Method Article
Derivation of a Human Brain Organoid with Microglia Development
In This Article
Summary
We present a protocol to generate a human brain organoid with resident microglia by incorporating Induced pluripotent stem cell (iPSC)-derived hematopoietic progenitor cells (HPCs) into organoid development.
Abstract
Three-dimensional (3D) brain organoid cultures derived from induced pluripotent stem cells (iPSC) provide an important alternative in vitro tool for studying human brain development and pathogenesis of neurological diseases. However, the lack of incorporation of microglia in the human brain organoids is still a major hurdle for 3D models of neuroinflammation. Current approaches include either the incorporation of fully differentiated microglia into mature brain organoids or the induction of microglial differentiation from the early stage of iPSC-derived embryoid bodies (EBs). The first approach misses the stage when microglial differentiation interacts with the adjacent neural environment, and the later approach is technically challenging, resulting in inconsistency among the final organoids in terms of the quantity and quality of microglia. To model brain organoids with microglia to study the early interactions between microglial and neuronal development, highly pure hematopoietic progenitor cells (HPC) differentiated from human iPSCs were incorporated into iPSC-derived EBs to make brain organoids. Using immunostaining and single-cell RNA sequencing (sc-RNA-seq) analysis, we confirmed that HPCs were incorporated into the 3D organoids, which eventually developed into brain organoids with both microglia and neurons. Compared to brain organoids without HPCs, this approach produces significant microglial incorporation in the brain organoids. This novel 3D organoid model, which consists of both microglial and neural development properties, can be used to study the early interactions between innate immune and nervous system development and potentially as a model for neuroinflammation and neuroinfectious disorders.
Introduction
Microglia are residential immune cells in the brain, playing critical roles in both brain development and homeostasis1,2. The activation of microglia results in the production of proinflammatory factors, elevated phagocytosis, and reactive oxidative stress, which removes the invading pathogens and compromised cells. However, overactivation or prolonged activation of microglia may, on the other hand, cause neurodegeneration as a mechanism of pathogenesis in many neurological disorders, including Parkinson's disease3,4. It is important that microglia are included in the relevant models for studying human neurological disorders. In recent years, human stem cells have been used to develop 3D organoids as in vitro models as an alternative to animal models and human subject studies5. Ideally, human organoids constitute multiple cell types and tissue structures similar to the corresponding human organs, better representing human physiology and pathogenesis than animal models but without the ethical concerns involved in studies of human individuals directly. They may represent the future of human disease modeling for the study of pathogenesis and drug development and for the guidance of individualized therapies6. As an example, 3D human brain organoids derived from human induced pluripotent stem cells (iPSCs) have prevailed in the field of neuroscience research, modeling neural infectious diseases including ZIKA, SARS-CoV-27, and neurodegenerative diseases including amyotrophic lateral sclerosis (ALS) and Alzheimer's disease8,9. However, conventional 3D neural organoids using dual-SMAD inhibition to induce neuronal differentiation10 produce brain organoids lacking microglia, as they are derived from progenitors recruited from the blood instead of the neuroectoderm lineage that neurons are from11,12. Without the presence of microglia, the organoids are inadequate to model CNS infections, inflammation, and the associated neurodegeneration.
To address this critical issue, attempts have been made to incorporate differentiated microglia into the brain organoids13 or inducing microglial differentiation within organoids from the beginning using alternative approaches instead of dual-SMAD inhibition13. However, by incorporating differentiated microglia into the brain organoids, the early interactions between neuronal and microglial development are missed. This could be important in CNS development or the pathogenesis of neuroinfectious disorders targeting infant brain development, such as in ZIKA virus infection14. On the other hand, differentiating innate microglia within iPSC-derived brain organoids without intermittent stages involves a prolonged process and has higher variability within the end products15. In this reported protocol, we incorporated the iPSC-derived hematopoietic progenitor cells (HPCs) into the iPSCs to make embryoid bodies (EBs), which were further differentiated into 3D organoids including both neurons and microglia.
Our protocol provides an easy approach that can be adopted to study the human central nervous system involving early neuron-microglial interactions and the pathogenesis of neural infectious disorders and neuroinflammation involving microglial activation.
Protocol
The original blood samples from healthy adult donors were collected at the Transfusion Medicine Blood Bank of the NIH, and signed informed consent forms were obtained in accordance with the NIH Institutional Review Board.
1. Producing hematopoietic progenitor cells (HPCs) from human iPSCs
NOTE: Human iPSC cells 510 and 507 were used to produce the representative results. The methods of generation and maintenance of the iPSCs can be found in a previous publication16.
- Day 0: Coat a 12-well cell plate by adding 500 μL/well of ice-cold Matrigel (basement membrane matrix [BMM]) solution diluted in DMEM/F12 medium and incubate it for at least 30 min at room temperature (RT).
- Remove the coating supernatant completely and replace it with 1 mL of E8 Flex medium per well.
- Check the iPSC culture in a 6 well plate under the microscope to confirm the iPSCs are of high quality and without signs of differentiation. Choose a middle-sized colony by marking it with a marker pen underneath the bottom of the plate.
- Remove the medium from the iPSC culture and add 500 μL of ethylenediaminetetraacetic acid (EDTA) iPSC dissociation buffer (0.5 mM EDTA, 0.45 g/L NaCl in DPBS) to the well.
- Observe under a microscope for signs of cell dissociation when three to four rows of cells from the edges of the colony start to shrink, with empty space appearing between cells. This usually takes 1-3 min.
- Discard the EDTA solution completely. Dislodge the marked iPSC colony by pipetting 1 mL of E8 Flex medium forcefully and directly onto it. Ensure that the colony detaches completely into cell patches containing 20-50 cells each after 1-3 times of pipetting.
- Collect the supernatant containing cell patches and add it to the first well of the coated wells in the 12-well plate.
- Mix the well by pipetting one or two times and transfer 1 mL of the cells into the second well that contains 1 mL of medium.
- Repeat step 1.8 twice to make serial diluted iPSC cultures in a total of four wells.
- Incubate the plate in a 37 °C, 5% CO2 incubator.
- After 24 h, on day 1, check the colonies under the microscope and count the numbers of colonies by going through all the fields in a well continuously. Choose one well that contains 10-20 iPSC colonies.
- Replace the medium with 1 mL of medium A from the hematopoietic Kit. Put the plate back into the incubator for 48 h.
- On day 3, remove 500 μL of spent medium and add 500 μL of fresh medium A.
- On day 4,observe under the microscope and notice significant cell growth and differentiation from the iPSC colonies. Replace the medium with 1 mL of medium B.
- Monitor the cell differentiation under the microscope and do half-medium change with 500 μL of Medium B every other day. The HPC-like cells appear on days 6-7.
- On day 10, observe under the microscope bright single round cells with the morphology of normal HPCs floating in the medium or loosely attached to the single bottom layer of flat cells, with some loose cell aggregates.
NOTE: The differentiated HPCs are ready for collection for characterization or further experiment. - Collect all the differentiated HPCs by pipetting up and down three times using a 1 mL pipette tip to break the cell aggregates and detach the HPCs from the plate surface.
- Add the cells into a 15 mL tube and centrifuge the cells at 300 x g for 5 min. Remove the supernatant and resuspend the cell pellet into 1 mL of medium B.
- Count the cells and adjust the concentration to 1 million cells/mL. At this stage, more than 1 million of HPCs are generated. When checked with flow cytometry, ensure that the purity is more than 85% of cells with CD34+/CD43+. Ensure no significant dead cells (more than 5%) are noticed.
2. Developing embryoid bodies from mixed iPSCs and HPCs
- On the same day of collecting the HPCs, make sure an iPSC culture also reaches 80% confluence in one well in a 6-well plate (or two wells in a 12-well plate).
- Treat one well of the microwell culture plate for EB formation with 500 µL of anti-adherence rinsing solution by adding it to minimize bubbles.
- Spin the plate at 2000 x g for 5 min in a swinging bucket rotor fitted with plate holders to remove possible bubbles.
- Remove the rinsing solution completely by pipetting and wash the wells twice with 1 mL of DPBS and then 1 mL of DMEM/F12 without generating bubbles.
- Dissociate the iPSCs from the plate by treating the cells with 1 mL of Accutase solution.
- Observe under the microscope until the cells show signs of separation but are still attached to the bottom. This usually happens within 1-3 min of Accutase treatment. Do not over-digest the iPSCs.
- Remove the Accutase solution completely without disturbing the cells and add1 mL of DMEM/F12 medium to the wells. Further, dissociate the cells into single cells by pipetting up and down a couple of times using a 1 mL tip.
- Collect the cells into a 15 mL tube and fill it to 5 mL with DMEM/F12 medium. Spin the cells at 300 x g for 5 min.
- Remove the supernatant by pipetting and resuspend the cells in 1 mL of E8 Flex medium. Count the cells and adjust the cell concentration to 1 million cells per mL in the E8 Flex medium.
- Mix iPSCs with HPCs in a ratio of 2:1 by adding 1 million of iPSCs (1 mL) and a half million of HPCs (500 µL) together. Add all the mixed cells into the previously treated well of the microwell culture plate.
- Add 1 µL of Rock inhibitor Y27632 stock solution (1 mM) per 1 mL medium into the supernatant. Shake the plate from side to side a few times to distribute the cells evenly.
- Spin the microwell culture plate at 300 x g for 5 min in a swinging bucket rotor fitted with plate holders. Observe under the microscope to see if the cells have settled into the microwells. (Supplementary Figure 1A).
- Without disturbing the cells, put the plate in a 37 °C, 5% CO2 cell incubator.
- On day 2, 48 h later, observe the cells under the microscope for the formation of EBs. When the EBs are clearly formed, continue to the next step (Supplementary Figure 1B).
- Treat a 24-well cell culture plate with anti-adherence rinsing buffer (500 µL) for 15 min to make a low attachment plate.
- Remove the rinsing buffer completely and wash the wells twice with 1 mL of DPBS, then add 1 mL of E8 Flex medium into the wells.
- Resuspend the EBs in the microwell culture plate by pipetting them with a 1 mL wide orifice pipette tip for a few times.
- For each well of the low adherence treated 24 well plates, collect and add 100 µL of medium containing EBs. This will result in 10-20 EBs per well. Add E8 Flex medium to the old well containing the leftover EBs as backups.
- Incubate the plate in a 37 °C, 5% CO2 cell incubator for 48 h.
3. 3D neural organoid induction, proliferation, and maturation
- On day 2 after EB formation, thaw aliquots of BMM on ice for at least 30 min.
- Use a pre-chilled 20 µL tip, and add 15 µL ice-cold BMM drop wisely on top of the medium to coat the EBs. This makes a film of BMM that EBs will attach and grow onto.
- Put the plate in a 37 °C, 5% CO2 cell incubator.
- On day 4, after the EB formation, remove 500 µL of medium carefully without discarding EBs and repeat BMM coating by following step 3.2.
- Immediately start neural induction by adding 500 µL of PSC Neural Induction Medium on top of cells.
- On days 6 and 8, perform half medium change by carefully removing 500 µL of medium without touching cells and adding 500 µL of neural induction medium.
- On days 10, 12, and 14, perform half medium change with neural stem cell (NSC) medium: Knockout DMEM/F12 + 1x Glutamax supplement + 1x Neural supplement + bFGF + EGF.
- On day 15, transfer the whole well of spheres with medium to a new 12-well plate treated with anti-adherence rinsing solution as previously described. Add 500 µL of neuronal maturation medium (DMEM/F12 + 1x N2 supplement + 1x B27 supplement + 1x antibiotics-antimycotic) on top of the culture.
- Perform half-medium change with neuronal maturation medium every other day.
- During the stage of maturation, if there are signs of nutrition depletion, such as color change of medium, move the organoid to 6 well plates, which hold up to 3 mL of medium per well.
- Optionally, 17 days after the EB formation, supplement the maturation medium with cytokines (IL-34, M-CSF, TGF-β1, CD200, CX3CL1) for 6 days to facilitate microglial maturation.
- On day 23, collect the resulting neural organoids for characterization or further experiments.
4. Clearance and immunostaining of 3D neural organoids
- Fix up to 10 organoids by immersion in 1 mL of 4% paraformaldehyde (PFA) at 4 °C for 24 h.
- Transfer the organoids per well to a 96-well clear bottom plate, and wash with 200 µL of DPBS 2 times for 1 h each time with gentle shaking.
- Permeabilize organoids by washing them once in 50% methanol in DPBS, 80% methanol in double distilled (dd) water, and 100% dry methanol for 10 min each, at 4 °C with gentle shaking.
- Wash the organoids serially in 20% DMSO/methanol, 80% methanol in dd water, 50% methanol in DPBS, in 100% DPBS, then in DPBS with 0.2% Triton X-100, for 10 min each at 4 °C with gentle shaking. Make an effort to remove the residual buffer completely after each washing.
- Incubate in permeabilizing buffer (DPBS with 0.2% Triton X100, 0.3 M glycine, and 20% DMSO) with gentle shaking for 40 min at RT.
- Block in blocking buffer (DPBS with 0.2% Triton X100, 6% Goat serum, and 10% DMSO) for 1 h at 37 °C with gentle shaking on a shaker at 80 rpm.
- Incubate with antibodies in antibody dilution buffer (IBA1 1:100, TREM2 1:100, βIII-tubulin, 1:1000, 100 µL /well in DPBS with 0.2% Tween 20, 100 µg/mL Heparin, 3% Goat serum and 5% DMSO) at 37 °C for 2 h or in cold room with gentle shaking at 80 rpm for 3 days.
- Wash with DPBS with 0.2% Tween 20, 100 µg/mL Heparin 5 times, 10 min each at RT with gentle shaking on a shaker at 80 rpm.
- Incubate with secondary antibody (1:200 goat anti-mouse Alexa488) at 37 °C for 1 h followed by RT overnight with gentle shaking on a shaker at 80 rpm.
- For nuclear contrast staining, perform DAPI staining together with antibody incubation or in a washing buffer.
- Wash the organoids 10 times in washing buffer, 10 min each time at 37 °C, with gentle shaking.
- Observe under a microscope. If the background is still high, keep washing it 5 times.
- Discard the supernatant as much as possible. Add 200 µL of organoid clearing solution and incubate it for 5 min before observation and take images using a confocal microscope.
Results
Our protocol follows a scheme to differentiate HPCs from iPSCs and then mix the HPCs with iPSCs to make EBs, followed by neural induction, differentiation, and maturation (Figure 1). High quality of HPC differentiation is critical for the success of EB formation and later organoid differentiation. A serial dilution culture technique is used to produce the appropriate numbers and size of iPSC colonies to start the HPC differentiation (Figure 2A). Normally, the iPSC colonies will undergo significant morphological changes at the end of culture in Medium A (Figure 2B) and further show HPC differentiation after 3 days in Medium B (Figure 2C), as homogenous round cells, floating just above or attached to the bottom of culture vessels. The HPC may expand to form colony-like cell clusters (Figure 2D). HPCs predominate at day 10 without showing significant debris (Figure 2E). High quality of HPC and iPSC mixture results in EB formation within 24 h, which continues to grow with minimal cell debris (Figure 3A). Low-quality HPCs with excessive debris may result in difficulty in EB formation. After EBs were transferred to a new plate, they continued to grow and eventually reached a plateau in the maturation medium (Figure 3B). The EBs will differentiate into organoids containing IBA1 and TREM2 positive microglia, with (Figure 4) or without treatment with microglial cytokines (Figure 5). The treatment with microglial growth cytokines may increase the number of TMEM119-specific microglia (Figure 6). The representative organoids show both βIII-tubulin-positive neurons and IBA1-positive microglia after clearance and immunostaining (Figure 7). When treated with lipopolysaccharide (LPS), an increase in microglial activation can be observed by microglial-specific markers and proinflammatory gene activation (Figure 8). Supplementary Figure 2 shows IBA-1 and TMEM-119 positive cells in organoids cultured without additional microglial cytokines.
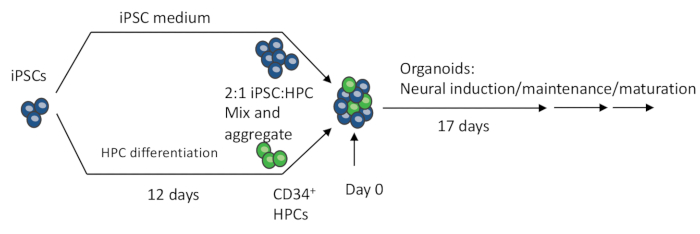
Figure 1: Schematic procedures of making the 3D organoid from iPSCs. EBs are made by mixing iPSCs and iPSC-differentiated HPCs. The EBs further undergo neural induction, differentiation, and maturation to produce organoids containing both neurons and microglia. Please click here to view a larger version of this figure.

Figure 2: Typical morphological changes from iPSCs to HPCs. Representative images are shown for the (A) iPSC colony, (B) at the end of Medium A treatment, (C) 3 days in Medium B, (D) day 10 in Medium B, and (E) the resulting floating HPCs. Images were taken under a fluorescence microscope with 10x or 4x objective. Please click here to view a larger version of this figure.
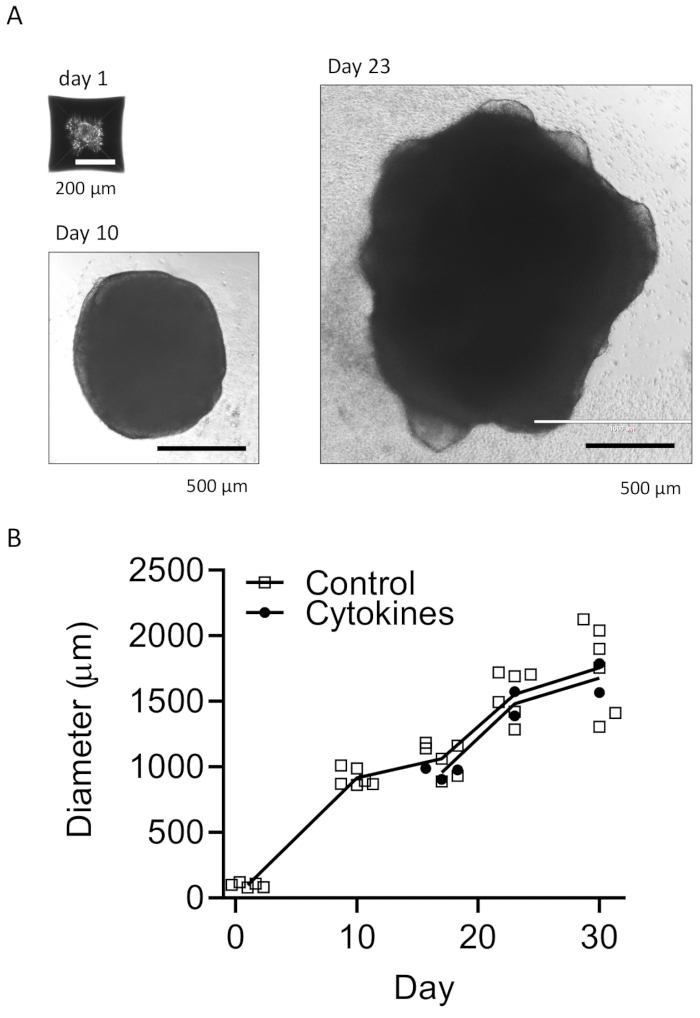
Figure 3: Develop EBs and mature organoids. (A) Representative photos show EB formation (Day 1), organoid growth (Day 10), and maturation (Day 23). A growth curve is shown for the sizes of organoids with or without microglial differentiation cytokines (B). Images were taken under a fluorescence microscope with 10x or 4x objective. Please click here to view a larger version of this figure.
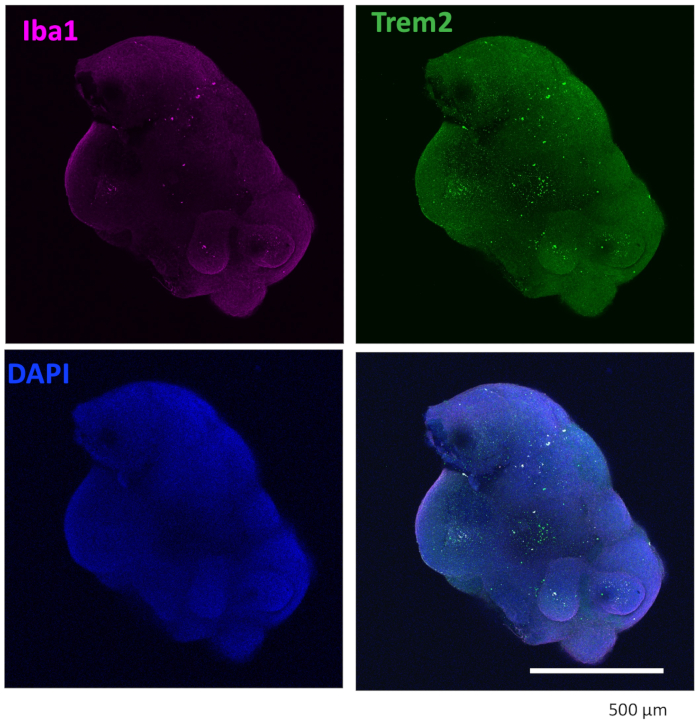
Figure 4: Organoids cultured with microglial differentiation cytokines contain microglia. After clearance and immunostaining, the organoid showed microglia were positively stained with both IBA1 and TREM2. Images taken under a 10x objective of a confocal microscope are shown. Please click here to view a larger version of this figure.
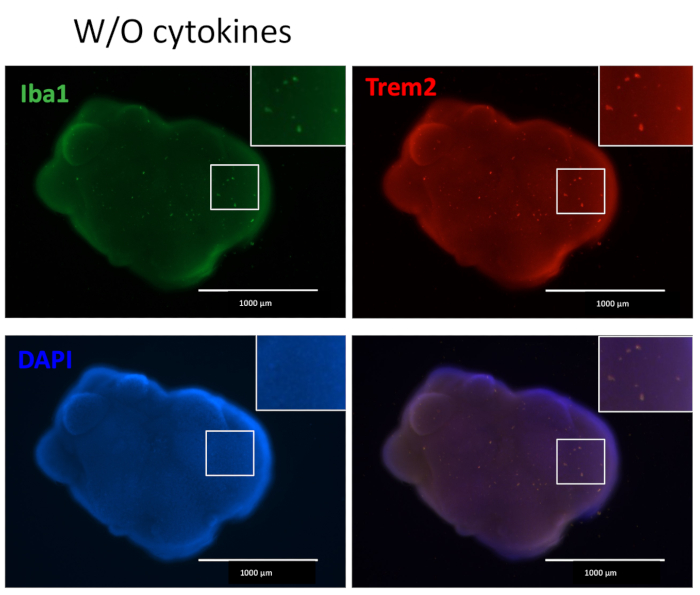
Figure 5: Organoids cultured without additional microglial differentiation cytokines contain microglia. Without the addition of external microglial differentiation cytokines, the organoids still produced microglia-positive stained with both IBA1 and TREM2, indicating the microenvironment of neuronal development in the organoid is enough to differentiate HPCs to microglia. Images were taken under a fluorescence microscope with a 4x objective. Please click here to view a larger version of this figure.

Figure 6: Single-cell RNA-seq result of microglia containing 3D organoids. (A) Plots showed IBA1(AIF1), TMEM119, and MAP2 distributions in an organoid without additional microglial differentiation cytokines. (B) IBA1 and TEME119 positive microglia were counted in two organoids cultured with and one without additional microglial differentiation cytokines. The numbers of IBA1 and TMEM119 positive cells change in representative organoids. Please click here to view a larger version of this figure.
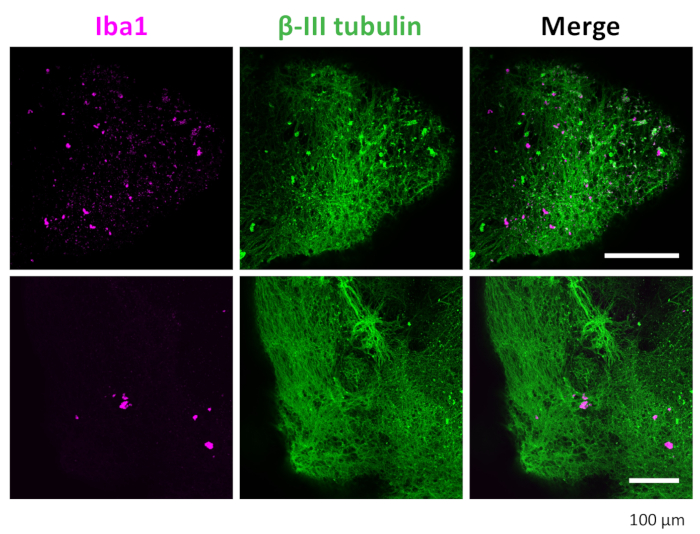
Figure 7: Immunostaining of neurons and microglia in the 3D organoids. After clearance and immunostaining, the organoid showed microglia positive stained with IBA1 and neurons stained with βIII-tubulin. Representative images taken under a confocal microscope with a 20x objective are shown. Please click here to view a larger version of this figure.
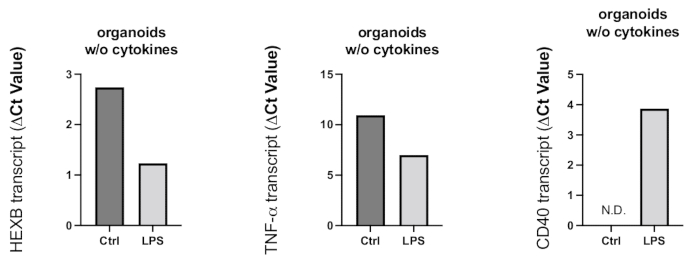
Figure 8: Inflammatory activation induced in the organoids by LPS treatment. After 24 h of treatment with LPS, organoids were collected, and RT-PCR was used to determine the microglial activation and proinflammatory reaction. Because Ct values are negatively related to the gene expression levels, as shown in the representative graphs, LPS treatment resulted in increased gene expression of microglial activation marker HEXB and proinflammatory cytokine TNF-α. Although CD40 gene expression was not detected in control organoids, LPS treatment resulted in detectable CD40 transcripts. Please click here to view a larger version of this figure.
Supplementary Figure 1: EBs in a microwell culture plate. (A) Mixed HPCs and iPSCs were settled in the microwells in a microwell culture plate well after centrifugation. (B) EBs were formed after 24 h incubation in the microwell culture plate when observed under a microscope. (C) CD34+ cells were seen in the forming organoids 8 days after EB formation. Confocal images were taken with a 20x objective. Please click here to download this figure.
Supplementary Figure 2: Confocal images showed IBA-1 and TMEM-119 positive cells in organoids cultured without additional microglial cytokines. Images were taken using a confocal microscope with 20x and 2x zoom-in to show the morphologies of cells. Please click here to download this figure.
Discussion
Here, a detailed protocol for making 3D neural organoids containing innate microglia from EBs derived from mixed iPSCs and iPSC-differentiated HPCs is presented. It is a relatively short and easy approach involving only cell culture techniques and equipment generally available in most laboratories.
The most critical factor for the success of this protocol is the quality of HPC differentiation. We adopted the published method17 using a commercial kit to differentiate HPCs from iPSCs with some modifications. Compared to the original protocol, we begin with a smaller sized and lower range of iPSC colonies and collect the differentiated HPCs after 10 days of differentiation instead of 12 days. This approach generates only 1-2 million HPCs per well but is still more than enough for the next step of 3D organoid production. Maintaining the right balance between these cells and iPSC colonies is important since excessive numbers of cells may result in dead cells/debris in the end, causing failure of EB formation. Although HPCs can be collected from day 10 to day 12 after induction, we found that collection at day 12 would risk having more dead cells and impurities as the rapid growth of the HPCs to the end of culture could exhaust nutritional factors easily and require close monitoring and additional medium changes. By collecting HPCs at day 10, there will be no need to do HPC purification by flow cytometry or magnet bead purification, which are necessary to enrich the lower purity of HPCs.
For normal iPSC subculture and HPC differentiation, EDTA dissociation buffer was used, for which the effect is mild and only dissociates the iPSC colonies into small cell patches, which are good for the reattachment of iPSCs after splitting. However, the cell patches may interfere with the EB formation. So, Accutase treatment is used to dissociate iPSCs and achieve a single-cell solution for more uniform EB formation. When making EBs by dissociating iPSCs, it is important not to overtreat the iPSCs with Accutase, which may result in excessive cell death. Leaving iPSCs at room temperature and in suspension for too long may also lead to massive cell death and abrogated differentiation. Thus, the process from steps 2.1 to 2.13 should be completed as soon as possible to avoid unnecessary delays.
To facilitate the neural differentiation and maturation, several EBs are cultured per well. This setting facilitates the fusion of EBs and decreases the time needed for organoid maturation. However, it is difficult to control the exact numbers of EBs for fusion and thus may introduce irregularity in the sizes and shapes of the resulting organoids. The irregularity can be minimized by shaking the plates from time to time or, if possible, using a bioreactor. It is also possible to seed single EB into wells of a 96-well plate to avoid the fusion altogether, but it will need extra effort and time for the organoids to grow and mature.
Contrary to embedding organoids individually with Matrigel in the most accepted approach18, we applied the Matrigel drop wisely on top of the medium several times. This approach is less labor intensive but produces a high quality of neural differentiation in the organoids. The protocol described in this study generates cortical neurons with microglia. However, by adjusting the growth factors and differentiation steps following published techniques19,20, it could be easily adapted to produce organoids representing other specific neuronal types or regions of the brain.
Recent publications have incorporated iPSC-differentiated microglia or macrophage-like cells into developing cortical organoids to make microglial-containing brain organoids21. As adult microglia are developed from primitive HPCs recruited into the brain during early embryonic days12, we incorporate HPCs into the EB formation and before the neural induction. It is interesting to find that without applying the specific cytokines for microglial differentiation, which are generally used for producing microglial differentiation in brain organoids22, we still observe decent microglial differentiation within the resulting brain organoids, albeit the TREM2 positive cells are lower in numbers, compared to the ones cultured with cytokines. It suggests that the inclusion of HPCs into the EB formation and the ensured early interactions of the neural development process and HPCs could be enough to differentiate the microglia in the brain organoid. It also suggests that our protocol is valuable as a model for studying the early microglial development in the brain.
As discussed, this protocol produces organoids mimicking the early human brain development. Although we have cultured organoids up to 6 weeks without noticing apparent deterioration based on morphologies of the organoids, and some cultures have reached more than 4 months, cell necrosis was found in the organoid cores since 8 weeks old. This is also common to other types of brain organoids, as the center cells in the growing organoids experience difficulties in accessing nutrition. Using bioreactors with continuous shaking may increase the viability and prolong the life of the organoids, allowing more mature cell types to differentiate. However, long term culture will almost certainly result in greater variabilities without certain automatic culture assistance. Thus, we recommend using this protocol to study microglial-related early brain development as well as the acute effects of neuroinfections on brain development. For more sophisticated brain modeling, we suggest researchers use this protocol as a basis and apply modifications to increase consistency and facilitate long-term cultures.
In summary, in the present protocol, we incorporate iPSC-differentiated HPCs into the EB formation and further induce neural differentiation. In less than a month, this protocol will produce organoids containing both neurons and microglia generated from the same iPSCs. It provides a convenient alternative to make brain organoids with early interactions between neuronal and microglial development, thus making it useful for studying the physiology of human brain development and the neural infectious and neuroinflammatory diseases involving innate microglia.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study is supported by NINDS intramural research funds.
Materials
| Name | Company | Catalog Number | Comments |
| 12 well cell culture plates | Corning | #3512 | |
| 24 well cell culture plate | SARSTEDT | #83.3922 | |
| Accutase | Thermo | A1110501 | |
| Aggrewell 400 plate | Stemcell technologies | #34411 | Referred to as microwell culture plate |
| Alexa Fluor 488 goat anti-mouse antibody | Life techniologies | A11001 | 1:400 dilution |
| Alexa Fluor 594 goat anti-rabbit antibody | Life techniologies | A11012 | 1:400 dilution |
| Allegra X-30R Centrifuge with rotor S6069 | Beckman Couler | ||
| Anti- Adherence Rinsing solution | Stem Cell Technologies | #07010 | |
| anti-CD34 antibody | Stem Cell Technologies | #60013 | 1:100 dilution |
| anti-Human CD43 antibody | Stem Cell Technologies | #60085 | 1:100 dilution |
| anti-IBA1 rabbbit antibody | Fujifilm | 019-19741 | 2.5 µg/mL |
| anti-TREM2 rat pAb | RD Systems | mab17291 | 2.5 µg/mL |
| Antibiotic-antimycotic | Gibco | 15240-062 | 1x |
| B27 supplement | Life technologies | 17504-044 | 1x |
| bFGF | Peprotech | 100-18B | 20 ng/mL |
| CD200 | Novoprotein | C311 | 100 ng/mL |
| CryoTube vials | Thermo | #368632 | |
| CX3CL1 | Peprotech | 300-31 | 100 ng/mL |
| DAPI | Sigma | D9542 | 1 µg/mL |
| DMEM/F12 | Life technologies | 12400-024 | 1x |
| DMSO | Sigma | D2650 | |
| DPBS | Gibco | #4190136 | 1x |
| E8 Flex medium kit | Thermo | A2858501 | |
| EDTA | Mediatech | 46-034-Cl | 0.5 mM |
| EGF | Peprotech | AF-100-15 | 20 ng/mL |
| EVOS FL Auto Microscope | Thermo | Fluorescence microscope | |
| FastStart Universal SYBR Green PCR master mix | Roche | #4913850001 | |
| Glutamax | Gibco | #35050079 | |
| Goat serum | Sigma | G9023 | 4% |
| IL-34 | Peprotech | 200-34 | 100 ng/mL |
| ImageXpress Micro Confocal | Molecular Devices | ||
| Knockout DMEM/F12 | Gibco | #10829018 | |
| M-CSF | Peprotech | 300-25 | 25 ng/mL |
| Matrigel | Corning | #354277 | Basement membrane matrix (BMM) |
| Mouse anti-βIII-tubulin antibody | Promega | G712A | 1:1000 dilution |
| Mr. Frosty container | Thermo | 5100-0001 | |
| N2 supplement | Life technologies | 17502-048 | 1x |
| Paraformadehyde | Sigma | P6148 | 4% |
| PSC Neural Induction Medium | Gibco | A1647801 | |
| Rock inhibitor Y27632 | Stemcell technologies | #72304 | 1 mM stock |
| RT LTS 1000 ul pipette tips | RAININ | #30389218 | for transferring organoids |
| STEMdiff Cerebral Organoid Kit | Stem Cell Technologies | #08570 | |
| STEMdiff Hematopoietic Kit | StemCell Technologies | #5310 | Referred to as hematopoietic Kit |
| StemPro Neural Supplement | Gibco | A1050801 | Referred to as neural supplement |
| TGF-β1 | Peprotech | 100-21 | 50 ng/mL |
| Total RNA Purification Plus Kit | Norgen | #48400 | |
| TritonX-100 | Sigma | T9284 | 0.10% |
| Visikol Histo-Starter Kit | Visikol | HSK-1 | Contains organoid clearing solution HISTO-M, washing buffer |
| Zeiss LSM 510-META Confocal Microscope | Zeiss |
References
- Sabate-Soler, S., et al. Microglia integration into human midbrain organoids leads to increased neuronal maturation and functionality. Glia. 70 (7), 1267-1288 (2022).
- Lazarov, T., Juarez-Carreño, S., Cox, N., Geissmann, F. Physiology and diseases of tissue-resident macrophages. Nature. 618 (7966), 698-707 (2023).
- Wang, T., Liu, B., Zhang, W., Wilson, B., Hong, J. S. Andrographolide reduces inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuron-glia cultures by inhibiting microglial activation. J Pharmacol Exp Ther. 308 (3), 975-983 (2004).
- Qian, L., Flood, P. M., Hong, J. S. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J Neural Transm (Vienna). 117 (8), 971-979 (2010).
- Clevers, H. Modeling development and disease with organoids. Cell. 165 (7), 1586-1597 (2016).
- Fakıoğlu, D. M., Altun, B. New therapeutic approaches in cystic fibrosis. Turk J Pharm Sci. 17 (6), 686-697 (2020).
- Mcmillan, R. E., Wang, E., Carlin, A. F., Coufal, N. G. Human microglial models to study host-virus interactions. Exp Neurol. 363, 114375 (2023).
- Scopa, C., et al. Jun upregulation drives aberrant transposable element mobilization, associated innate immune response, and impaired neurogenesis in Alzheimer's disease. Nat Commun. 14 (1), 8021 (2023).
- Tamaki, Y., et al. Spinal cord extracts of amyotrophic lateral sclerosis spread TDP-43 pathology in cerebral organoids. PLoS Genet. 19 (2), e1010606 (2023).
- Chambers, S. M., et al. Highly efficient neural conversion of human es and IPS cells by dual inhibition of smad signaling. Nat Biotechnol. 27 (3), 275-280 (2009).
- Kim, S. H., Chang, M. Y. Application of human brain organoids-opportunities and challenges in modeling human brain development and neurodevelopmental diseases. Int J Mol Sci. 24 (15), 12528 (2023).
- Ginhoux, F., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 330 (6005), 841-845 (2010).
- Ormel, P. R., et al. Microglia innately develop within cerebral organoids. Nat Commun. 9 (1), 4167 (2018).
- Abdelmalek, C. M., et al. Building a growing genomic data repository for maternal and fetal health through the ping consortium. medRxiv. , (2024).
- Wei, Z., et al. Human IPSC-derived brain organoids: A 3D mini-brain model for studying HIV infection. Exp Neurol. 364, 114386 (2023).
- Wang, T., et al. Regulation of stem cell function and neuronal differentiation by HERV-K via mTOR pathway. Proc Natl Acad Sci U S A. 117 (30), 17842-17853 (2020).
- Mcquade, A., et al. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener. 13 (1), 67 (2018).
- Lancaster, M. A., Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 9 (10), 2329-2340 (2014).
- Fiorenzano, A., et al. Single-cell transcriptomics captures features of human midbrain development and dopamine neuron diversity in brain organoids. Nat Commun. 12 (1), 7302 (2021).
- Qian, X., et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 165 (5), 1238-1254 (2016).
- Park, D. S., et al. IPS-cell-derived microglia promote brain organoid maturation via cholesterol transfer. Nature. 623 (7986), 397-405 (2023).
- Schafer, S. T., et al. An in vivo neuroimmune organoid model to study human microglia phenotypes. Cell. 186 (10), 2111-2126.e20 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved