified by the lineage cluster differentiation markers (CD)8,9. However, in pathology, notably in cancer10 and graft rejection11,12, MVs are shed from the damaged tissue and bear noxious procoagulant and proinflammatory features. These are detected in the systemic circulation, making them useful probes for monitoring protective or rejuvenation therapies, and possible pharmacological targets13. Because MVs circulate as a dynamic storage pool reflecting vascular and tissue cell homeostasis in health and disease, a better understanding of their remote action also needs to be investigated in vivo, after IV injection or nasal instillation in small animals14,15. Indeed, MVs have been considered major contributors to the intricate pathways coupling exaggerated inflammation and thrombosis16.
Periodontitis is an inflammatory disease of infectious origin affecting tooth-supporting tissues17,18 and is associated with thrombotic risk. It is characterized by gingival bleeding, alveolar bone destruction, tooth mobility and can ultimately lead to tooth loss. Periodontitis is highly prevalent worldwide and affects more than 50% of the population, with 11% presenting a severe form19. Periodontitis is induced by bacterial dysbiosis of the subgingival biofilms, which sustain an exacerbated inflammatory response that triggers tissue destruction20. Over the last decade, periodontitis has been linked to systemic diseases such as cardiovascular disorders, diabetes, and rheumatoid arthritis. The possible explanations are the action of the periodontal pathogens at a distance and/or the increased systemic inflammation mediated by proinflammatory cytokines such as interleukin (IL-1, IL-6) and TNF-α21,22.
Among pathogens associated with the periodontitis onset and development, Porphyromonas gingivalis (P. gingivalis)23 is found in most severe lesions that harvest several virulence factors, including lipopolysaccharide (LPS)24 inducing Toll-like-receptor (TLR)-mediated inflammatory responses25 and the recruitment of neutrophils and polymorphonuclear leukocytes (PMNs) at the site of the initial infection26. The LPS from P. gingivalis activates TLR-4 or TLR-2, facilitating immune detection and bacterial survival evasion27. At the vascular level, activation of TLR2 by LPS is associated with immunothrombosis. The unique ability of P. gingivalis to prompt TLR-2 signaling while TLR-4-dependent recognition is significantly impaired favors persistent low-grade infection28,29.
In vivo procedures to study the MV responses to low-grade pathogen chronic and sustained infection are scarce. Methodological approaches for tissular MVs extraction are poorly described in the literature and generally concern the harvest of MVs from pathological tissues like solid tumors, liver steatosis, atherothrombotic plaques, or grafts11,29,30, while the spleen senses bacteria and viruses in the bloodstream. It also stores erythrocytes, platelets, lymphocytes, monocytes, basophils, and eosinophils involved in the immune response. Granulocytes like neutrophils from the red pulp also generate reactive oxygen species (ROS) and proteases that destroy pathogens and prevent infection from spreading31,32. Amazingly, and to the best of our knowledge, spleen MVs are not investigated in the context of pathogen-induced tissue insults. There is a global unmet need to study the variations of tissular MVs in physiopathology.
In vitro data from our laboratory showed that LPS induces the shedding of procoagulant MVs from rat splenocytes, which in turn prompt the senescence of cultured coronary endothelial cells and a consecutive proinflammatory and proinflammatory procoagulant endothelial phenotype11. The feasibility of the pharmacological control of the spleen MVs was further demonstrated after treating the animal with an optimized omega-3 formula. The oral gavage of middle-aged and aged rats was found to be protective against both the shedding of procoagulant MVs from splenocytes and their prosenescent noxious properties towards the coronary endothelium33.
Compared to blood, the spleen offers the advantage of being an important source of leukocytes in one individual. In addition, a splenic-cardiac axis has recently been proposed3,34, making the spleen a possible contributor to the infection-related cardiovascular risk of beneficial interest for pharmacological control. The assessment of the SMVs properties or release is key in understanding pathogen-induced or inflammatory responses. Interestingly, it can be achieved in treated animals and in different physiopathological models (age, hypertension, diabetes). Indeed, by comparing middle-aged and aged rats33, the differences in spleen MVs properties and release can be evidenced following a simple 24 h splenocyte culture.
Given the above evidences of the alteration of the effector properties of spleen MVs with the physiopathological condition and the feasibility of their pharmacological control in rats, a standardized protocol is described herein for the isolation of murine spleen MVs. The procedure would better fit in-depth investigations of the in vivo mechanisms supporting SMVs-mediated effects, eventually in engineered mice. The procedure was established in C57BL/6 mice using a local intraperitoneal infection by P. gingivalis, to establish proof of a remote action of the pathogen on spleen MV (SMV) effector properties.
Protocol
All experimental protocols were approved by and followed the relevant guidelines of the local Ethics Committee (APAFIS#28745-2020121815051557) and animal care of the home University and of the INSERM. Male Young C57BL/6 mice, 6-8 weeks of age, were used for the experiments. Mice were regularly examined to evaluate pain and stress, and their weights were monitored daily. Unless otherwise stated, all extracting buffers and solutions must be sterile and at room temperature.
1. Animal preparation
- Administer the mice with six intraperitoneal injections of P. gingivalis (PG) every 2 days for 2 weeks (three injections of 5 x 107 bacteria/week, Supplementary File 1).
- Anesthetize the mice using a combination of 100 mg/kg of ketamine and 10 mg/kg of xylazine.
- Sacrifice the anesthetized mice and harvest the spleen after laparotomy.
NOTE: The specific conditions for P. gingivalis infection and the sacrifice of the animals for spleen harvest are detailed in the "animal experiment" section of Supplementary File 1. The schematic representation of the protocol steps is shown in Figure 1.
2. Extraction of splenocytes
- Using a scalpel, mince the spleen tissue into ~1 mm slices in a Petri dish already filled with 3 mL of RPMI containing antibiotics (Streptomycin (100 U/mL)/Penicillin (100 U/mL), Fungizone (250 mg/mL), L-glutamine (2 mM)), and supplemented with 10% Fetal Bovine Serum (FBS) (see Table of Materials).
- Transfer the slices to a sieve (see Table of Materials), which is from beforehand attached to a 50 mL polystyrene tube.
- Use the plunger of a syringe as a pestle to crush the tissues on the sieve.
- Rinse the sieve with the RPMI medium (3 mL).
- Centrifuge the cell suspension eluate at 450 x g for 5 min at room temperature and discard the supernatant with caution by backflow.
3. Removal of erythrocytes via an osmotic shock
- Add 1 mL of ACK buffer (see Table of Materials), mix well, incubate for 3 min at room temperature and shake gently by hand rotation.
- Dilute by adding 9 mL of RPMI and mix well using aspiration and backflow.
- Centrifuge at 450 x g for 5 min at room temperature.
- Discard the supernatant by aspiration and backflow, and resuspend the pellet in 5 mL of RPMI medium.
- Mix gently (aspiration/backflow) and eventually remove any lipidic debris by pipetting or using a sieve.
4. Adjustment of isolated splenocyte concentration
- Fill a 1.5 mL microtube containing 900 µL of RPMI.
- Using a micropipette, take 100 µL of the cellular suspension, add to the 1.5 mL microtube containing 900 µL of RPMI medium, and mix well.
- Transfer 10 µL of the diluted cells into a microtube, add 10 µL of the trypan blue exclusion dye, and mix well.
- Place 10 µL of the solution (step 3.3) into the counting slide of the automatic cell counter (see Table of Materials) and determine the cell count.
NOTE: The device software gives two values: the total cell number and the living cell number and percentage. - Adjust the concentration of living cells to 3 or 5 x 106 cells/mL (Vmax = 20 mL; max total capacity is 100 x 106 cells/mL).
- Seed the cells in a 75 mL flask containing RPMI.
- Incubate the flask vertically in a humidified incubator at 37 °C for 24 h.
CAUTION: Trypan blue is a mutagenic and carcinogenic agent35,36. Ensure to wear gloves while counting, even outside of the hood. Immediately discard tips and fluids into containers and wash the bench surface.
NOTE: If the collected cells are less than 50 x 106, use a 25 mL culture flask. The cell number should not vary much from one individual to the other. The percentage of living cells is generally superior to 80% at this step.
5. Isolation of splenocyte microvesicles
- After 24 h, pour the flask content into a 50 mL polypropylene or polystyrene tube.
- Centrifuge at 450 x g for 15 min at room temperature to remove cells and debris.
- After centrifugation, keep 100 µL of the 1st supernatant (SN1) for an eventual measurement of the initial MV concentration to calculate the isolation yield. Note the SN1 volume.
- Centrifuge the SN1 at 450 x g for 15 min at room temperature.
- Discard pellet and centrifuge again at 450 x g for 15 min at room temperature. Rapidly withdraw and collect SN2 (note the volume).
- Transfer SN2 into 1.8 mL microtubes with conical shape bottoms. Keep 100 µL of SN2 for an eventual measurement of the initial MV concentration to calculate the isolation yield in case too much debris eventually impairs MV measurement in SN1.
- Add 5 mL of RPMI medium to the pellet (cells) and count the splenocytes.
NOTE: The cell suspension can also be fixed for flow cytometry characterization in 0.1% paraformaldehyde (final concentration)37. - Centrifuge SN2 at 14,000 x g for 1 h at 4 °C and eventually refrigerate the rotor in advance by running the empty rotor at 4 °C. This step generates SN3.
- Immediately withdraw SN3 from each microtube by inverting over a single sterile 50 mL tube (under the hood). Note the SN3 volume.
- Gently mix SN3 using a pipette under the hood. Keep aliquots for eventual measurements in the suspension.
NOTE: The solution is now depleted of MVs but contains the soluble mediators and exosomes. - Scrape each microtube (closed) on a spiked rack to mechanically favor the resuspension of the MVs in the pellet.
- Add 200 µL of Hank's balanced saline solution without Ca2+ and Mg2+ (HBSS) in the first microtube (see Table of Materials).
- Resuspend the MV pellet of the first tube by gentle aspiration/backflow cycles (~10 times) using a 1 mL micropipette. Take great care to avoid foaming as this will promote the oxidation of the vesicles and other moieties in the suspension.
- Harvest the first 200 µL of resuspended MVs and pour them into the second tube over the pellet. Gently mix as above, and then pour again into the third tube and mix gently. The suspension becomes more and more turbid from tube to tube. Close the third tube.
- Initiate the collection of the next three microtubes as above (step 4.14).
- Collect all the resuspended MVs in a single (or two) collector microtube (2 mL with conical shape bottom). Keep 50 µL for the eventual calculation of the yield of this step. Note the suspension volume.
- Centrifuge the collector tube at 14,000 x g for 1 h at 4 °C. This step generates SN4.
- Immediately discard the SN4 (pour over a supernatant collecting tube). Keep an aliquot of SN4 to calculate the yield of the purification step and gently resuspend the pellet in 500 µL of HBBS (without Ca2+ and Mg2+). Keep a 50 µL aliquot of the resuspended pellet to calculate the purification yield and note its volume.
- Store the resuspended pellet at 4 °C for up to one month for functional assays under sterile conditions or at -20 °C for structural assays.
- Calculate the purification yield (see NOTE below) of each step or the whole procedure by measuring the concentration of MVs in supernatants and/or pellet suspensions.
NOTE: Purification Yield (%): ([MV1] x SN1 volume) / ([MV4] x final suspension volume) x 100.
This yield needs to be rationalized to the number of splenocytes isolated from the culture flask and counted in step 4.4. The present experiment's purification yield is ~70% starting from 111 x 106 cells and 60 mL of supernatant.- Evaluate the loss of SMVs caused by the procedure by measuring the SMVs remaining in the supernatants depleted of SMVs and mainly containing exosomes (SN2 and SN4).
- Measure the MV concentration by TRPS or prothrombinase assay38 for high sensitivity and specificity.
CAUTION: Harvest the MVs after centrifugation by the rapid upside-down pouring of the supernatant. Waiting or using U-shape microtubes will prompt MV loss on the microtube wall. Never use a micropipette to discard the supernatant; the pellet can be aspirated instead. Never delay the process after the centrifuge is stopped. Overspeed centrifugation will result in contamination of the MV pellet by exosomes.
NOTE: The centrifugation speeds and duration are optimized for an MV enrichment with minimal contamination by exosomes. This protocol is not reliable to study spleen monocyte/macrophage MVs because these cells can phagocyte and rapidly recapture their own MVs39. The protocol cannot be paused and restarted later because of loss of cells due to cell viability.
Results
The data provided give a full representation of the whole procedure, using two main animal conditions: control untreated young C57BL/6 mice and their littermates subjected to six intraperitoneal (IP) PG injections every 2 days, for 2 weeks. They also show the remote action of an intraperitoneal PG injection on the spleen response after 2 weeks. Splenocyte microvesicles were quantified by either prothrombinase enzymatic assay or by measuring their proteins and RNA concentration by spectrophotometric, and the proportion of neutrophils was determined by flow cytometry33,38 in the splenocyte suspension (sections 2-4 of Supplementary File 1 and Supplementary Figure 2).
Spleen weight and cell number increase after 2 weeks of IP injection in P. gingivalis
In young adult mice, the PG injection leads to a significant 2.6-fold increase in spleen weight after 2 weeks, likely due to the immune response. Indeed, the total splenocyte count was also raised by 2.3 folds (Figure 2, n = 5 mice per each condition, p < 0.0001 by Student's T-test).
Splenocyte viability is not impaired by 2 weeks of injection in P. gingivalis
Similar viability rates were measured between treated and untreated mice after splenocyte isolation or 24 h after cell culture. Nevertheless, when the initial percentage of living cells at t0 was below 76%, viability was reduced in both groups by ~50% after 24 h. (see Ctl 1-3, Table 1, as an example). Conversely, and whatever the animal condition, the 24 h recovery was enhanced when the proportion of living cells reached 90% or more at t0, pointing at the crucial role of the first isolation steps (Figure 3).
Splenocyte neutrophil count is enhanced in P. gingivalis-injected mice
After double staining, neutrophils were identified by flow cytometry as Mac1+ and LYG-6-(Gr1)+ (see Table of Materials and section 4 in Supplementary File 1). In response to PG, the neutrophil spleen count was significantly enhanced by 40-folds just after cell isolation (t0, 4.8% ± 0.6 vs. 0.86% ± 0.06). Nevertheless, it is worth considering that after 24 h culture, the neutrophil proportion in the splenocyte suspension increases from 4.8% ± 0.6 to 14.67% ± 2.02 (Figure 4).
The shedding of MVs from splenocytes increases in mice infected by P. gingivalis
Following the protocol, we proved that the isolated splenocytes released more MVs when mice were subjected to PG infection. By comparison, to control individuals and the measurement assay, MVs shed in the supernatant were roughly tripled in PG-injected mice, using either the detection by prothrombinase assay that refers to the total surface of procoagulant anionic phospholipids borne by SMVs (Figure 5A), the protein content of SMVs measured by spectrophotometry (Figure 5B), or the RNA content of SMVs assessed by spectrophotometry (Figure 5C, n = 5, Prothrombinase: p = 0.002; protein content: p = 0.003; RNA content, p = 0.008, by Student's T-test). The higher fold-range extent was obtained using the prothrombinase assay that showed a very low background.
Interestingly, a correlation can be established between the SMVs protein content and SMVs measured by prothrombinase assay (Supplementary Figure 3). In addition, the SMVs size distribution did not vary as determined in preliminary experiments (Supplementary Figure 4) comparing SMVs from PG-stimulated and unstimulated mice (median diameter 193 nm vs. 189 nm). These diameter values are close to those of SMVs isolated from rat splenocytes in response to a 24 h incubation with LPS (5 µg/mL) or Phorbol myristate acetate (25 ng/mL) and ionophore (1 µM), (median diameter, 222 nm vs. control 212 nm)11 (Supplementary Figure 5). In addition, the protein content of the SMVs can be assessed by western blot, which enables the characterization of specific cytoplasmic or membrane proteins preferentially exported into the SMVs (Supplementary Figure 5).
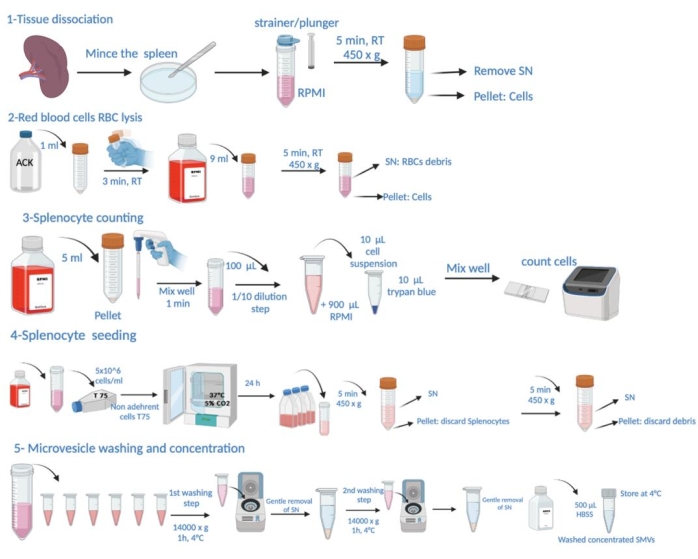
Figure 1: Schematic representation of the protocol steps. This consists of tissue dissection, red blood cell (RBC) lysis, splenocyte counting, seeding, microvesicle harvest from supernatant, washing and concentration determination. Please click here to view a larger version of this figure.

Figure 2: IP injection of P. gingivalis significantly enhances spleen weight (A) and splenocyte count (B). CTL: untreated mice, IP: mice subjected to PG injection for 2 weeks. Data are expressed as mean ± SEM. n = 5: ***: p < 0.0001 and 0.0005, respectively. Please click here to view a larger version of this figure.

Figure 3: Splenocyte viability is reduced 24 h after splenocyte culture, whereas IP injection of P. gingivalis does not alter cell viability. CTL: untreated mice, IP: mice subjected to PG injection for 2 weeks, t0: viability measured immediately after isolation, t24: after 24 h culture. Data are expressed as mean ± SEM. n = 5, ***: p < 0.0001 by Student's T-test. Please click here to view a larger version of this figure.
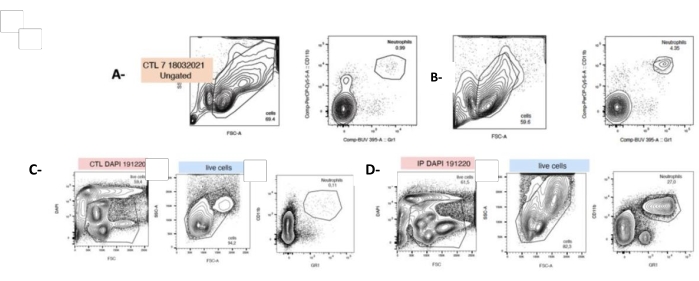
Figure 4: P. gingivalis injection increases the neutrophil count at t0 and after 24 h of cell culture. Cells were freshly isolated from the harvested spleen and labeled with fluorescent antibodies directed against granulocytes (LyG-6 Gr1) or neutrophils (LY6-C) and identified in the CD11b+ gate that delineates monocytes, granulocytes, and neutrophils. Neutrophils were identified as LY6-C+, CD11b+, and LYG-6 Gr1 low. Representative plots showing SSC/FSC and CD11b+, and LYG-6 Gr1 double staining for each condition at t0 (A,B) or 24h after seeding (C,D). A,C: untreated mice splenocytes; B,D: splenocytes from mice subjected to IP injection of P. gingivalis. FSC: Forward Scatter; SSC: Side Scatter; CTL: unexposed mice. n = 5, ***: p < 0.0001, 10000 events acquired per sample. Please click here to view a larger version of this figure.

Figure 5: P. gingivalis favors enhanced MV splenocyte shedding after 24 h culture. Splenocytes were isolated from P. gingivalis infected and control mice and cultured for 24 h and SMVs measured by different assays. Black bars: untreated mice (CTL), grey bars (IP): mice subjected to P. gingivalis injection for 2 weeks. A: Quantification by prothrombinase assay, B: protein concentration of the intact SMVs determined by spectrophotometry, C: RNA content of SMVs determined by spectrophotometry. Data are expressed as mean ± SEM. n = 5, **: p < 0.0001, by Student's T-test. Please click here to view a larger version of this figure.
| Animal condition | Spleen weight | Splenocytes number | Viability (%) | Viability (%) |
| after cell isolation | ||||
| (mg) | (106 cells) | After 24 h cell culture | ||
| (t0) | ||||
| Ctl 1 | 108 | 25 | 75 | 24 |
| Ctl 2 | 112 | 55 | 70 | 26 |
| Ctl 3 | 118 | 52 | 73 | 26 |
| Ctl 4 | 83 | 38 | 91 | 46 |
| Ctl 5 | 78 | 55 | 96 | 46 |
| IP 1 | 243 | 150 | 91 | 43 |
| IP 2 | 250 | 138 | 93 | 46 |
| IP 3 | 283 | 85 | 90 | 46 |
| IP 4 | 270 | 100 | 91 | 43 |
| IP 5 | 300 | 125 | 91 | 43 |
Table 1: Comparison of splenocyte viability rates between the treated and untreated mice after splenocyte isolation.
Supplementary Figure 1: How procoagulant MVs are procoagulant? For details, see Supplementary File 1. Please click here to download this File.
Supplementary Figure 2: The microparticles (MP) captured-based prothrombinase assay. For details, see Supplementary File 1. Please click here to download this File.
Supplementary Figure 3: Correlation plot between measurements of SMVs by prothrombinase assay and by spectrophotometric protein assay in untreated or P. gingivalis treated mice. Please click here to download this File.
Supplementary Figure 4: Analysis of the size distribution of SMVs isolated from mice (A) and rat splenocytes (B). The suspension of SMVs was obtained from the spleen of untreated mice (Control MVs) or individuals subjected to gum damage without LIG MVs or with local addition of P. gingivalis (LIG-PG MVs). SMVs from rats were harvested in the supernatant of isolated splenocytes subjected to a 24 h stimulation by LPS (LPS MVs) or PMA (PMA MVs) as described elsewhere37. Mean dia: Mean diameter; Mode dia: median value of the diameter. Measurements were performed using a 400 nanopore to assess the largest size distribution range. Please click here to download this File.
Supplementary Figure 5: Identification of the protein content of MVs from rat splenocytes isolated from young rats by western blot. For details, see Supplementary File 1. Please click here to download this File.
Supplementary File 1: Details on the experimental procedures and explanations for Supplementary Figures 1,2,5. Please click here to download this File.
Discussion
The present study confirms that the spleen is a major and reliable source of MVs with physiopathological relevance compared to other sources like blood, of limited volume in mice. Provided precautions are taken, the method is easy to set up and does not require expensive equipment. Since no alternate way other than in vivo assessment is available, the current model appears to be a valuable method to study the impact of pro-drugs on MV shedding. Importantly, the standardized protocol for harvesting murine splenic microvesicles (SMVs) presented here should fit the unmet needs for the in vivo study of the control of SMV-mediated effects, eventually in engineered mice after injection or nasal instillation.
The present method also shows that SMVs are relevant preclinical tools to investigate remote effects of local infections, like P. gingivalis IP infection. Indeed, using multiple approaches, significant variations between SMVs released from P. gingivalis-treated vs. untreated animals were measured, suggesting a remote effect of the local IP injection, already detectable after 2 weeks. Preliminary data from our laboratory using other remote P. gingivalis infection sites confirmed that P. gingivalis may initiate a 1.7-fold SMV elevation as early as day-7 post-infection.
It is also observed that the P. gingivalis-induced remote effects were confirmed by spleen analysis with significant variations in weight, cell viability, and total count. Furthermore, P. gingivalis prompted a 6.5-fold increase in the spleen neutrophil count when measured by immunostaining just after splenocyte isolation (t0). Accordingly, variations of the SMV cell origin profile would indicate the shedding of parental cells caused by the initial condition of the animal.
Because SMVs released in the supernatant of 24 h splenocyte cultures were tripled when isolated from IP-injected mice, they appear reliable biomarkers of remote spleen activation by P. gingivalis, making them eventual tools to monitor pharmacological control.
In vitro, the splenocytes culture can be performed using 10% FBS, depleted or not from exosomes. In the current experiments, no differences were observed, and variations between untreated splenocytes remained detectable to the same extent with both types of FBS.
In the present procedure, a 2-week duration did not lead to significant variations of the spleen weight in untreated mice. It is, however, important to keep in mind that the spleen weight may vary with age, as previously reported in a series of young, old, and middle-aged control rats.1 In such a case, the SMV quantification needs to be expressed as a cell count ratio.
The present procedure was optimized for a murine model and suitable for further transgenic mice investigations. Although the major drawback of the murine model is a small spleen weight compared to rats33, the adapted splenocyte and SMV isolation procedures described herein prevent cell damage and loss, while all contaminating erythrocytes are fully discarded. Washing is crucial for a good splenocyte purification yield and needs to be performed gently with no more than 1 mL of ACK lysis buffer per spleen in 9 mL of RPMI to enable extensive elimination of red blood cells. This step may be repeated until the pellet is white. This procedure does not allow the investigation of monocytes and should be adapted for adherent cells in such eventuality.
After 24 h culture, the SMV concentration measured in cell culture supernatants reflects the balance between MV generation and MV recapture by the cultured splenocytes. Therefore, in adapted versions of the above procedure, special attention needs to be given to the presence of cells with professional or accessory phagocyte activity (monocytes, endothelial cells, etc.), and kinetics of the SMV release in supernatant should be carefully established accordingly.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Authors are indebted to Claudine Ebel from the Service commun de cytométrie en flux (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg) for expert assistance and formation to complex flow cytometry analysis of the spleen and Ali El Habhab for initial training on rat spleen cells labelling. Deniz Karagyoz helped in digging gathering literature. This work was partly supported by two ANR grants COCERP (N°A17R417B), ENDOPAROMP(N°ANR-17-CE17-0024-01).
Materials
| Name | Company | Catalog Number | Comments |
| 2 mL tubes type Eppendorf | Dutscher | 54816 | Conical bottom stériel microtubes |
| Allegra 64 R Centrifuge | Beckman Coulter | ||
| Automatic cell counter | Biorad | ||
| Bovine serum albumin | Euromedex | 04-100-812-E | Prepared, filtered with 0.22 µm sieve and stored at 4 °C under sterile conditions by using the following formulas: 2 mM EDTA, 0,5% BSA and sterile PBS |
| CD11 (Mac-1) | e-Biosciences | 45-00112-80 | Conjugated to eFluor 450; λmax excitation 405 nm λmax emission 445 nm |
| CD16/32 | BD Biosciences | 553142 | unconjugated |
| EDTA | Calbiochem | Calbiochem | S 6381-92-6 |
| Falcon tube | Cell star | 227261 | 50 mL |
| Fetal Bovine serum | Dutscher | S1810-500 | Batch number = S14028S1810 |
| Fortessa Aria | BD Biosciences | for cell sorting | |
| Fortessa flow cytometer | Becton-Dickinson. | ||
| Fungizone | PAN biotech | P06-01050 | |
| HBSS | Gibco | 14175-053 | Without phenol red, without Ca+2 and Mg+2 |
| ICAM-1 | abcam | ab171123 | |
| LYG-6 (Gr-1) | BD Biosciences | 566218 | Conjugated to BUV395; λmax excitation 348 nm, λmax emission 395 nm |
| Lysis buffer erythrocytes (ACK) | Sigma | Prepared, filtered with 0.22 µm sieve and stored at 4°C under sterile conditions by using the following formulas: NH4Cl, 0.15 M (molarity), 53.491 (mw) 4 g KHCO3 1 mM (molarity) 100.115 (mw), 50 mg EDTA 0.1 mM (molarity), 292.24 (mw), 14.6 g pH: 7.2–7.4 | |
| NanoDrop 1000 spectrophotometer | Thermoscientific | ||
| PBS | Lonza | 17-516F | Without Ca+2 and Mg+2 |
| Plastic petri dish | 100 mm | ||
| Polystyren tube | Falcon | 352070 | |
| q-Nano Gold | iZON science | ||
| RPMI 1640 culture medium: 2 g/L glucose | PAN biotech | p04-18047 | Supplemented withsupplemented with Streptomycin (100 U/mL) /Penicillin (100 U/mL), Fungizone (250 mg/mL), L-glutamine (2 mM) and FBS 10%. |
| Scalpels | |||
| Sieve Nylon | Falcon USA | 352360 | 100 µm |
| Streptomycin/Penicillin | PAN biotech | P06-07100 | |
| Syringe | 2 mL | ||
| Trypan Blue | Biorad | 1450013 | |
| VCAM1 | abcam | ab215380 |
References
- Loyer, X., et al. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circulation Research. 123 (1), 100-106 (2018).
- Aupeix, K., Toti, F., Satta, N., Bischoff, P., Freyssinet, J. M. Oyxsterols induce membrane procoagulant activity in monocytic THP-1 cells. Biochemical Journal. 314 (3), 1027-1033 (1996).
- Emami, S., et al. Antibiotic resistance pattern and distribution of pslA gene among biofilm producing Pseudomonas aeruginosa isolated from waste water of a burn center. Jundishapur Journal of Microbiology. 8 (11), 23669 (2015).
- Burger, D., et al. Microparticles: biomarkers and beyond. Clinical Science. 124 (7), 423-441 (2013).
- Braeckmans, K., et al. Sizing nanomatter in biological fluids by fluorescence single particle tracking. Nano Letters. 10 (11), 4435-4442 (2010).
- Chironi, G. N., et al. Endothelial microparticles in diseases. Cell and Tissue Research. 335 (1), 143-151 (2009).
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Current Biology. 28 (8), 435-444 (2018).
- Chen, T. S., et al. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research. 38 (1), 215-224 (2010).
- Pirro, M., et al. Microparticles derived from endothelial progenitor cells in patients at different cardiovascular risk. Atherosclerosis. 197 (2), 757-767 (2008).
- Zahra, S., Anderson, J. A., Stirling, D., Ludlam, C. A. Microparticles, malignancy and thrombosis. British Journal of Haematology. 152 (6), 688-700 (2011).
- Amoura, L., et al. Assessment of plasma microvesicles to monitor pancreatic islet graft dysfunction: Beta cell- and leukocyte-derived microvesicles as specific features in a pilot longitudinal study. American Journal of Transplantation. 20 (1), 40-51 (2020).
- De Rop, C., et al. Evaluation of tissue factor bearing microparticles as biomarkers in allogeneic stem-cell transplantation. Transplantation. 92 (3), 351-358 (2011).
- de Abreu, R. C., et al. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nature Reviews Cardiology. 17 (11), 685-697 (2020).
- Beitler, J. R., et al. Advancing precision medicine for acute respiratory distress syndrome. The Lancet Respiratory Medicine. 10 (1), 107-120 (2022).
- Porro, C., et al. Proinflammatory effect of cystic fibrosis sputum microparticles in the murine lung. Journal of Cystic Fibrosis. 12 (6), 721-728 (2013).
- Boisrame-Helms, J., et al. Lipid emulsions differentially affect LPS-induced acute monocytes inflammation: in vitro effects on membrane remodeling and cell viability. Lipids. 49 (11), 1091-1099 (2014).
- Kinane, D. F., Stathopoulou, P. G., Papapanou, P. N. Authors' reply: Predictive diagnostic tests in periodontal diseases. Nature Reviews Disease Primers. 3 (1), (2017).
- Kinane, D. F., Stathopoulou, P. G., Papapanou, P. N. Periodontal diseases. Nature Reviews Disease Primers. 3 (1), 1-14 (2017).
- Kassebaum, D. K., Tedesco, L. A. The 21(st)-century dental curriculum: a framework for understanding current models. Journal of Dental Education. 81 (8), 13-21 (2017).
- Hajishengallis, G., Chavakis, T., Hajishengallis, E., Lambris, J. D. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. Journal of Leukocyte Biology. 98 (4), 539-548 (2015).
- Suh, J. S., et al. Periodontitis-induced systemic inflammation exacerbates atherosclerosis partly via endothelial-mesenchymal transition in mice. International Journal of Oral Science. 11 (3), 21 (2019).
- Bugueno, I. M., et al. Porphyromonas gingivalis triggers the shedding of inflammatory endothelial microvesicles that act as autocrine effectors of endothelial dysfunction. Scientific Reports. 10 (1), 1778 (2020).
- Hajishengallis, G., Lamont, R. J. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. European Journal of Immunology. 44 (2), 328-338 (2014).
- Lamont, T., Worthington, H. V., Clarkson, J. E., Beirne, P. V. Routine scale and polish for periodontal health in adults. The Cochrane Database of Systematic Reviews. 12, (2018).
- Huck, O., et al. Reduction of articular and systemic inflammation by Kava-241 in a Porphyromonas gingivalis-induced arthritis murine model. Infection and Immunity. 86 (9), 00356 (2018).
- Sochalska, M., Potempa, J. Manipulation of neutrophils by Porphyromonas gingivalis in the development of periodontitis. Frontiers in Cellular and Infection Microbiology. 7, 197 (2017).
- Kocgozlu, L., Elkaim, R., Tenenbaum, H., Werner, S. Variable cell responses to P. gingivalis lipopolysaccharide. Journal of Dental Research. 88 (8), 741-745 (2009).
- Slocum, C., et al. Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation. PLOS Pathogens. 10 (7), 1004215 (2014).
- Thietart, S., Rautou, P. E. Extracellular vesicles as biomarkers in liver diseases: A clinician's point of view. Journal of Hepatology. 73 (6), 1507-1525 (2020).
- Witek, R. P., et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 136 (1), 320-330 (2009).
- Tahir, F., Ahmed, J., Malik, F. Post-splenectomy sepsis: a review of the literature. Cureus. 12 (2), 6898 (2020).
- Hussain, M., Stover, C. M., Dupont, A. P. gingivalis in periodontal disease and atherosclerosis - scenes of action for antimicrobial peptides and complement. Frontiers in Immunology. 6, 45 (2015).
- Qureshi, A. W., et al. Ageing enhances the shedding of splenocyte microvesicles with endothelial pro-senescent effect that is prevented by a short-term intake of omega-3 PUFA EPA:DHA 6:1. Biochemical Pharmacology. 173, 113734 (2020).
- Dunford, A., Keramida, G., Anagnostopoulos, C. D., Michael Peters, A. The cardiosplenic axis: another obscure pathophysiological function of the spleen and its investigation using molecular imaging. Nuclear Medicine Communications. 38 (3), 205-208 (2017).
- Ford, R. J., Becker, F. F. The characterization of trypan blue-induced tumors in Wistar rats. The American Journal of Pathology. 106 (3), 326-331 (1982).
- Field, F. E., et al. Trypan blue: identification and teratogenic and oncogenic activities of its coloured constituents. Chemico-Biological Interactions. 16 (1), 69-88 (1977).
- Covarrubias, R., et al. Optimized protocols for isolation, fixation, and flow cytometric characterization of leukocytes in ischemic hearts. American Journal of Physiology - Heart and Circulatory Physiology. 317 (3), 658-666 (2019).
- El Habhab, A., et al. Significance of neutrophil microparticles in ischaemia-reperfusion: Proinflammatory effectors of endothelial senescence and vascular dysfunction. Journal of Cellular and Molecular Medicine. 24 (13), 7266-7281 (2020).
- Freyssinet, J. M. Cellular microparticles: what are they bad or good for. The Journal of Thrombosis and Haemostasist. 1 (7), 1655-1662 (2003).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved