Method Article
Laparoscopic Left Hemihepatectomy Combined with Caudate Lobe Resection
In This Article
Summary
Intrahepatic cholangiocarcinoma of the caudate lobe is a challenge for many surgeons due to its unique location. Here, we present a protocol to show the step-by-step details of laparoscopic left hemihepatectomy combined with caudate lobe resection for cholangiocarcinoma.
Abstract
Intrahepatic cholangiocarcinoma (ICC) is the common malignant tumor of the liver. Radical surgical resection is the mainstay of potentially curative treatment for ICC. Anatomical liver resection for ICC in the caudate lobe is one of the most difficult liver resections. Because the tumor is located deep and easily invades surrounding blood vessels, such as the left hepatic pedicle, right hepatic pedicle, and middle hepatic vein. Laparoscopic anatomic hepatectomy of the caudate lobe not only ensures a negative incision margin but also offers a more minimally invasive approach for patients. This technique is poised to become the preferred choice for radical surgery of the caudate lobe in the future. In this surgical protocol, a 65-year-old male patient with intrahepatic cholangiocarcinoma (sized about 3.2 × 1.9 cm2) located in the left caudal lobe underwent laparoscopic left hemihepatectomy combined with caudate lobe resection successfully without any postoperative complications. Postoperative pathological examination showed a cholangiocarcinoma with a tumor thrombus visible in the vasculature. The patient was discharged on the 14th postoperative day. Laparoscopic left hemihepatectomy combined with caudate lobectomy for the treatment of caudate lobe ICC can be performed safely and does not add significantly to the morbidity or mortality of the procedure.
Introduction
Intrahepatic cholangiocarcinoma originates from intrahepatic bile duct epithelial cells and is a cholangiocarcinoma occurring above the secondary bile duct of the liver. The incidence of ICC is second only to that of hepatocellular carcinoma (HCC), accounting for 10% to 15% of primary liver cancer and about 20% of bile duct cancer1. The incidence of ICC has been increasing year by year in the world, increasing by 140% in the past 40 years2. Surgical resection remains the mainstay of potentially curative treatment for ICC. However, only 20% to 30% of patients have the possibility of surgical resection. After surgical resection, the 5-year overall survival rate of ICC patients is only 20% to 35%3. This is because even if radical resection is performed, only a few patients can obtain negative margins4. It is particularly important for early ICC patients to obtain curative surgery. The curative surgery for ICC patients is closely related to surgical margins. Two recent meta-analyses5,6 indicated that a surgical margin width of >1 cm is associated with better overall survival. A study7 involving 126 patients showed that a margin ≥1.0 cm was associated with better overall survival (OS) and recurrence-free survival (RFS).
In ICC surgery, anatomical liver resection, which is important for the prognosis of ICC patients8, not only can obtain a safe surgical margin but also can excise the violated liver pedicle. A study9 reported that the most common infiltrating pathway for caudate lobe cholangiocarcinoma is through fibrous connective tissue along the Glisson system, not the bile duct. In a prior study, Si et al.10 reviewed data from 702 ICC patients and found that the incidence of complications was similar between anatomical resection and non-anatomical resection, and anatomical resection was associated with better disease-free survival and overall survival at 1, 3, and 5 years. Another propensity matching study11 about ICC identified nonanatomical resection as an independent risk factor for OS (p < 0.05).
However, surgical resection of the caudate ICC can be challenging for the surgeon. It is very difficult for surgeons to explore the caudate lobe because of its unique anatomical location, such as its deep penetration into the liver parenchyma and its proximity to major vessels12 (the inferior vena cava, middle or right hepatic veins, portal vein, and ligamentum venosum). These can make it difficult to obtain a broad field of view during surgery and get an invisible cutting edge. In recent years, with the detailed study of liver anatomy, the rapid development of laparoscopic technology, and the continuous promotion of the concept of precise liver resection, ICC surgical management has grown from isolated caudate lobectomy13,14 to laparoscopic hemihepatectomy combined with caudate lobectomy15, which is rarely reported in the literature. There is a great need for videos of successful operations to guide the widespread use of such operations in the future. Here, we present a laparoscopic left hemihepatectomy combined with caudate lobe resection to treat an intrahepatic cholangiocarcinoma in the caudate lobe.
A 65-year-old Chinese man was admitted to the hospital with an incidentally detected hepatic mass by abdominal ultrasonography. Physical examination showed no significant abnormalities. Laboratory examinations, including routine blood testing, liver function tests, coagulation, and tumor indicators (AFP, CEA, CA199), were normal. Enhanced computed tomography (CT) of the upper abdomen showed a 3.2 1.9 cm2 abnormal enhancement shadow at the S1/4/8 junction and showed that the middle hepatic vein (MHV) and left hepatic vein (LHV) shared a common trunk (Figure 1A). Enhanced magnetic resonance imaging (MRI) of the upper abdomen also revealed a 2.5 2.0 cm2 nodule in the same area, but suggesting ICC (Figure 1B). After completing the preoperative evaluation, we decided to perform a laparoscopic left hemihepatectomy combined with caudate lobe resection to ensure a negative surgical margin.
Protocol
This protocol follows the guidelines of the human research ethics committee of Meizhou People's Hospital. Informed consent was obtained from the patients to release information and data related to this treatment.
1. Preoperative preparation
- Prohibit the patient from eating for 6 h and drinking for 2 h before the operation.
- Apply the antibiotic (1 g of Ceftriaxone sodium) through an elbow intravenous injection to prevent infection preventively 30 min before cutting through the skin.
- Use tracheal intubation under general anesthesia. Puncture and catheterize the right radial artery (catheter size, 20 G) and the internal jugular vein (catheter size, 8 Fr) under ultrasound guidance.
- Sterilize the skin with 0.5% iodine-based scrub and sterile towel sheets to fully expose the surgical area. Disinfect the surgical area with iodophor three times.
2. Surgical technique
- Operation setting
- Cut the skin about 10 mm longitudinally below the umbilicus, then insert a disposable pneumoperitoneum needle through the incision. Inject CO2 after the pneumoperitoneum needle is connected to the pneumoperitoneum machine to establish the pneumoperitoneum.
- Then, insert a 10 mm iron trocar into the incision. Change the patient from a supine position to a supine position with legs apart, head raised 30°, and feet lowered after examining no puncture damage.
NOTE: The pneumoperitoneum pressure was set to 12 mmHg. - Place two 12 mm trocars to the right and left midclavicular line above the umbilical 4 fingers (B and C), two 5 mm trocars to right anterior axillary line subcostal and left midclavicular line subcostal (D and E), see Figure 2.
- Perform abdominal exploration laparoscopically. Check for abdominal injury and puncture bleeding; check for the presence of significant extrahepatic metastases to evaluate the feasibility of radical surgery.
- Exploration phase
- Free the left half of the liver and divide the round and falciform ligaments back to the level of the hepatic vein fossa. Divide the left triangular ligament and the left coronary ligament, extending until the lateral border of the left hepatic vein is identified.
- Clamp the proximal round ligament of the liver.
- Reveal the hepatoduodenal ligament upon accessing the lesser omentum and routinely position a hepatic blood flow occlusion band. If necessary, utilize the Pringle method to intermittently block hepatic blood flow.
- Dissection phase
- Dissect the left hepatic pedicle using the extrathecal approach.
- After lifting the left lateral lobe, expose the caudate lobe by fully opening it to the lesser omentum. Identify, sling, and dissect the left hepatic pedicle using a nontraumatic grasper and a 10 mm right angle dissecting forceps.
- A few minutes later, create a marked pre-resection line with an electrocautery based on the ischemia line of the left and right halves of the liver (Figure 3A, B).
- Use an ultrasonic knife to incise the liver tissue, starting on the upper liver surface from the front to the back, until the level of the left and right hepatic pedicle is fully exposed along the marked line (Figure 3C).
- Fix the pipe structured >4 mm in diameter with clips and dissociate it with an ultrasonic knife at the distal end.
- Utilizing the time to activate the liver, dissect the space between the caudate lobe and the inferior vena cava (IVC) through a dorsal approach. When encountering the short hepatic vein (VHS), clamp it with a hem-o-lok and then disconnect it at the distal end.
- After fully exposing IVC, mark the right paracaval plane, which is the imaginary right margin of the caudate lobe (Figure 3D).
- After removing the suspension line of the left hepatic pedicle, disconnect the left hepatic pedicle by linear cutler reloads (Figure 4A).
- Along the broken end of the left hepatic pedicle, look for the hepatic pedicle of the caudate lobe and dissect it, then clamp its proximal end with two hem-o-loks and cut its distal end with an ultrasonic knife through the left-side approach (Figure 4B).
- Pull the first hepatic portal towards the right to expose the right paracaval plane. Along the right margin of the caudate lobe, as marked, cut off the liver parenchyma beneath the junction of the left and right liver pedicles until the common trunk of MHV and left hepatic vein (LHV) was revealed (Figure 4C).
- When encountering the caudate vein, clamp it with hem-o-lok and disconnect at its distal end.
- Subsequently, complete the transection of the common trunk of MHV and LHV by using linear cutler reloads to completely separate the left liver and caudate lobe, taking care to avoid traction injury to the middle hepatic vein and the vena cava.
- After thorough hemostasis of the wound, place the specimen in a bag and remove it through a 6 cm vertical incision around the navel in the lower abdomen. Place two drainage tubes on the liver section and the hepatorenal recess, respectively.
- Dissect the left hepatic pedicle using the extrathecal approach.
Results
The relevant outcome of this operation is shown in Table 1. The patient's total caudate lobe and left liver were removed in 200 min with 50 mL of blood loss and about 1500 mL of fluid replacement. The intraoperative urinary output was 150 mL. The time of transecting the liver parenchyma, including dissecting the hepatic pedicle and the space between the caudate lobe of the liver and the inferior vena cava, was 84 min. The pringle maneuver was performed three times (the time of hepatic hilar occlusion was 10 min, 20 min, and 15 min, respectively). The entire recovery period after surgery went smoothly, with no signs of postoperative bile leakage and bleeding. Two drains were removed on the 6th and 10th day after surgery. The patient was discharged on the 14th postoperative day.
The pathological analysis revealed a cholangiocarcinoma (pT1N0M0, stage I, AJCC 8th edition)16, with a tumor thrombus visible in the vasculature. MVI grade: M2 (high-risk group). The surgical margin of the liver was negative. Immunohistochemistry indicated positivity CK7, CK19, EMA, CK8, MSH6, MSH2, PMS2, MLH1, Ki67 (40%). According to the AJCC 8th edition16, the patient returned to the hospital 1 month after surgery to receive regular chemotherapy because of MVI grade.
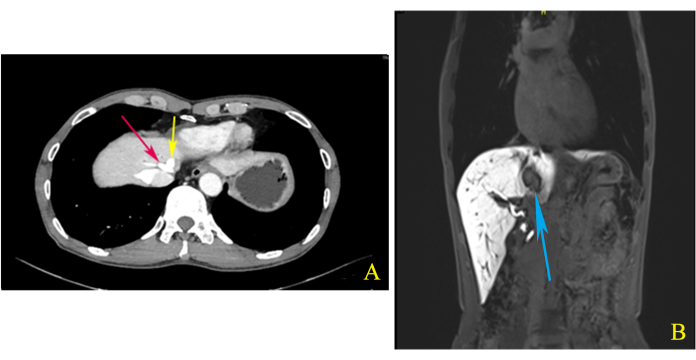
Figure 1: Computed tomography (CT) and magnetic resonance imaging (MRI). (A) The middle hepatic vein (MHV) and left hepatic vein (LHV) shared a common trunk (red and yellow arrows), as shown by enhanced computed tomography (CT) of the upper abdomen during the delay phase. (B) The coronal view imaging of enhanced magnetic resonance imaging (MRI) of the upper abdomen showed that the tumor located in the S1/4/8 junction is closely related to MHV (blue arrow). Please click here to view a larger version of this figure.

Figure 2: Specimen extraction incision and trocar placement. (A) The observation hole. (B) The trocar of the right midclavicular line was the main operation hole for the operator. (C) The trocar of the left midclavicular line was the auxiliary operation hole for the assistant. (D) The trocar of the subcostal right anterior axillary line was the auxiliary operation hole for the operator. (E) The trocar of the subcostal left midclavicular line was the main operation hole for the assistant, and (red line) the vertical incision to remove the specimen. Please click here to view a larger version of this figure.
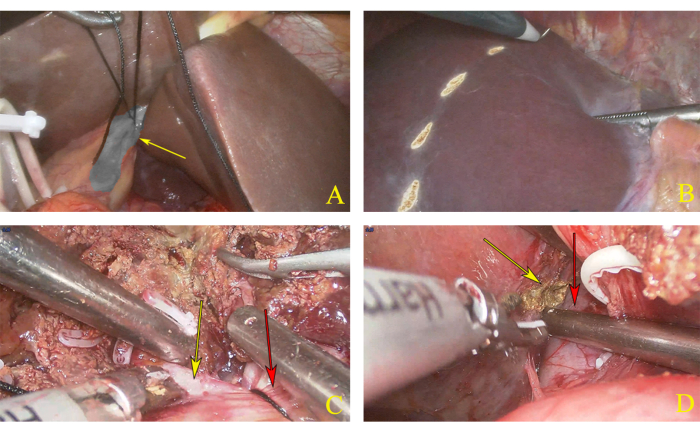
Figure 3: Dissection of left hemihepatectomy. (A) Ligation of left hepatic pedicle. The yellow arrow indicates the left hepatic pedicle. (B) Pre resection line marked according to the ischemic line. (C) The transection plane. The yellow arrow indicates the right hepatic pedicle, and the red arrow indicates the left hepatic pedicle. (D) The yellow arrow indicates the right edge of the caudal lobe, and the red arrow indicates the inferior vena cava (IVC). Please click here to view a larger version of this figure.
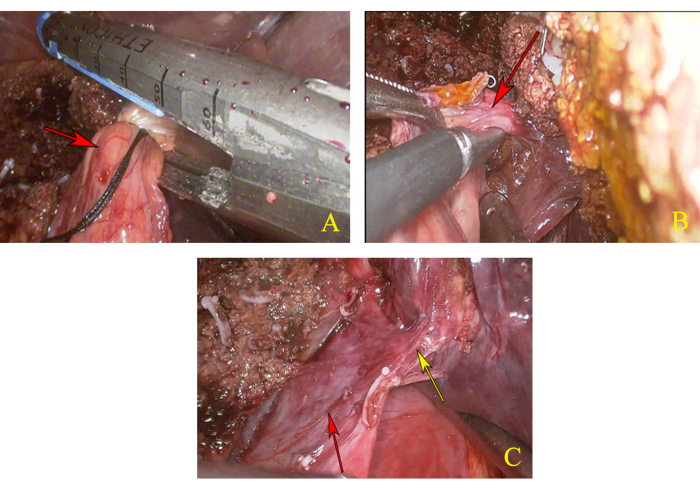
Figure 4: Dissection of caudal lobe. (A) Disconnect the left hepatic pedicle. The red arrow indicates the left hepatic pedicle. (B) Anatomy and dissection of the hepatic pedicle of the caudate lobe. The red arrow indicates the hepatic pedicle of the caudate lobe. (C) Full exposure of the common trunk of the middle hepatic vein (MHV) and left hepatic vein (LHV). The yellow arrow indicates the common trunk of MHV and LHV, and the red arrow indicates IVC. Please click here to view a larger version of this figure.
| Variable | Outcome | |
| Intraoperative | Operative time (min) | 200 |
| Intraoperative blood loss (mL) | 50 | |
| Blood transfusion (mL) | 0 | |
| Fluid replacement (mL) | 1500 | |
| urinary output (mL) | 150 | |
| the time of hepatic hilar occlusion (min) | 10, 20, 15 | |
| Postoperative | Discharge time (days) | 14 |
| Postoperative complications | None | |
| Pathological | Diagnosis | Intrahepatic cholangiocarcinoma |
| Positive markers | CK7, CK19, EMA, CK8, MSH6, MSH2, PMS2, MLH1, Ki67(40%) | |
| Negative markers | CEA, Hep, Arginase-1, HER-2 | |
| MVI grade | M2 | |
Table 1: Surgical outcomes and postoperative details of the patient.
Discussion
The caudate lobe ICC often invades surrounding liver segments or adjacent liver pedicle, which treatment principle is similar to some hilar cholangiocarcinoma17, and due to its anatomical location and biological characteristics, the caudate lobe ICC is prone to damage surrounding structures during surgery, leading to massive bleeding and postoperative bile leakage. So, laparoscopic caudate lobectomy is one of the most difficult liver resections, which needs high skill and experience. We reported a successful case of laparoscopic left hemihepatectomy combined with caudate lobectomy for the treatment of caudate lobe ICC. The patient was discharged without any postoperative complications. We enhanced the safety and feasibility of the surgical procedure by refining certain techniques throughout the operation. A comprehensive preoperative assessment and meticulous surgical planning are paramount. We employed continuous hemihepatic occlusion in conjunction with intermittent Pringle maneuver, which delineates the resection plane through ischemic demarcation, thereby optimizing the preservation of residual liver function and minimizing intraoperative blood loss. Crucially, we integrated multiple surgical approaches to facilitate better exposure of the hepatic caudate lobe and its surrounding critical vascular structures, ensuring a clearer anatomical view and enhancing overall surgical safety.
Due to several factors like challenging resection for combined liver segment resection or even semi-liver resection, tumor vascular infiltration, and the need for lymph node dissection, the adoption of laparoscopy treatment for ICC, compared to hepatocellular carcinoma, has been relatively delayed. Nonetheless, laparoscopic resection for ICC has shown superior short-term outcomes and comparable long-term results when contrasted with the laparotomic approach18. This is because laparoscopy can provide a variety of surgical approaches, allowing for better exposure and control of vital vessels. Moreover, it is easy to distinguish abdominal implantation metastasis during laparoscopic exploration, which makes tumor staging more accurate. In addition, hidden liver lesions can also be identified during laparoscopic surgery through fluorescence staining or intraoperative ultrasound19. A recent meta-analyses20 indicated laparoscopic caudate lobe resection had multiple advantages over open surgery, especially intraoperative blood loss, and hospital stays. Two studies21,22 about the treatment of ICC suggested that laparoscopic resection for the caudate lobe is a feasible and safe procedure. However, further studies are necessary to establish the real benefits of laparoscopy because laparoscopic left hemihepatectomy combined with caudate lobe radical surgery for the treatment of caudate lobe ICC is still relatively rare.
Determining the caudate lobe's right margin is extremely challenging. The traditional approach is to utilize the right posterior Glisson pedicle as the right ventral plane of the caudate lobe and to use the IVC as a reference point for transecting the hepatic parenchyma between the middle and right hepatic veins in order to determine the right boundary for simple caudate lobe resection. However, it is important to note that surgical decisions should not be solely based on this conceptual plane. Kogure et al.23 emphasized that hepatic veins are delineated by the right liver and caudate process. Kumon et al.24 suggested that the short hepatic veins in the paravaval portion serve as boundaries, but these can be challenging to operate on intraoperatively. Ho et al.25 established a boundary between the caudate process and posterior lobe using occlusion of the right lower hepatic pedicle. Most scholars26 think that the right paraventral plane is typically employed to define the right margin of the caudate lobe, which aligns with the surgical approach described here.
There is controversy over whether lymph node dissection is necessary during radical surgery for patients with cholangiocarcinoma. Although metastatic lymph nodes are associated with a negative prognostic value, extensive lymphadenectomy is not routinely conducted in cases where the lymph nodes appear macroscopically non-suspicious8. Ratti F et al.27 conducted a study that showed that there is no significant difference in the effectiveness of laparoscopic lymph node dissection compared to open surgery, and the difference in long-term efficacy between the two is also not statistically significant. Laparoscopic lymph node dissection does not lead to an increased or even lower incidence of complications, and there is no significant difference in the number of lymph node dissections compared to open surgery.
For patients with resectable caudate lobe ICC, laparoscopic caudate lobe resection, or even combined resection of other liver segments, which is safe and feasible, represents a valuable treatment opportunity. However, further research is needed to clarify the long-term efficacy of the surgery, determine the appropriate margin for resection of the caudate lobe, and assess whether lymph node dissection is necessary.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank the anaesthesiologists and operating room nurses who assisted with the operation.
Materials
| Name | Company | Catalog Number | Comments |
| Bipolar radiofrequency excision hemostatic device | ERBE | 20195-136 | |
| Disposable trocar | Kangji Medical | 101Y.611 | |
| Endoscopic linear cutter reloads | Ethicon, LLC | ECR60W | |
| Laparoscopic system | STORZ | 26003BA | |
| Laparoscopic system | STORZ | TC200 | |
| Non-absorbable polymer ligation clips (Hem-o-lok) | Teleflex Medical | 544240 | |
| Pneumoperitoneum needle | Kangji Medical | 101Y.611 | |
| Ultrasound knife | Johnson | GEN11 | |
| Video system | SONY | LMD-3252SC |
References
- Rebecca, L., Angela, N. S., Ahmedin, G., , J. Cancer statistics, 2024. CA Cancer J Clin. 74 (1), 12-49 (2024).
- Paramita, D., et al. Global trends in incidence rates of primary adult liver cancers: A systematic review and meta-analysis. Front Oncol. 10, 171 (2020).
- Dimitrios, M., et al. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 73 (2), 198-222 (2022).
- Nuzzo, G., Giuliante, F., Ardito, F., Giovannini, I. Intrahepatic cholangiocarcinoma. Ann Surg. 249 (3), 541-542 (2009).
- Jiang, J. H., Fang, D. Z., Hu, Y. T. Influence of surgical margin width on survival rate after resection of intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. BMJ Open. 13 (5), e067222 (2023).
- Dai, Y. S., et al. The influence of resection margin width in patients with intrahepatic cholangiocarcinoma: A meta-analysis. World J Surg Oncol. 21 (1), 16 (2023).
- Zhu, H., et al. Prognostic value of resection margin length after surgical resection for intrahepatic cholangiocarcinoma. Am J Surg. 222 (2), 383-389 (2021).
- Lauterio, A., et al. Current surgical management of peri-hilar and intra-hepatic cholangiocarcinoma. Cancers (Basel). 13 (15), 3657 (2021).
- Jiang, N., et al. Patterns of caudate lobe invasion of hilar cholangiocarcinoma: A panoramic histologic study of liver. Ann Surg Oncol. 29 (11), 6804-6812 (2022).
- Si, A., et al. Impact of anatomical versus non-anatomical liver resection on short- and long-term outcomes for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 26 (6), 1841-1850 (2019).
- Wang, C., et al. Anatomical resection improved the outcome of intrahepatic cholangiocarcinoma: A propensity score matching analysis of a retrospective cohort. J Oncol. 2022, 4446243 (2022).
- Huang, J., Sun, D., Xu, D., Zhang, Y., Hu, M. A comprehensive study and extensive review of the caudate lobe: The last piece of "jigsaw" puzzle. Asian J Surg. 47 (1), 1-7 (2024).
- Parikh, M., Han, H. S., Cho, J. Y., D'silva, M. Laparoscopic isolated caudate lobe resection. Sci Rep. 11 (1), 4328 (2021).
- Guo, L., et al. An inferior vena cava-priority approach in laparoscopic isolated hepatic caudate lobectomy. Langenbecks Arch Surg. 409 (1), 106 (2024).
- Wang, Z., et al. Laparoscopic right hemi-hepatectomy plus total caudate lobectomy for perihilar cholangiocarcinoma via anterior approach with augmented reality navigation: A feasibility study. Surg Endosc. 37 (10), 8156-8164 (2023).
- Chun, Y. S., Pawlik, T. M., Vauthey, J. N. 8th edition of the AJCC cancer staging manual: Pancreas and hepatobiliary cancers. Ann Surg Oncol. 25 (4), 845-847 (2018).
- Wang, D., et al. The value of total caudate lobe resection for hilar cholangiocarcinoma: A systematic review. Int J Surg. 110 (1), 385-394 (2024).
- Ratti, F., et al. Intrahepatic cholangiocarcinoma as the new field of implementation of laparoscopic liver resection programs. A comparative propensity score-based analysis of open and laparoscopic liver resections. Surg Endosc. 35 (4), 1851-1862 (2021).
- Dorovinis, P., et al. Safety and efficacy of laparoscopic caudate lobectomy: A systematic review. J Clin Med. 10 (21), 4907 (2021).
- Ding, Z., et al. Safety and feasibility for laparoscopic versus open caudate lobe resection: A meta-analysis. Langenbecks Arch Surg. 406 (5), 1307-1316 (2021).
- Wan, H. F., et al. Laparoscopic caudate lobectomy for cholangiocarcinoma of caudate lobe invading middle hepatic vein. Ann Surg Oncol. 27 (11), 4181-4185 (2020).
- Hu, Y. F., Hu, H. J., Ma, W. J., Jin, Y. W., Li, F. Y. Laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma: A systematic review of propensity score-matched studies. Updates Surg. 75 (8), 2049-2061 (2023).
- Kogure, K., et al. The caudate processus hepatic vein: A boundary hepatic vein between the caudate lobe and the right liver. Ann Surg. 247 (2), 288-293 (2008).
- Kumon, M., et al. Definition of the caudate lobe of the liver based on portal segmentation. Glob Health Med. 2 (5), 328-336 (2020).
- Ho, K. M., et al. Laparoscopic total caudate lobectomy for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A. 27 (10), 1074-1078 (2017).
- Shen, X. Y., et al. Can we delineate preoperatively the right and ventral margins of caudate lobe of the liver. Ann Surg Treat Res. 97 (3), 124-129 (2019).
- Ratti, F., et al. Perioperative and long-term outcomes of laparoscopic versus open lymphadenectomy for biliary tumors: A propensity-score-based, case-matched analysis. Ann Surg Oncol. 26 (2), 564-575 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved