Method Article
The Creation of a Rat Model for Osteosarcopenia via Ovariectomy
In This Article
Summary
This current protocol outlines a procedure for creating a rat model of osteosarcopenia using ovariectomy.
Abstract
Osteosarcopenia (OS), a complex degenerative disorder, is characterized by the concurrent decline in skeletal muscle mass and bone mineral density (BMD), posing an enormous health hazard for the elderly population. Despite its clinical relevance, the pathophysiological mechanisms underlying OS are not fully understood, underscoring the necessity for a deeper comprehension of its etiology to facilitate effective treatment strategies. The development of a reliable animal model is pivotal in this endeavor. This study presents a refined protocol for the induction of postmenopausal osteosarcopenia in rats through bilateral ovariectomy, a method known to accelerate the onset of age-related muscle and bone loss. In this study, rats aged 12 weeks were stratified by body weight and randomly assigned to either a sham operation group or an ovariectomized (OVX) group. Tissue samples from the quadriceps and triceps muscles of the left hind limb, as well as the left femur, were systematically collected at 4, 8, and 12 weeks post-surgery. This methodical approach ensures a comprehensive evaluation of the effects of ovariectomy on muscle and bone health. Histological evaluation of muscle fiber atrophy and femoral morphology was conducted using hematoxylin and eosin (HE) staining, while bone mineral density was quantified using dual-energy X-ray absorptiometry (DXA). The temporal progression of OS was meticulously monitored at the aforementioned intervals, providing insights into the dynamic interplay between muscle and bone degeneration. This model not only accurately reflects the clinical manifestations of OS but also serves as a robust platform for investigating novel therapeutic approaches and their underlying mechanisms.
Introduction
Osteosarcopenia is a multifaceted degenerative condition that encapsulates the clinical manifestations of both osteoporosis and sarcopenia1,2,3,4. Osteoporosis, a prevalent skeletal disorder, is characterized by diminished bone mass, compromised microarchitecture, and heightened susceptibility to fractures. Sarcopenia, often referred to as muscle wasting syndrome, is typified by a reduction in muscle strength and mass5,6. Maryam's7 findings revealed that osteosarcopenia increased the risk of death by 30% over sarcopenia alone and by 8% over low BMD alone. Research has shown that 16.4% of community-dwelling individuals aged 60 and above are affected by osteosarcopenia8. In South Korea, the incidence of osteosarcopenia among elderly individuals aged 60 and above who have sustained hip fractures is reported to be 27.2%9. Individuals with OS face higher risks of falls, fractures, hospitalization, and institutionalization, which burdens the healthcare system and society10,11. Given the gravity of these consequences, it is crucial to develop and implement efficient measures for the prevention and treatment of OS. Despite the urgency, research in this field remains nascent, with ongoing debates surrounding diagnostic criteria and the efficacy of various treatment modalities. The development of reliable animal models is thus essential for dissecting the pathogenesis of OS and uncovering the molecular underpinnings that could inform more efficacious treatment approaches.
Currently, commonly used models for preclinical studies on osteosarcopenia include the aging model, which simulates the human aging process without drug intervention. This approach is closer to the natural process and is cost-effective; however, it demands a significant investment of time for maturation12. The chemical drug injection method offers certain benefits, such as a short modeling cycle, stable results, and low cost. However, it also presents challenges, including the precise determination of hormone dosage, the technical skill required for injection, and the variable effects of hormonal interventions13,14. Genetic engineering models may involve genetically modified organisms that can be both genetically defective and costly. Although these models are highly specific, they are notably more complex and expensive to produce15. Disuse models simulate the effects of prolonged bed rest on clinical patients16. Disuse models are effective and cost-efficient for addressing muscle loss but are associated with complications such as blood clots and pressure sores. These models are routinely monitored to prevent limb necrosis17,18 and hormone-deficient models; there is a prevailing agreement within the scientific community that bilateral ovariectomy serves as an effective method for establishing an animal model of osteoporosis19,20.
Research indicates that bone and muscle tissues can also interact with each other through autocrine, endocrine, and paracrine mechanisms21. The accumulation of adipose tissue in muscle and bone marrow serves as an indicator of reduced bone and muscle mass in the context of osteosarcopenia2. Sarcopenia in older adults is directly associated with a reduction in bone density and the deterioration of bone microarchitecture. Additionally, diminished muscle mass serves as an independent risk factor for the degradation of bone microstructure22. This methodology has been recognized as a viable strategy for the modeling of sarcopenia23,24, which could potentially serve as a combined model for both conditions25. Despite the limited body of research concerning the application of ovariectomy as a means to induce osteosarcopenia, this approach demonstrates potential efficacy. The benefits of utilizing ovariectomy in preclinical studies encompass a swift modeling process, the elimination of pharmacological interventions, the creation of a stable experimental model, straightforward implementation, and cost-effectiveness.
The present study aims to delineate the procedure for creating a preclinical model in female rats through the removal of a segment of both fallopian tubes and ovaries in non-pregnant individuals. This approach serves as a valuable tool for investigating the molecular underpinnings of OS and for assessing the therapeutic benefits of interventions in a controlled experimental setting.
Protocol
Female Sprague Dawley rats (n = 36), aged 12 weeks and weighing approximately 200-240 g, were housed individually in ventilated cages in a specific-pathogen-free (SPF) animal room with a 12-h light/dark cycle. They had free access to SPF feed and sterile water. The rats were allowed to acclimate to the environment for a week before the experiments. Using random allocation, the rats were divided into ovariectomized (OVX) groups (each with 6 rats) and sham groups (each with 6 rats) for 4, 8, and 12 weeks post-surgery. All animal procedures were carried out following the approved guidelines of the animal welfare committee at Liaoning University of Traditional Chinese Medicine (No. 21000042021040).
1. Ovariectomy in rats
NOTE: The surgical apparatus used in this protocol is in Figure 1.
- Keep the rats in the SPF animal room and follow all necessary procedures using sterilized equipment in a sterile environment.
- Mix sodium pentobarbital, a white powder, with distilled water or a 0.9% normal saline solution to create an anesthetic solution. The standard dose is 30 mg/kg; fill the syringe accordingly.
NOTE: It is important to note that the solution is unstable and should be used immediately. Prepare the required amount for one experiment at a time. - Elevate the rat's abdomen above its head to shift the viscera to the upper abdomen. Using the dominant hand, position a syringe 1-1.5 cm from the left (or right) side of the midline of the abdomen and insert it at a 45° angle into the rat's body. After the drug solution is administered, rotate the needle and then withdraw.
- Following the administration of anesthesia, carefully monitor the rat's breathing and pinch their toes to confirm that it is completely anesthetized.
NOTE: If there are any signs of spasms or convulsions, it is advisable to wait longer before proceeding. - Position the rat on the operating table, secure its limbs, and remove the hair on both sides of its back using a trimmer (Figure 2A).
NOTE: If the hair removal effect is not ideal, hair removal cream can be used for hair removal. - Disinfect the area where the hair was removed using cotton balls soaked in Iodine.
NOTE: The process of surgical disinfection involves starting from the center and moving outwards in a circular pattern, typically repeated three times. - Make an incision on the back, about 1.0 cm away from the centerline. Make the incision close to the juncture between the rib cage curvature and the border of the spinal column, slightly lower by 0.5-1 cm by separating the skin, fascia, and muscle on both sides (Figure 2B).
NOTE: For accessing the abdominal cavity through the weaker muscle layer of the posterior abdominal wall, the incision is kept as minimal as feasible. - Finding the ovary can be challenging at first. Start by locating the oviduct and tracing it to the ovary's terminal end, which is encased in a layer of loose adipose tissue.
NOTE: The right ovary is positioned on the side of the 4th to 5th lumbar vertebrae, 7-12 mm behind the kidney and 15 mm away from the center line. The left ovary is situated on the side of the 5th to 6th lumbar vertebrae, 3-5 mm behind the kidney and 11 mm from the center line. - Carefully lift the ovary and the end of the oviduct out of the body (Figure 2C). Apply the hemostatic forceps at the most constricted region between the uterine end and the ovary. Use a surgical thread to tie it off, and then excise the ovary completely with scissors.
NOTE: It is crucial to be gentle when handling the oviduct and the uterus during the procedure, avoiding excessive pulling. The ligature used prior to ovariectomy must be firmly secured, as the soft lipid tissue around the ovary can easily cause it to come loose. This precaution is necessary to prevent postoperative bleeding, which could result in the rats' death. In the sham group, adipose tissue of equal volume and size adjacent to the ovary was excised, followed by suturing of the muscle and skin. - Release the hemostatic forceps and gently return the uterus to the abdominal cavity.
- Administer penicillin to the abdominal wounds where the ovaries and fallopian tubes are ligatured to avoid infection.
NOTE: Administer penicillin 80,000 units/rat once a day for 3 consecutive days. - Individually suture (size 3-0) the skin and muscle layers (Figure 2D).
NOTE: Sterilization should be conducted 24-48 h post-surgery, spaced 1-2 days apart. - Place the rat back in a sanitized cage and monitor it until it completely regains consciousness from the anesthesia.
NOTE: Continue to provide heat support during the procedure until the animal has fully recovered from anesthesia. - To avoid wound infection, administer the rats in each group with an intramuscular injection of penicillin sodium 80,000 units/rat once a day for 3 consecutive days26.
2. Collection of bone tissue and muscle tissues
NOTE: Rats were euthanized with an overdose of sodium pentobarbital (100-200 mg/kg) at 4, 8, and 12 weeks after the modeling surgery. A total of 36 samples were collected.
- Expose the triceps brachii and quadriceps muscles of the left calf. Carefully identify and dissect these muscles at their points of origin and endpoint to preserve their integrity. Following this, record and calculate the average of the wet weights of the muscles to determine the wet weight coefficients of the muscles.
NOTE: Animal body weight and skeletal muscle wet weight coefficient = rat muscle wet weight/body weight. - Fully detach the femur by slicing the joint capsule upwards along the femur. Then, eliminate the nearby muscle and ligament tissue.
3. Pathological examination
- Immerse the muscle tissues in a receptacle containing a 10% neutral buffered formalin solution for a duration of 24 h. Following this, rinse the muscle tissues extensively under flowing water to remove the fixative.
- Place the left femur in a 4% paraformaldehyde solution for 1 week, then soak it in a sufficient amount of ethylenediaminetetraacetic acid (EDTA) decalcification solution to remove calcium deposits, with the buffer being changed daily.
- Measure the bone mineral density values using a dual-energy X-ray absorptiometry (DXA) bone densitometer. Place the femur in a dual-energy X-ray. Set the measurement accuracy to Fine, adjusting the mode to Small Animal-Specific Mode, and analyze the BMD of rat femurs using the accompanying BMD analysis software.
- Place the sample in paraffin wax. Section the samples for the routine histological examination27.
4. Statistical analysis
- Present the continuous variables as mean ±± standard deviation (SD) and compare between the two groups using the independent sample t-test. All statistical analyses followed a two-sided approach, with statistical significance set at P < 0.05. Use appropriate data analysis software to perform data analyses.
Results
This protocol provides a detailed description of the bilateral ovariectomy procedure for establishing a rat model of Osteosarcopenia. Figure 3 demonstrates a decrease in the wet weight coefficient of the quadriceps muscle in the OVX group in comparison to the sham group. Although there was no statistically significant variance in BMD between the two groups 4 weeks after surgery, the BMD in the OVX group was significantly lower than that in the sham-operated group at 8 and 12 weeks post-surgery.
In Figure 4, significant atrophy of the triceps brachii muscle is observed in the OVX group, with a wider muscle fiber gap compared to the sham group at 12 weeks post-modeling. Figure 5 demonstrates that at 4 weeks post-surgery, the trabecular density of the femoral head in both the OVX and sham operation groups was similar, showing a regular and dense arrangement with good connectivity. However, by 8 weeks post-surgery, the trabeculae in the OVX group started to decrease in number, becoming sparsely arranged with an increased bone marrow cavity area. The quantity of adipocytes in the bone marrow cavity was higher than in the sham group. At the 12-week postoperative, the trabeculae in the OVX exhibited a marked reduction, displaying incomplete interconnections, a notable expansion in the bone marrow cavity area, and a significantly elevated adipocyte count in comparison to the sham group.

Figure 1: Surgical instruments. (A) Straight needle holder. (B) Straight Mayo scissors. (C) Disposable medication changing tray. (D) Sterile cotton ball (E) Syringes. (F) Iodine. (G) Suture needle. (H) Suture line. Please click here to view a larger version of this figure.
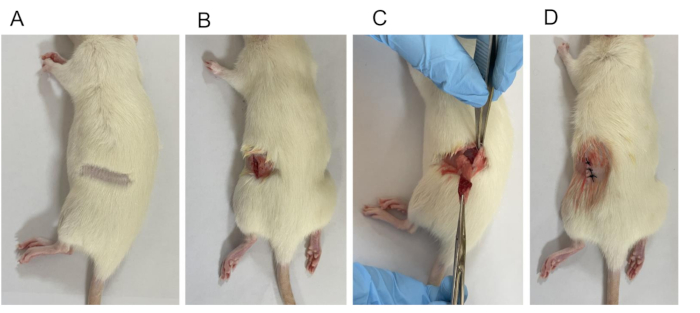
Figure 2: Establishment of OVX model. (A) Hair removal. (B) A 1 cm long surgical opening was made from the skin to the subcutaneous layer. (C) Ligation involving the removal of ovaries and a portion of the fallopian tubes. (D) Closed wound. Please click here to view a larger version of this figure.
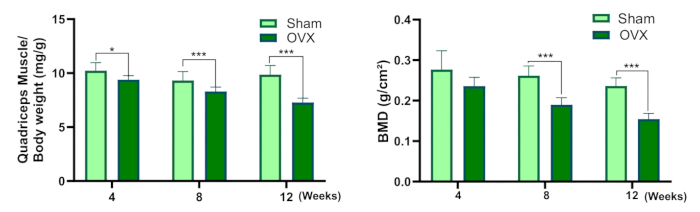
Figure 3: Quadriceps muscle/body weight and bone mineral density. (A) Quadriceps muscles wet weight coefficient. (B) Bone mineral density (Compared to the sham group, * P<0.05, ** P<0.01, *** P<0.001). Please click here to view a larger version of this figure.
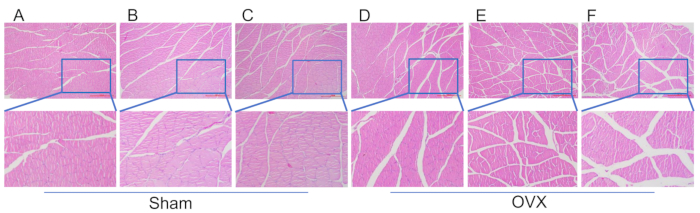
Figure 4: HE staining of tissue sections from the muscle fibers of the calf triceps muscle. (A) Muscle morphology 4 weeks following the modeling procedure in the sham group. (B) Muscle morphology 8 weeks following the modeling procedure in the sham group. (C) Muscle morphology 12 following the modeling procedure in the sham group. (D) Muscle morphology 4 weeks after modeling in the OVX group. (E) Muscle morphology 8 weeks after modeling in the OVX group. (F) Muscle morphology 12 weeks after modeling in the OVX group. Scale bars: 1000 µm. Please click here to view a larger version of this figure.

Figure 5: HE staining of tissue sections from the femoral head. (A) Femoral morphology 4 weeks following the modeling in the sham group. (B) Femoral morphology 8 weeks after modeling in the sham group. (C) Femoral 12 weeks after modeling in the sham group. (D) Femoral morphology 4 weeks after modeling in the OVX group. (E) Femoral morphology 8 weeks after modeling in the OVX group. (F) Femoral morphology 12 weeks after modeling in the OVX group. Scale bars: 1000 µm. (G) Quantified adiposity area. Please click here to view a larger version of this figure.
Discussion
The bilateral ovariectomized animal model is instrumental in elucidating the mechanisms underlying osteosarcopenia and assessing potential therapeutic interventions. Ovariectomy-induced osteoporosis in rats, which mirrors the abrupt decrease in estrogen levels seen in postmenopausal women, is commonly employed as a model for osteoporosis research. Furthermore, research has highlighted a significant association between osteoporosis and sarcopenia in older individuals, with concurrent muscle and bone loss frequently observed. Consequently, numerous studies have utilized this model to investigate sarcopenia28,29. As a result, the present study successfully establishes an animal model of Osteosarcopenia.
Several important factors must be taken into account when establishing a reliable model. A suitable animal model must be characterized by convenience, relevance, and specificity30. SD rats are the most commonly used animals in osteoporosis modeling. After the removal of the ovaries, this mechanism of transformation occurring in the bones of rats closely resembles the process of postmenopausal bone loss observed in humans31,32. Research indicates that rats aged between 11 weeks and 36 weeks are optimal for replicating either the osteoporosis or sarcopenia models33,34,35,36. In terms of gender, the prevalence of osteosarcopenia among females was higher at 28% than among males at 14%21; therefore, we selected female rats. Rats become sexually mature at about 6 weeks of age37, so we selected 12-week-old rats. A research study found that the volumes of the quadriceps muscles were significantly reduced in older adults in comparison to younger individuals, suggesting that aging has a more detrimental impact on quadriceps volume38. Osteoporosis uses femoral bone mineral density as the gold standard32. So, we selected the quadriceps muscle and the femoral bone.
Consistency in surgical technique is crucial, with it being recommended that the same individual performs all procedures to ensure uniformity in incision localization and size. The entire procedure consists of several crucial steps. First, the intraperitoneal injection of anesthetic should avoid puncturing internal organs. Prior to drug administration, it is essential to aspirate to ensure the needle has not entered a blood vessel, to push the syringe plunger accurately, and to maintain stability and speed throughout the process. Second, identifying the ovary quickly after cutting the peritoneum can be challenging, necessitating a comprehensive understanding of rat anatomy by the operator. Once the ovary is located, ligating and removing part of the oviduct and ovary is a vital step for the rat's postoperative survival. Due to the soft adipose tissue near the ovary, the suture may easily come loose after tying, leading to bleeding and potentially death post-surgery. Finally, preceding suturing, the application of penicillin to the surgical site is recommended, with additional intramuscular administration 3 days post-surgery as a preventive measure against infection.
As a result of surgical procedures and the administration of anesthesia, rats may experience severe pain or even death, necessitating their placement in a warm, hygienic, and well-ventilated setting until they regain consciousness. Vigilant monitoring is imperative during the initial postoperative week, with attention to the rats' behavioral activity throughout the study.
The benefits of this model include its user-friendly nature, efficient modeling capabilities, cost-effectiveness, and ability to mimic the natural development of osteoporosis and muscle loss. Nonetheless, there are certain constraints associated with this model, such as the rapid decline in estrogen levels post-ovarian removal surgery, with estrogen not being recognized as a direct contributor to sarcopenia. The present experiment was conducted on female rats and did not involve male rats. Despite these limitations, the bilateral ovariectomy animal model has emerged as a valuable resource for investigating OS and exploring avenues for disease advancement.
Disclosures
Each author declares no competing financial interests.
Acknowledgements
This work is supported by grants from (1) National Nature Science Foundation (82305275). (2) Liaoning Provincial Natural Science Foundation program (2022-YGJC-80 and 2022-YGJC-79). (3) High-level Chinese Medicine Key Discipline Construction Project of National Administration of TCM (zyyzdxk-2023040).
Materials
| Name | Company | Catalog Number | Comments |
| Double lion Irradiated Rodent Diet | Suzhou Shuangshi Experimental Animal Feed Technology Co., Ltd. | GB 14924.3 | Animal feed |
| Disposable medication changing tray | Yangzhou Chenglin Medical Technology Co., Ltd. | RVnpFXLc | |
| Dual Energy X-ray Bone Densitometer | Xuzhou PinyuanElectronic Technology Co., Ltd. | DXA-800E | |
| Iodine | Shanghai Likang Sterilization Hi-Tech Co., Ltd. | LK-310512 | |
| IVCs rat cage | Suzhou Monkey King Animal Experimental Equipment Technology Co., Ltd. | HH-MMB-2 | Animal barrier |
| Penicillin sodium | North China Pharmaceutical Group Limited Liability Co., Ltd. | H13020654 | |
| sodium pentobarbital | Sigma-Aldrich, St. Louis, MO | P3761-5G | |
| Sterile cotton ball | Henan Piaoan Group Co., Ltd | 20140017 | |
| Straight Mayo scissors | Shenzhen Huayang Biotechnology Co., Ltd. | 18-0410 | |
| Straight needle holder | Shanghai Simplicity Biotechnology Co., Ltd. | 32100-14 | |
| Suture line | Shenzhen Huayang Biotechnology Co., Ltd. | 18-5902 | |
| Suture needle | Shenzhen Huayang Biotechnology Co., Ltd. | 18-5036 | |
| Syringes | Shenzhen Huayang Biotechnology Co., Ltd. | 21-3021 |
References
- Binkley, N., Buehring, B. Beyond FRAX®: It's time to consider "Sarco-Osteopenia. J Clin Densitom. 12 (4), 413-416 (2009).
- Hirschfeld, H. P., Kinsella, R., Duque, G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 28 (10), 2781-2790 (2017).
- Kaplan, S. J., et al. Association of radiologic indicators of frailty with 1-year mortality in older trauma patients. JAMA Surg. 152 (2), e164604 (2017).
- Nielsen, B. R., Abdulla, J., Andersen, H. E., Schwarz, P., Suetta, C. Sarcopenia and osteoporosis in older people: a systematic review and meta-analysis. Eur Geriatr Med. 9 (4), 419-434 (2018).
- Cruz-Jentoft, A. J., et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on sarcopenia in older people. Age Ageing. 39 (4), 412-423 (2010).
- Polito, A., Barnaba, L., Ciarapica, D., Azzini, E. Osteosarcopenia: A narrative review on clinical studies. Int J Mol Sci. 23 (10), 5591 (2022).
- Pourhassan, M., et al. Three-year mortality of older hospitalized patients with osteosarcopenia: Data from the OsteoSys study. Nutrients. 16 (9), 1328 (2024).
- Salech, F., et al. Osteosarcopenia predicts falls, fractures, and mortality in Chilean community-dwelling older adults. J Am Med Dir Assoc. 22 (4), 853-858 (2021).
- Yoo, J. I., Ha, Y. C. Review of epidemiology, diagnosis, and treatment of osteosarcopenia in Korea. J Bone Metab. 25 (1), 1-7 (2018).
- Inoue, T., et al. Related factors and clinical outcomes of osteosarcopenia: A narrative review. Nutrients. 13 (2), 291 (2021).
- Teng, Z., et al. The analysis of osteosarcopenia as a risk factor for fractures, mortality, and falls. Osteoporos Int. 32 (11), 2173-2183 (2021).
- Scheuren, A. C., et al. Hallmarks of frailty and osteosarcopenia in prematurely aged PolgA(D257A/D257A) mice. J Cachexia Sarcopenia Muscle. 11 (4), 1121-1140 (2020).
- Gasparini, S. J., et al. Continuous corticosterone delivery via the drinking water or pellet implantation: A comparative study in mice. Steroids. 116, 76-82 (2016).
- Pal, S., et al. A butanolic fraction from the standardized stem extract of Cassia occidentalis L delivered by a self-emulsifying drug delivery system protects rats from glucocorticoid-induced osteopenia and muscle atrophy. Sci Rep. 10 (1), 195 (2020).
- Mito, T., et al. Mitochondrial DNA mutations in mutator mice confer respiration defects and B-cell lymphoma development. PLoS One. 8 (2), e55789 (2013).
- Thomsen, J. S., et al. Cancellous bone structure of iliac crest biopsies following 370 days of head-down bed rest. Aviat Space Environ Med. 76 (10), 915-922 (2005).
- Papadopoulou, S. K., et al. Exercise and nutrition impact on osteoporosis and sarcopenia-The incidence of osteosarcopenia: A narrative review. Nutrients. 13 (12), 4499 (2021).
- Du, F., et al. A hind limb disuse model inducing extensor digitorum longus atrophy in rats: tail suspension-immobilization. Aviat Space Environ Med. 82 (7), 689-693 (2011).
- Gomes, R. M., et al. Strength training reverses ovariectomy-induced bone loss and improve metabolic parameters in female Wistar rats. Life Sci. 213, 134-141 (2018).
- Ma, J., et al. Establishment of a rat model of osteosarcopenia. Chin J Osteoporos. 28 (1), 1-5 (2022).
- Huang, T., et al. Prevalence and risk factors of osteosarcopenia: a systematic review and meta-analysis. BMC Geriatr. 23 (1), 369 (2023).
- Qi, H., et al. mineral density and trabecular bone score in Chinese subjects with sarcopenia. Aging Clin Exp Res. 31 (11), 1549-1556 (2019).
- Shu, H., et al. An integrated study of hormone-related sarcopenia for modeling and comparative transcriptome in rats. Front Endocrinol. 14, 1073587 (2023).
- Nakaoka, K., Yamada, A., Noda, S., Goseki-Sone, M. Influence of dietary vitamin D deficiency on bone strength, body composition, and muscle in ovariectomized rats fed a high-fat diet. Nutrition. 60, 87-93 (2019).
- Chong, L., Xiaonan, Q., Hao, Z., Xiaosheng, Y. Castration method was used to construct a rat model of kidney-yang deficiency sarcopeniaosteoporosis and explore the mechanism. Chin Arch Tradit Chin Med. , (2024).
- Ma, X., et al. (S)-10-hydroxycamptothecin inhibits EMT-evoked osteosarcoma cell growth and metastasis by activating the HIPPO signaling pathway. Combin Chem High Throughput Screen. 27 (15), 2239-2248 (2024).
- Yamazaki, I., Yamaguchi, H. Characteristics of an ovariectomized osteopenic rat model. J Bone Miner Res. 4 (4), 13-22 (1989).
- Lee, H., et al. MiR-141-3p promotes mitochondrial dysfunction in ovariectomy-induced sarcopenia via targeting Fkbp5 and Fibin. Aging (Albany NY). 13 (4), 4881-4894 (2021).
- China, S. P., et al. Globular adiponectin reverses osteo-sarcopenia and altered body composition in ovariectomized rats. Bone. 105, 75-86 (2017).
- Rodgers, J. B., Monier-Faugere, M. C., Malluche, H. Animal models for the study of bone loss after cessation of ovarian function. Bone. 14 (3), 369-377 (1993).
- Matsushita, M., et al. Age-related changes in bone mass in the senescence-accelerated mouse (SAM). SAM-R/3 and SAM-P/6 as new murine models for senile osteoporosis. Am J Pathol. 125 (2), 276-283 (1986).
- Cheng, M., et al. A traditional Chinese herbal preparation, Er-Zhi-Wan, prevent ovariectomy-induced osteoporosis in rats. J Ethnopharmacol. 138 (2), 279-285 (2011).
- Roch, P. J., et al. Ostarine and ligandrol improve muscle tissue in an ovariectomized rat model. Front Endocrinol. 11, 556581 (2020).
- Bei, M. J., et al. Raloxifene retards cartilage degradation and improves subchondral bone microarchitecture in ovariectomized rats with patella baja-induced - patellofemoral joint osteoarthritis. Osteoarthritis Cartilage. 28 (3), 344-355 (2020).
- Wen, K., et al. Fecal and serum metabolomic signatures and microbial community profiling of postmenopausal osteoporosis mice model. Front Cell Infect Microbiol. 10, 535310 (2020).
- Shah, F. A., Stoica, A., Cardemil, C., Palmquist, A. Multiscale characterization of cortical bone composition, microstructure, and nanomechanical properties in experimentally induced osteoporosis. J Biomed Mater Res A. 106 (4), 997-1007 (2018).
- Andreollo, N. A., Santos, E. F., Araújo, M. R., Lopes, L. R. Rat's age versus human's age: what is the relationship. Arq Bras Cir Dig. 25 (1), 49-51 (2012).
- Fuchs, C. J., et al. Thigh muscles are more susceptible to age-related muscle loss when compared to lower leg and pelvic muscles. Exp Gerontol. 175, 112159 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved