Method Article
Electroporation-Based CRISPR-Cas9-Mediated Gene Knockout in THP-1 Cells and Single-Cell Clone Isolation
In This Article
Summary
The THP-1 cell line is widely used as a model to investigate the functions of human monocytes/macrophages across various biology-related research areas. This article describes a protocol for efficient CRISPR-Cas9-based engineering and single-cell clone isolation, enabling the production of robust and reproducible phenotypic data.
Abstract
The human acute monocytic leukemia (AML) THP-1 cell line is widely used as a model to study the functions of human monocyte-derived macrophages, including their interplay with significant human pathogens such as the human immunodeficiency virus (HIV). Compared to other immortalized cell lines of myeloid origin, THP-1 cells retain many intact inflammatory signaling pathways and display phenotypic characteristics that more closely resemble those of primary monocytes, including the ability to differentiate into macrophages when treated with phorbol-12-myristate 13-acetate (PMA). The use of CRISPR-Cas9 technology to engineer THP-1 cells through targeted gene knockout (KO) provides a powerful approach to better characterize immune-related mechanisms, including virus-host interactions. This article describes a protocol for efficient CRISPR-Cas9-based engineering using electroporation to deliver pre-assembled Cas9:sgRNA ribonucleoproteins into the cell nucleus. Using multiple sgRNAs targeting the same locus at slightly different positions results in the deletion of large DNA fragments, thereby increasing editing efficiency, as assessed by the T7 endonuclease I assay. CRISPR-Cas9-mediated editing at the genetic level was validated by Sanger sequencing followed by Inference of CRISPR Edits (ICE) analysis. Protein depletion was confirmed by immunoblotting coupled with a functional assay. Using this protocol, up to 100% indels in the targeted locus and a decrease of over 95% in protein expression were achieved. The high editing efficiency makes it convenient to isolate single-cell clones by limiting dilution.
Introduction
THP-1 is a human monocyte-derived cell line isolated from a patient suffering from acute leukemia (AML), which displays phenotypic features closely resembling those of primary monocytes1. As compared to primary monocyte-derived macrophages, which do not divide and display both limited lifespan and inter-/intra-donor variability in phenotype, THP-1 cells can be cultured virtually forever and have a more homogeneous behavior that favors results reproducibility2,3,4,5,6. Notably, THP-1 cells can be differentiated towards a macrophage-like phenotype with phorbol-12-myristate 13-acetate (PMA), making them a widely used in vitro model to investigate the responses of monocytes/macrophages to inflammatory signals7,8,9,10,11,12,13 or infection by clinically relevant human pathogens, including HIV14,15,16. The possibility to genetically engineer THP-1 cells is of interest across many biology-related research areas.
Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR associated protein 9 (CRISPR-Cas9) is a prokaryotic adaptive immune system relaying on RNA-guided nuclease to degrade invading viral genomes, which has been reprogrammed as a genetic engineering tool17. The process of genome editing proceeds in three steps: recognition, cleavage, and repair. A single-guide RNA (sgRNA) recruits the Cas9 nuclease to a specific genomic locus through base pairing with its 20-bp guide sequence. The presence of a Protospacer Adjacent Motif (PAM) sequence directly 3' of the 20-bp genomic target sequence triggers the Cas9-mediated unwinding and cleavage on both DNA strands between positions 17 and 18 (3-bp 5' of the PAM). The resulting double-strand break (DSB) is processed by two major repair pathways. In the absence of a repair template bearing homology with the damaged locus, the error-prone Non-Homologous End Joining (NHEJ) pathway will introduce random nucleotide insertions and/or deletions (indels), potentially leading to frameshift mutations and/or the introduction of premature termination codons (PTC). In turn, PTC-containing mRNAs are targeted by degradation by the nonsense-mediated mRNA decay (NMD) pathway, ultimately disrupting protein expression/function18,19,20. Alternatively, the template-dependent Homology-Directed Repair (HDR) pathway can operate and faithfully repair the DSB. This mechanism has been harnessed to achieve precise gene editing, including knock-ins and base substitutions. It is worth noting that the cell cycle status is an important factor influencing the choice of DSB repair pathway. Indeed, NHEJ is active at all stages of the cell cycle, while HDR is mainly restricted to the S/G2 phases21.
THP-1 cells grow in suspension and are notoriously difficult to transfect with plasmid DNA, a procedure that possibly also alters their viability and/or differentiation capacity22,23. Transduction with HIV-1-based lentiviral vectors encoding both Cas9 and the sgRNA is often employed to knockout (KO) a gene of interest24. Integration of the Cas9/sgRNA cassette into the cellular genome ensures prolonged expression and efficient KO, but is also a persistent source of off-target effects25. Alternatively, the pre-assembled Cas9:sgRNA ribonucleoproteins (RNPs) are delivered by electroporation, a method involving the temporary formation of pores in both the plasma and nuclear membranes upon application of electric impulses. Preserving cell viability is an important challenge when undertaking this approach.
Here, a THP-1 cell line stably expressing GFP (THP-1_GFP) was produced to serve as a tool to establish a protocol to achieve efficient CRISPR-Cas9-based editing. After designing a strategy to inactivate the EGFP gene using three sgRNAs simultaneously (multi-guide approach), KO efficiency among several electroporation conditions was determined using GFP expression as a readout. Cell proliferation was monitored in parallel. Gene editing was confirmed by both a T7 endonuclease I (T7EI) assay and Sanger sequencing, followed by analysis with the Inference of CRISPR Edits (ICE) algorithm26. Parameters that yielded up to 95% GFP expression decrease, with THP-1 cells recovering normal growth rates after electroporation, were successfully employed to inactivate an endogenous gene (SAMHD1) and produce single-cell THP-1 clones.
Protocol
The details of the reagents and the equipment used in this study are listed in the Table of Materials.
1. Guide design with CRISPOR (Figure 1.1)
NOTE: SnapGene Viewer software may be used in steps 4, 7, and 10 to annotate the editing target site and the location of the PCR primer hybridization within the gene of interest.
- Go to the Ensembl website (www.ensembl.org). In the Search box, select a species and enter the name of the gene of interest. Click on Go. Select the result that corresponds to the gene (not a transcript).
- Click on Show transcript table and then select the Transcript ID that corresponds to the gold labeled Protein coding in the Biotype column. Once on the transcript page, click on Exons in the left menu.
- Scroll down and click on Download sequence. Make sure the File format is FASTA. In Settings - Included Sequences, deselect everything except Genomic sequence.
Enter the number "500" in Flanking sequence at either end of transcript box. Click on Preview, select the whole sequence (just the nucleotides without the header), and copy it (Ctrl+C). - Open SnapGene Viewer and click on New > DNA file. Paste the sequence (Ctrl+V) into the Create a sequence box. Uncheck Detect common features and select Linear in Topology.
Rename the file and click on Create. In the left menu, deselect Show enzymes (first icon). At the bottom of the window, select the Sequence tab.
NOTE: This step allows retrieval of the complete gene sequence, including exons, introns, and 500 bp flanking sequences (optional). This latter information is useful for designing PCR primers for the amplification of a target site located within the first exon. - Back on the Ensembl website, scroll up on the file preview and click Back. Now, change the File format to RTF. In Settings - Included Sequences, deselect everything except Exons. In Show variants, select No. Click on Download at the top of the page.
- Open the downloaded file (with Word), which contains the sequence of exons and shows the coding sequence in blue, starting with the initial ATG. Choose the exon to be targeted by the CRISPR-Cas9-directed editing (see below for recommendations), select it, and copy it (Ctrl+C).
- The targeted exon is either an early exon or an exon encoding a functionally important domain of the protein. It is worth noting that the establishment of a PTC in a late exon close to the 3' UTR will likely fail to elicit NMD, leading to the expression of a C-terminally truncated protein. Conversely, the introduction of a PTC in an early exon proximal to the native initiation site is associated with a risk of illegitimate translation (ITL, aka alternative translation initiation (ATI)), yielding the unexpected expression of an N-terminally truncated protein that begins at an in-frame translation initiation sites (TIS) downstream of the first ATG codon. To mitigate this latter risk, it is advised to assess the occurrence of alternative TIS using ATGpr27 (atgpr.dbcls.jp) and/or NetStart 1.028 (services.healthtech.dtu.dk/services/NetStart-1.0/).
- Ensure that the target exon contains a coding sequence. However, it might prove useful to choose a sgRNA annealing with the 5'UTR region upstream of the initial ATG to include it in the deleted fragment.
- Ideally, the targeted exon should be common to all the protein-coding transcript variants of the gene. Check if that is the case on the NCBI's Genome Data Viewer (www.ncbi.nlm.nih.gov/gdv/) by searching the gene of interest.
Clicking on the gene name (in green) on the display window will show the transcript variants (in purple). Exons are represented by a rectangle.
- In SnapGene Viewer, press Ctrl+F, Ctrl+V, and then Enter to search for the sequence of the exon. Press Ctrl+T to add a new feature, name it, change the type to "exon" and click on OK.
- Go to the CRISPOR website (http://crispor.gi.ucsc.edu/) and paste the exon sequence in Step 1. First, select a reference genome in Step 2 and then the type of PAM to target in Step 3, typically 20bp-NGG for SpCas9. Click on SUBMIT.
- Select two sgRNAs so that they are separated by up to 150 bp, then select a third sgRNA in between. Here are some guidelines for sgRNA selection:
- The MIT specificity score is related to off-target effects. A higher score indicates fewer potential off-targets. The right column displays three predicted off-target sites ranked from the most to the least likely, along with their locations (in an exon, an intron, or an intergenic region). The full list of predicted off-target sites can be accessed by clicking show all. If possible, select sgRNAs with an MIT score >80, prioritizing those without off-targets for 0, 1, or 2 mismatches. Additionally, sgRNAs with off-targets in an exon, which have the greatest potential to affect phenotype, should be avoided.
- For predicted efficacy, refer to the Doench '16 score. Note that a high Doench '16 score simply indicates that the sgRNA is more likely to be effective. Actual efficacy must be determined experimentally. For this reason, it is always useful to select several sgRNAs, even if they are not intended to be used together.
- A GC content that is either too high or too low, as well as certain motifs, can be detrimental to sgRNA efficiency and should be avoided. These parameters are highlighted by CRISPOR.
- Repeat step 1.7 to add the sgRNA sequence and the associated PAM sequence to the gene sequence in SnapGene Viewer. At the same time, paste the sgRNA sequence (without the PAM) into a text or Excel file to retain the 5'-3' orientation required when ordering the oligonucleotide.
- For PCR-based amplification of the target site, design a couple of primers surrounding it. The amplicon size should be between 800 and 1000 bp. PrimerQuest was used to design the primers for this protocol (https://eu.idtdna.com/pages/tools/primerquest). Alternatively, CRISPOR provides a list of primers to amplify the targeted genomic region as well as the off-target sites. After displaying the full list of potential off-target sites (step 1.9.1), click on Off-target primers at the bottom right corner.
2. Reagent and cell preparation for electroporation (Figure 1.2)
- Prepare a 24-well culture plate to recover cells after electroporation by filling the wells with 500 µL of RPMI 1640 medium supplemented with 20% heat-inactivated (56 °C, 30 min), filtered (0.20 µm) fetal bovine serum (FBS). Do not add antibiotics. Keep the plate in a humidified incubator at 37 °C and 5% CO2 for 24 h until electroporation.
- If the sgRNAs are shipped dry, rehydrate them with TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) to a final concentration of 100 µM (i.e. 10 µL of TE buffer per 1 nmol of sgRNA). Vortex for 30 s, incubate at 4 °C overnight to allow complete rehydration and, after a brief pipette homogenization, store the sgRNA stock solution at -20 °C. Depending on the final volume, make aliquots to avoid multiple freeze-thaw cycles.
- Prepare a working sgRNA solution at a final concentration of 30 µM by diluting the 100 µM stock solution in nuclease-free water. Vortex for 30 s and incubate 5 min at room temperature.
- Assemble the Cas9:sgRNA RNPs at a 1:9 molar ratio by simultaneously diluting 1 µL of each of the three 30 µM sgRNAs and 0.5 µL of 20 µM Cas9 solutions in 3.5 µL of resuspension buffer R, included in the electroporation kit (final volume of 7 µL, for one experimental condition; scale accordingly). Vortex briefly and incubate for 5 min at room temperature.
- In the meantime, prepare an unedited control by adding 0.5 µL of 20 µM Cas9 to 6.5 µL of resuspension buffer R. Vortex briefly and incubate for 5 min at room temperature.
- Add 5 µL of resuspension buffer R to all samples for a final volume of 12 µL per electroporation condition.
- Prepare the THP-1 cells for electroporation.
- To assess concentration and viability by Trypan blue exclusion test. Dilute the cells 1:2 in a 0.4% trypan blue staining solution. After 30 s incubation, homogenize well and add 10 µL in a wall of a counting chamber with a Neubauer-improved grid style. Count three large squares and divide the count by 100 to obtain the cell concentration (x106 cells/mL).
NOTE: The health of the cells influences their sensitivity to electroporation. Care should be taken to perform the cell culture in optimal conditions. Time outside the incubator must be limited, and all reagents and solutions should be prepared and warmed up in advance. - For each condition, collect a volume equivalent to 0.2 x 106 cells and centrifuge (336 x g, 5 min, 20 °C).
- Aspirate the supernatant using a pipette and resuspend the pellet in 500 µL of PBS. Centrifuge again (336 x g, 5 min, 20 °C).
- Aspirate the supernatant carefully using a pipette and resuspend the THP-1 cell pellet with the 12 µL of RNP solution (step 2.6).
- To assess concentration and viability by Trypan blue exclusion test. Dilute the cells 1:2 in a 0.4% trypan blue staining solution. After 30 s incubation, homogenize well and add 10 µL in a wall of a counting chamber with a Neubauer-improved grid style. Count three large squares and divide the count by 100 to obtain the cell concentration (x106 cells/mL).
3. Electroporation system set-up and nucleofection (Figure 1.3)
- Place the pipette station under a biosafety cabinet, put an electroporation tube in the support, and add 3 mL of buffer E, included in the electroporation kit.
- After switching on the electroporation device, use the touch screen to set the following electroporation parameters: Voltage = 1 500 V, Duration = 10 ms, Number = 3.
- Equip the electroporation pipette with a tip and aspirate 10 µL of the RNP/THP-1 solution (step 2.7.4). Insert the pipette into the electroporation tube and press Start on the electroporation device screen. Wait for the message Complete to appear and remove the pipette from the tube.
- Transfer the cells to a well of the pre-heated 24-well plate and homogenize gently. Put the plate back in the humidified incubator and let them rest undisturbed for 72 h.
NOTE: Take care not to make any bubbles when pipetting the RNP/THP-1 suspension, as they will interfere with the electroporation procedure. If an error message appears in the absence of a visible electric arc, remove the electroporation pipette from the tube and press Start again. However, if an electric arc in the form of a brief bright spark is observed, it might indicate the presence of bubbles. The electroporation procedure will likely fail, even in the absence of an error message.
4. THP-1 recovery 72 h post-electroporation (Figure 1.4)
- Count the cells to assess concentration (step 2.7.1) 72 h after the electroporation.
- If there are enough cells (i.e.,≥0.6 x 106 cells/mL), dilute them at least a factor 2 with RPMI supplemented with 20% FBS and 1% Penicillin-Streptomycin and bring the concentration between 0.3-0.5 x 106 cells per mL. Otherwise, allow the cell to recover for another 72 h.
- Passage and amplify the cells until there is enough for KO validation. In the meantime, isolation of single-cell clones may be initiated (step 7).
5. Gene editing validation by T7EI mismatch assay (Figure 1.5)
NOTE: The assay might underestimate the editing efficiency given that T7EI recognizes mismatches larger than 1 bp. Thus, the T7EI assay is not useful for screening homozygous cell populations (i.e., single-cell clones) unless appropriately modified (step 5.7).
- Assess the cell concentration (step 2.7.1) and withdraw a volume equivalent to 0.1 x 106 cells in a 1.5 mL tube. Centrifuge (336 x g, 5 min, 20 °C), aspirate the supernatant, and resuspend the pellet in 500 µL of PBS. Centrifuge again and, using a pipette, aspirate as much supernatant as possible without disturbing the pellet. Snap-freeze the sample and store at -20 °C.
- Extract the genomic DNA to serve as a matrix for PCR amplification.
- Resuspend the pellet with 50 µL of DNA extraction solution, homogenize, and transfer the entire volume in a 0.2 mL PCR tube. Vortex and centrifuge briefly (pulse for 3 s).
- Place the tube in a thermal cycler and heat it at 65 °C for 15 min, followed by 98 °C for 10 min.
- Dilute the extracted DNA with 90 µL of ultrapure water. Vortex and centrifuge briefly (5,000 x g, 3 s).
- Dilute the PCR primer (see Table of Materials) in ultrapure water for a final concentration of 10 µM (i.e., 10 pmol/µL).
- Prepare the PCR mix (following the NOTE below) in a 0.2 mL PCR tube (final volume = 50 µL, for one condition). Usually, there will be at least two conditions: the KO and the unedited control with Cas9 only.
NOTE: Purified genomic DNA: 10 µL; Forward and reverse primer (10 µM): 2.5 µL each, the final concentration of 500 nM; Reaction Buffer (5x): 10 µL. Vortex well before adding. Mix dNTP (25 mM of each): 0.6 µL, final concentration of 0.3 mM for each dNTP. DNA polymerase: 0.5 µL; Ultrapure water: 23.9 µL. Vortex and centrifuge briefly (pulse for 3 s). - Place the tubes in the thermal cycler and run the PCR program with the following settings:
NOTE: One cycle at 95 °C for 5 min, followed by 30 cycles [98 °C for 20 s (denaturation step), X °C for 15 s (annealing step), 72 °C for 45 s (elongation step)], then one final cycle at 72 °C for 2 min. At the end of the amplification, remove the tubes, vortex, and centrifuge briefly (pulse for 3 s). The annealing temperature (X) is the melting temperature (Tm) of the primers minus 5 °C. - For a polyclonal edited population: in a new 0.2 mL tube, add 17.5 µL of the PCR amplicon and 2 µL of NEBuffer 2 (10x) for a final volume of 19.5 µL. Vortex and centrifuge briefly (pulse for 3 s). Alternatively, to screen single-cell clones, mix 1:1 of the PCR products from both the edited and the unedited control cells (step 5.6) (Supplementary Figure 1).
- For the heteroduplex formation, place the tubes in the thermal cycler and run the following program: one cycle at 95 °C for 10 min, one with a ramp of -2 °C/s from 95 to 85 °C, one with a ramp of -0.3 °C/s from 85 to 25 °C and one final cooling cycle at 10 °C on HOLD.
- After the heteroduplex formation, add 0.5 µL of T7EI solution into the tube. Incubate at 37 °C for 30 min.
- Prepare a 1.2% agarose gel:
- Weight the agarose in a glass bottle and add the appropriate volume of 1x TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.3). Place it in a microwave paying attention not to screw the cap firmly. Heat several times until the agarose crystals are completely dissolved.
NOTE: If required, mix the solution. Do not allow it to boil, or volume will decrease, changing the agarose concentration. - Add the DNA gel stain diluted at 1/20,000 and mix well.
- Pour the agarose solution into a mold, add a comb, and let solidify at room temperature.
- Weight the agarose in a glass bottle and add the appropriate volume of 1x TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.3). Place it in a microwave paying attention not to screw the cap firmly. Heat several times until the agarose crystals are completely dissolved.
- Prepare samples for gel electrophoresis by mixing 5 µL of the T7EI-digestion products (step 5.9) or the undigested PCR product with 5 µL of water and 2 µL of 6x DNA loading dye. Load the samples and size ladder and migrate at 80 V for 45 min (time can be adapted depending on the expected size of the DNA fragments). Once migration is over, acquire an image of the gel with an appropriate imaging system.
6. Gene editing validation by Sanger sequencing analysis (Figure 1.6)
- To characterize the genetic modification, purify the PCR products (step 5.4) and, after Sanger sequencing, analyze the results with the ICE tool (https://www.synthego.com/products/bioinformatics/crispr-analysis).
- Control whether a protein can be produced despite the modifications.
- Download the ICE results and open the contribs.txt file.
- Compare the edited sequences with the WT counterpart. Map the indels and verify that they arose at the targeted genomic region. Assess their size. If the indel length is not a multiple of three, a frameshift will occur, and a PTC will possibly be introduced. The mutated mRNA will expectedly undergo NMD-mediated degradation.
7. Single-cell clone isolation by limiting dilution (Figure 1.7)
NOTE: Isolation of single-cell clones is not mandatory. However, if choosing to do so, it is important to characterize multiple clones and compare their phenotype with the original polyclonal population.
- Take a sample of cell suspension, dilute it 1/2 with culture medium, and assess the concentration (step 2.7.1). Dilution increases the counting accuracy.
- Perform 2 to 3 serial dilution steps to reach a concentration of 7 cells/mL. Dispense 100 µL of the cell suspension per well in a round-bottom 96-well plate (i.e., 0.7 cells per well). Let the cell decant for a few hours before observing the plates at the microscope to identify wells containing one cell.
- Monitor regularly colony formation and growth and transfer cells to a larger culture plate or flask when needed.
8. Functional characterization of THP-1 KO SAMHD1 cells by HIV-1 restriction assay
- Seed THP-1 in a 24-well culture plate with 0.25 x 106 cells per well in 300 µL of RPMI supplemented with 10% FBS, 1% Penicillin-Streptomycin (complete RPMI) and containing 300 ng/mL PMA for differentiation. Keep the plate in a humidified incubator (37 °C, 5% CO2) for 24 h.
- Replace the medium with a PMA-free complete RPMI medium and put the plate back in the incubator for another 24 h.
- Using a vacuum pump, aspirate all the culture medium. Add 250 µL of VSVg-pseudotyped HIV-1-GFP viral stock-containing solution (MOI = 0.5 IFU/cell). Include a non-infected control. Place the plate at 4 °C for 2 h to synchronize the infection.
- Wash the cells once with RPMI. Add 500 µL of complete RPMI to each well and incubate for 48 h in standard conditions (time can be adjusted).
- After removing the culture medium, wash the cells once with cold 1x PBS. Then, add 100 µL of 0.05% Trypsin-EDTA in each well. Place the plate in an incubator at 37 °C until the cells are completely detached (5 min should be enough) before adding 200 µL of complete RPMI to inactivate the enzyme. Transfer 250 µL from each well into a 96-well round-bottom plate (ensure the flow cytometer accepts culture plates).
- Centrifuge the plate (757 x g, 3 min, deceleration = 6, 20 °C) and aspirate the supernatant using a multichannel pipette. Resuspend the pellet with 100 µL of 4% PFA and incubate at 4 °C for 10 min. Add 100 µL of cold 1X PBS.
- Analyse the infection rate, represented by GFP-positive cells, by flow cytometry (see Supplementary Figure 2 for a suggested gating strategy).
Results
A THP-1 cell line was generated stably expressing the GFP reporter protein (THP-1_GFP) (Figure 2A) and used as a tool to establish a protocol for an efficient CRISPR-Cas9-mediated gene edition. To this aim, 3 sgRNA targeting the EGFP gene was designed with the CRISPOR web tool29 (Figure 2B), which were simultaneously complexed with Cas9 at a molar ratio of 9:1 to form RNPs before delivery into the cells by electroporation using different settings. Next, cells were grown in either 20% FBS-containing RPMI medium alone (Figure 2C, upper panel) or diluted 1:1 with conditioned media (Figure 2C, lower panel). Cell proliferation and GFP expression, as a readout of the EGFP KO efficiency, were monitored over time. For several conditions, the percentage of GFP-positive cells sharply declined, reaching >90 % reduction on day 7 post-electroporation (pe). At day 3 pe, the number of cells had halved, likely due to the negative impact of electroporation on cell viability. However, the cell number rose again, and doubling time returned to a normal rate by day 7 pe (Figure 2C, upper panel). The use of conditioned media did not favor cell recovery under our experimental conditions (Figure 2C, lower panel). Based on these results, three 10 ms pulses of 1500 V were chosen for follow-up experiments.
The CRISPR-Cas9-mediated EGFP KO was then characterized at the genomic level. For this, the genomic DNA of THP-1_GFP cells was extracted, electroporated with either the Cas9:sgRNA RNPs (edited) or Cas9 alone (unedited), and used as a template to PCR-amplify an 891 bp region containing the target site. Parental THP-1 cells lacking EGFP were also included as negative control. Next, the PCR product of edited THP-1_GFP cells was prepared for mismatch detection by the T7EI assay, followed by the separation of the DNA fragments by agarose gel electrophoresis. A ~900 bp band corresponding to the expected size of the WT amplicon was readily visualized for unedited THP-1_GFP cells but not for the parental THP-1 control (Figure 2D, compare lines 2 and 3). In the case of the edited THP-1_GFP cells, the ~900 bp band became undetectable and replaced by a faster migrating one, whose size matched the loss of a ~75 bp fragment consequent to CRISPR editing (Figure 2D, line 4). This banding pattern was altered upon T7EI digestion, causing the appearance of smaller DNA fragments, further confirming the successful editing of the target site (Figure 2D, line 5). To gain insight into the editing efficiency and the specific genotypes of the edited population, the PCR amplicon was sequenced using the Sanger method, followed by analysis with the ICE tool26. The unedited control sample was processed in parallel. We retrieved indel and KO scores of 100%, corresponding to the percentage of non-WT sequences in the edited sample and the proportion of indels that likely lead to a functional KO, respectively (Figure 2E, left). Detailed analysis of the indel size spectrum and frequency showed the presence of two dominant populations bearing a 62-bp or 76-bp deletion and accounting for 57% or 37% of the total sequences, respectively (Figure 2E, right). These findings suggest that sgRNA #1, in combination with either sgRNA #2 or #3, triggered concurrent DSB in the target gene (Figure 2B).
This protocol was then applied to inactivate the endogenous SAMHD1-coding gene (Figure 3A). Characterization of the SAMHD1 KO THP-1 polyclonal cell line included monitoring cell proliferation over time, which matched that of unedited THP-1 cells (Figure 3B). The outcome of gene editing was assessed by PCR-based amplification of the target sequence, which revealed a smaller band for the SAMHD1 KO cells as compared to the Cas9-only control (Figure 3C, compare lines 2 and 4), indicative of a DNA sequence loss. These observations were confirmed by the T7EI assay, showing the appearance of cleavage products for the SAMHD1 KO, but not unedited, cells (Figure 3C, compare lines 3 and 5). Analysis of the Sanger sequencing data with the ICE tool returned indel and KO scores of 100% and 97%, respectively. The major modifications within the SAMHD1 locus were 170 bp, 93 bp, or 104 bp sequence deletions in 46%, 13%, and 10% of the sequences, respectively (Figure 3D). The protein expression levels in the crude cell lysate were also assessed by immunoblotting. The ~70 kDa band consistent with the size of endogenous SAMHD1 becomes virtually undetectable after editing, corresponding to an estimated reduction of >97 % (Figure 3E). Finally, phenotypic characterization of the SAMHD1 KO THP-1 polyclonal cell lines was also assessed by testing the susceptibility to HIV-1 infection. In agreement with its well-known antiviral role30,31, inactivation of the SAMHD1 locus was accompanied by an increased HIV-1 infection rate (Figure 3F).
In parallel, single-cell clones by limiting dilution were generated. Amplification of the genomic target site from the SAMHD1 KO clones yielded PCR products with increased electrophoretic mobility as compared to those of unedited control cells (Figure 4A). ICE-based analysis of the Sanger sequencing data returned an indel score of 100% for all selected single-cell clones but one (clone A2) (Figure 4B, left). Characterization of the editing outcomes at the genomic level returned a single sequence for clone A3 with a 98-bp deletion located 28-pb downstream of the initial ATG, thus leading to an out-of-frame change (Figure 4B, right). The other clones contained three (clone B1) or more sequences, indicative of the presence of alleles having undergone different editing events and/or more than one cell per well at the limiting dilution step (Figure 4B, right). Notably, 11% of the sequences of clones A4 and A7 bear indels upstream of the initial ATG, leaving the coding sequence unaltered. Moreover, 1% of clone A7 sequences lacked a 123-bp fragment 9-bp downstream of the initial ATG, resulting in an in-frame deletion of 41 amino acids. Consistent with these findings, functional studies revealed that clones with a disrupted SAMHD1 locus (A3, A8, B1, B3), thereby lacking SAMHD1 expression, were highly permissive to infection by HIV-1 (Figure 4C). Conversely, clones A4 and A7, where SAMHD1 is intact, were as refractory as parental or unedited control THP-1 clones (Figure 4C). In conclusion, the successful editing of the SAMHD1 locus in clone A3 was confirmed.
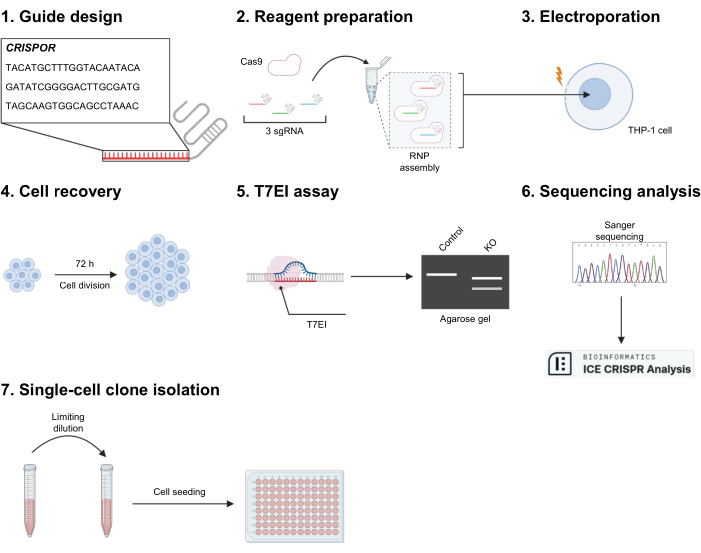
Figure 1: Experimental outline. (1) CRISPOR is used to generate sgRNA sequences against the targeted exon. Three of them are selected according to high on-target efficacy and weak off-target prediction. (2) The three gRNAs are mixed with Cas9 to assemble the ribonucleoprotein (RNP), resulting in a mix of three different RNPs. (3) Cells are electroporated to allow entry of the RNPs. (4) The cells are transferred to a 24-well culture plate and let to rest for at least 72 h. (5) Gene editing is first tested qualitatively by a T7EI digestion assay, and (6) for the validated conditions, further characterized by Sanger sequencing and ICE analysis. (7) If necessary, cells can be seeded after limiting dilution to produce clonal populations. Please click here to view a larger version of this figure.
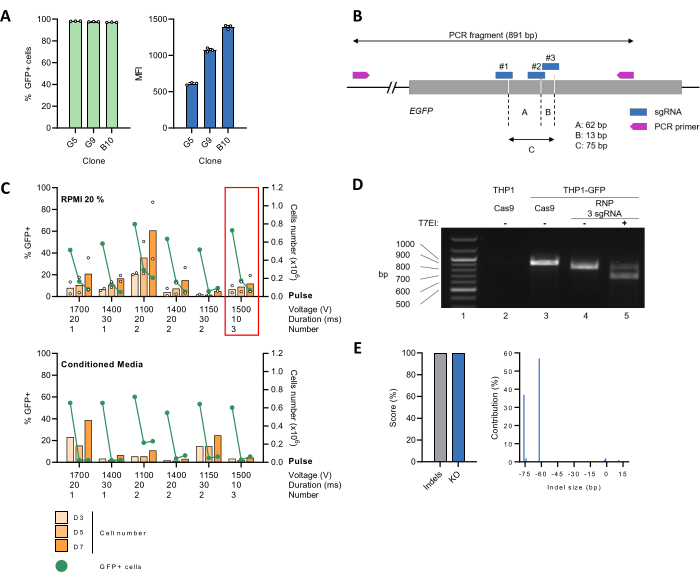
Figure 2: Setup of electroporation settings for EGFP KO in THP-1 cells and gene editing validation. (A) THP-1 cells were transduced with lentiviral vectors encoding the EGFP reporter gene. Single-cell clones were obtained by limiting dilution followed by expansion in culture for 30 days. Analysis by flow cytometry confirmed that virtually the whole cell population expressed GFP. The median fluorescence intensity (MFI) of the GFP-positive population, indicative of the integrated EGFP copy number, varied among several cell clones. Clone G5 was chosen for follow-up studies. (B) Schematic representation of the EGFP gene, including the binding sites for both sgRNA and primers. The Cas9 cut sites are indicated by the dashed lines. (C) Undifferentiated THP-1_GFP (2 x 105) cells were mixed with pre-assembled Cas9:sgRNA RNPs before electroporation with the indicated settings. Next, cells were transferred in a 24-well plate containing 500 µL of pre-heated 20% FBS-containing RPMI medium alone or diluted 1:1 with conditioned medium produced by a 48-h THP-1 cell culture. Proliferation (orange bars) and the percentage of GFP-positive cells (green line) were monitored over time. Data represent the mean for n = 2 biological replicates. The red frame highlights the condition selected for subsequent experiments. (D) The genomic DNA of edited (RNP 3 sgRNA) or unedited (Cas9) THP-1_GFP cells was extracted, and the region surrounding the target locus amplified by PCR. Parental THP-1 cells were used as the negative control. The amplicons underwent a denaturation/renaturation cycle, leading to the formation of a heteroduplex. Next, mismatches were detected by the T7EI assay. DNA fragments were separated by 1.2% agarose gel electrophoresis. (E) Purified PCR products were sequenced by the Sanger method, followed by analysis with the ICE algorithm. The Indel and KO scores indicate the percentage of non-WT sequences in the sample, and the proportion of cells with either a frameshift or 21+ bp indel, respectively. Please click here to view a larger version of this figure.
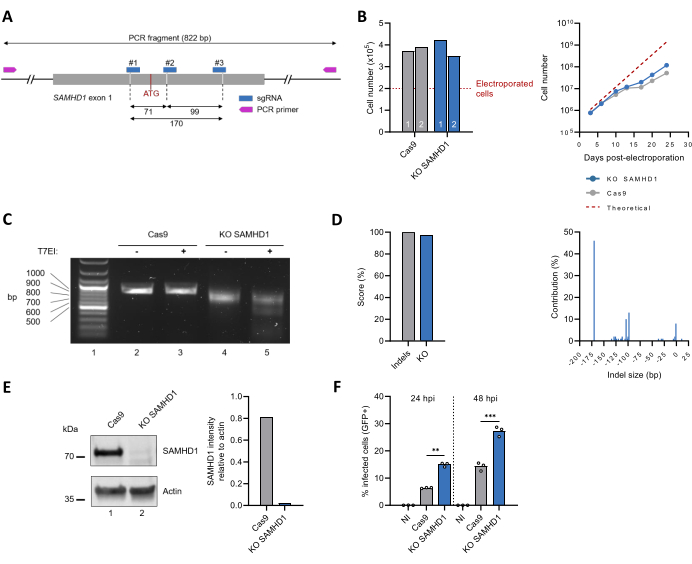
Figure 3: Production and characterization of a polyclonal SAMHD1 KO THP-1 cell population. (A) Schematic representation of SAMHD1 exon 1, including the binding sites for sgRNA and primers. The Cas9 cut sites are indicated by the dashed lines, and the initial ATG codon is shown in red. (B) Three days after electroporation, viable cells (n = 2) were quantified using the Trypan blue exclusion test (left diagram). Cell growth was monitored over 25 days (right diagram). The dashed line corresponds to the theoretical cell number based on an estimated doubling time of 49 h. (C) Editing outcomes at the genomic level were assessed, as shown in Figure 2D. (D) Genotypes following CRISPR editing were identified and quantified by ICE-based analysis of Sanger sequences. (E) The protein contained in the crude lysate of both edited and unedited THP-1 cells was separated by migration on SDS-PAGE gel and, next, visualized by immunoblotting using antibodies against SAMHD1. Actin was used as the loading control. The intensity of SAMHD1 and Actin bands was quantified by densitometry with ImageJ software. (F) Unedited (Cas9) and KO SAMHD1 THP-1 cells were differentiated by treatment with PMA (300 ng/mL, 24 h) and next challenged with a VSVg-pseudotyped HIV-1 virus expressing GFP as a reporter. The proportion of GFP-positive cells was scored by flow cytometry at 24 h and 48 h post-infection (hpi). NI: not infected. **P < 0.01; ***P < 0.001 by unpaired t-test with Welch's correction. Please click here to view a larger version of this figure.

Figure 4: Production and characterization of SAMHD1 KO single-cell clones. (A) The editing outcome for selected SAMHD1 KO single-cell clones was assessed by monitoring the mobility of the DNA fragments produced by PCR amplification of the region containing the target site by agarose gel electrophoresis. (B) Sanger sequencing data were analyzed with the ICE tool, as in Figure 2E. The bars in the right graph represent the diversity of indels in each cell population. The green bar represents the dominant sequence and the total size of the associated deletion. Numbers in brackets indicate the size of non-continuous gaps. (C) The permissiveness of unedited and SAMHD1 KO single-cell clones to HIV-1 infection was tested as in Figure 3F, and compared to that of non-electroporated parental THP-1 cells or unedited cell clones (Cas9). The infection rate was scored at 48 hpi. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns P≥ 0.05 by Brown-Forsythe and Welch ANOVA with Dunnett's multiple comparisons test. Please click here to view a larger version of this figure.
Supplementary Figure 1: Assessment of the editing outcome for KO single-cell clones by adapted T7EI test. The genomic DNA of KO single-cell clones or unedited cells was purified, and the region surrounding the target locus was amplified by PCR. The amplicons of each single-cell clone were mixed at a 1:1 ratio with those of unedited control cells. After heteroduplex formation and T7EI digestion, the product was analyzed by agarose gel electrophoresis. The edited clones produce multiple digestion fragments (clones A, B, C, D, and E), while the non-edited ones show a single WT band (clones F and G). Please click here to download this File.
Supplementary Figure 2: Flow cytometry gating strategy to quantify the percentage of HIV-1 infected PMA treated THP-1 cells. Using the forward scatter area (FSC-A) against the side scatter area (SSC-A), a first gate is defined to eliminate debris and select the PMA-treated THP-1 cells. Next, single cells are selected with an SSC-A against an SSC-H plot. Finally, GFP-positive cells are detected with GFP-A against FSC-H. Please click here to download this File.
Discussion
Here, a protocol is described to obtain a successful CRISPR-mediated editing of the THP-1 cell line. The approach relies on the transfer of pre-assembled sgRNA/Cas9 RNPs by electroporation/nucleofection. This strategy was chosen to limit the off-target effects that potentially arise upon lentiviral-mediated integration of the sgRNA/Cas9 cassette, yielding persistent expression of the nuclease. Multiple sgRNAs targeting the gene of interest were selected to achieve reliable and efficient editing, which increases the likelihood of creating genomic indels, leading to loss of protein expression and functional KO32. Special care was paid to the electroporation settings to achieve optimal permeabilization of the cell membrane, which was required to ensure efficient delivery of the sgRNA/Cas9 RNPs while preventing excessive cell death33. In this regard, a crucial parameter is the vigor of the cell culture prior to the electroporation (i.e., low passage number).
The CRISPR-Cas9-mediated editing was validated at the genetic level with the T7EI mismatch detection assay, which provides a cost-effective and rapid assessment of multiple samples for pre-selection before Sanger sequencing. This method was initially developed to estimate the editing efficiency using a single sgRNA by comparing the intensity of full-length amplicons and cleavage products34,35. Noteworthy, the large indels (>70 bp) produced when using multiple sgRNA can be detected by comparing the mobility of the DNA fragments obtained by PCR amplification of the targeted loci from edited versus unedited cells by standard gel electrophoresis. Nevertheless, the T7EI assay might be useful to ascertain the presence of sequences harboring small indels (>1bp) in either a polyclonal or, using the adapted protocol, a monoclonal cell population.
The presence of on-target out-of-frame mutations, which interrupt the coding sequence, was confirmed by a straightforward approach, where the genome of the whole cell population is extracted and used as a matrix for PCR amplification. Next, purified amplicons undergo Sanger sequencing in bulk, followed by bioinformatics analysis with the ICE tool. Overall, this method overcomes the need for subcloning the PCR products into a plasmid and transforming the ligation products into bacteria to isolate single-cell colonies, which are individually sequenced.
Once inactivation of the targeted locus is validated, a thorough characterization of the edited cell line is required to confidently attribute a given phenotype to the lack of the protein of interest and rule out unanticipated effects stemming from off-target, but also on-target changes that might impact mRNA structure and/or function.
The absence of protein expression should be confirmed by immunoblotting and/or immunofluorescence using possibly two antibodies recognizing different epitopes. Indeed, the introduction of a PTC in an early exon close to the canonical ATG codon is associated with the risk of ITL events, which yield N-terminally truncated protein variants36. Of note, Tuladhar et al. reported the detection of aberrant protein species in a significant proportion (~50%) of CRISPR-edited cells of either commercial or in-house origin18,19. The occurrence of novel protein products was ascribed to diverse and potentially cell type-specific processes such as skipping of the indel-containing exon or emergence of internal ribosome entry sites (IRES) causing initiation of protein synthesis at alternative TIS downstream of the canonical ATG codon. There are also hints that ITL is coupled with an escape from NMD under certain circumstances37,38. Similarly, genome-editing strategies targeting a late exon might fail to trigger NMD of the mutated mRNA underlying ineffective ablation of gene expression20. Finally, if Cas9 is guided by multiple sgRNAs, complex chromosomal rearrangements might arise, including inversions or large DNA-fragment deletions39, which might alter the structure and the expression of the genome, particularly when regulatory elements are targeted40.
To further mitigate the impact of unintended genome-editing outcomes, functional studies are needed to confirm the relationship between the loss of protein expression and the ensuing phenotype (i.e., antiviral activity). If edited cells are subcloned, the behavior of multiple clones among themselves and with the polyclonal population should be compared. Performing rescue experiments, which consist of restoring gene expression to reverse the phenotype of the edited cells to a WT phenotype, will also strengthen specificity and exclude possible off-target events.
Although this protocol was conceived for the editing of THP-1 cells, it provides a general structure for the CRISPR-Cas9 KO workflow that can be adapted to other cell lines. Adjusting the electroporation parameters might be required, and if a voltage higher than 1800 V is applied, a resuspension buffer T should be used, according to manufacturer instructions. Another variable to consider is the concentration ratio between Cas9 and sgRNA during RNP assembly. Although the 1:9 ratio worked well in this case, modifying it may improve editing efficiency in other circumstances. Finally, while not beneficial here, the use of conditioned media for cell recovery after electroporation should be tested, as the effect could be different with different cell lines.
Disclosures
All authors have no conflicts of interest.
Acknowledgements
We are grateful to JP Concordet (MNHN, U1154/UMR7196, Paris), G. Bossis (IGMM, Montpellier), and D. Schlüter (Hannover Medical School, Germany) for sharing protocols and for discussion. This project has received funding from the European Union's Horizon 2020 research and innovation program (grant agreement No 101017572 to AZ) and ANRS (grant ECTZ162721 to AZ). The Infectious Disease Model and Innovative Therapies (IDMIT) research infrastructure is supported by the "programme investissement d'avenir (PIA)" under reference ANR_11_INSB_0008.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2 µm syringe filter | ClearLine | 146560 | _ |
| 0.4 % trypan blue | Beckman Coulter | 383200 | _ |
| 1.5 mL tube | Eppendorf | 3810X | _ |
| 24-well plate | Corning | 353047 | _ |
| 6x TriTrack DNA Loading Dye | Thermo scientific | R1161 | _ |
| 75 cm² Culture Flask Vented Cap | Corning | 353136 | _ |
| 8-Strip PCR Tubes with Caps | Life technologies | AM12230 | _ |
| 96-well plates Flat bottom | Corning | 353072 | _ |
| 96-well plates Round bottom | Corning | 353077 | _ |
| Agarose | Euromedex | D5 | _ |
| ATGpr | _ | _ | https://atgpr.dbcls.jp/ |
| ChemiDoc Imaging System | BIO-RAD | 12003153 | _ |
| Counting slide | NanoEntek | DHC-N04 | _ |
| CRISPOR | _ | _ | http://crispor.gi.ucsc.edu/ |
| DPBS | Gibco | 14190094 | _ |
| Ensembl | EMBL-EBI | _ | https://www.ensembl.org/index.html |
| Fetal Bovine Serum | Sigma-Aldrich | F7524 | _ |
| FlowJo | BD Life Sciences | v10.10 | _ |
| GeneRuler 100 bp Plus DNA Ladder | Thermo scientific | SM0323 | _ |
| Genome Data Viewer | NCBI | _ | https://www.ncbi.nlm.nih.gov/gdv/ |
| GraphPad Prism | Dotmatics | _ | Version 9.3.1 |
| Herculase II Fusion DNA Polymerases | Agilent | 600679 | _ |
| ICE CRISPR Analysis Tool | Synthego | _ | https://www.synthego.com/products/bioinformatics/crispr-analysis |
| Image Lab Touch | BIO-RAD | _ | Version 2.4.0.03 |
| NEBuffer 2 | New England Biolabs | B7002S | Included with T7EI M0302S |
| Neon Kit, 10 µL | Invitrogen | MPK1025K | Electroporation kit containing tips, tubes, buffer R and E |
| Neon Transfection System | Invitrogen | MPK5000 | _ |
| NetStart 1.0 | _ | _ | https://services.healthtech.dtu.dk/services/NetStart-1.0/ |
| Nuclease-free Water | Synthego | _ | _ |
| PCR primer (EGFP) | Eurofins | _ | Fw : GGAATGCAAGGTCTGTTGAATG ; Rev : CACCTTGATGCCGTTCTTCT |
| PCR primer (SAMHD1) | Eurofins | _ | Fw : CGGGATTGATTTGAGGACGA ; Rev : GGGTGGCAAGTTAGTGAAGA |
| Penicillin-streptomycin (10,000 U/mL) | Gibco | 15140122 | _ |
| PFA | Electron Microscopy Sciences | 15714 | _ |
| PMA | Sigma-Aldrich | P8139 | _ |
| PrimerQuest | IDT | _ | https://eu.idtdna.com/pages/tools/primerquest |
| QIAquick PCR Purification Kit | Qiagen | 28104 | _ |
| QuickExtract DNA Extraction Solution | Biosearch Technologies | QE09050 | _ |
| RPMI 1640, GlutaMAX | Gibco | 61870010 | _ |
| SnapGene Viewer | Dotmatics | _ | Version 7 |
| SpCas9 2NLS Nuclease | Synthego | _ | _ |
| SYBR Safe DNA Gel Stain | Invitrogen | S33102 | _ |
| Synthetic sgRNA (EGFP) | Synthego | _ | #1 : CGCGCCGAGGUGAAGUUCGA ; #2 : UUCAAGUCCGCCAUGCCCGA ; #3 : CAACUACAAGACCCGCGCCG |
| Synthetic sgRNA (SAMHD1) | Synthego | _ | #1 : AUCGCAACGGGGACGCUUGG ; #2 : GCAGUCAAGAACCUCGGCGC ; #3 : CCAUCCCGACUACAAGACAU |
| Syringe Plastipak Luer Lock | BD | 301229 | _ |
| T100 Thermal Cycler | BIO-RAD | 1861096 | _ |
| T7 endonuclease I | New England Biolabs | M0302S | _ |
| TAE buffer UltraPure, 10x | Invitrogen | 15558026 | 400 mM Tris-Acetate, 10 mM EDTA |
| THP-1 cells | ATCC | TIB-202 | _ |
| Trypsin-EDTA (0,05 %) | Gibco | 25300054 | _ |
| ZE5 Cell Analyzer | BIO-RAD | 12014135 | _ |
References
- Tsuchiya, S., et al. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 26 (2), 171-176 (1980).
- Danis, V. A., Millington, M., Hyland, V. J., Grennan, D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-lRa) gene polymorphism. Clin Exp Immunol. 99, (1995).
- Bol, S. M., et al. Donor variation in in vitro HIV-1 susceptibility of monocyte-derived macrophages. Virology. 390 (2), 205-211 (2009).
- Appleby, L. J., et al. Sources of heterogeneity in human monocyte subsets. Immunol Lett. 152 (1), 32-41 (2013).
- O'Neill, M. B., et al. Single-cell and bulk RNA-sequencing reveal differences in monocyte susceptibility to Influenza A virus infection between Africans and Europeans. Front Immunol. 12, 768189 (2021).
- Wen, Y., et al. Comparability study of monocyte-derived dendritic cells, primary monocytes, and THP1 cells for innate immune responses. J Immunol Methods. 498, 113147 (2021).
- Inagaki, Y., et al. Interferon-g-induced apoptosis and activation of THP-1 macrophages. Life Sci. 71 (21), 2499-2508 (2002).
- Boonkaewwan, C., Toskulkao, C., Vongsakul, M. Anti-inflammatory and immunomodulatory activities of stevioside and its metabolite steviol on THP-1 cells. J Agric Food Chem. 54 (3), 785-789 (2006).
- Chanput, W., et al. β-Glucans are involved in immune-modulation of THP-1 macrophages. Mol Nutr Food Res. 56 (5), 822-833 (2012).
- Cui, J., et al. USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors. Cell Res. 24 (4), 400-416 (2014).
- Chen, S., et al. SAMHD1 suppresses innate immune responses to viral infections and inflammatory stimuli by inhibiting the NF-κB and interferon pathways. Proc Natl Acad Sci USA. 115 (16), E3798-E3807 (2018).
- Pradhananga, S., Spalinskas, R., Poujade, F. A., Eriksson, P., Sahlén, P. Promoter anchored interaction landscape of THP-1 macrophages captures early immune response processes. Cell Immunol. 355, 104148 (2020).
- Mezzasoma, L., Talesa, V. N., Romani, R., Bellezza, I. Anp and BNP exert anti-inflammatory action via npr-1/cgmp axis by interfering with canonical, non-canonical, and alternative routes of inflammasome activation in human THP1 cells. Int J Mol Sci. 22 (1), 1-17 (2021).
- Martinat, C., et al. SUMOylation of SAMHD1 at Lysine 595 is required for HIV-1 restriction in non-cycling cells. Nat Commun. 12 (1), 4582 (2021).
- Rensen, E., et al. Clustering and reverse transcription of HIV-1 genomes in nuclear niches of macrophages. EMBO J. 40 (1), e105247 (2021).
- Ikeda, T., et al. APOBEC3 degradation is the primary function of HIV-1 Vif determining virion infectivity in the myeloid cell line THP-1. mBio. 14 (4), e0078223 (2023).
- Jinek, M., et al. A programmable dual RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337, 816-821 (2012).
- Kurosaki, T., Maquat, L. E. Nonsense-mediated mRNA decay in humans at a glance. J Cell Sci. 129 (3), 461-467 (2016).
- Tuladhar, R., et al. CRISPR-Cas9-based mutagenesis frequently provokes on-target mRNA misregulation. Nat Commun. 10 (1), 4056 (2019).
- Embree, C. M., Abu-Alhasan, R., Singh, G. Features and factors that dictate if terminating ribosomes cause or counteract nonsense-mediated mRNA decay. J Biol Chem. 298 (11), 102592 (2022).
- Xue, C., Greene, E. C. DNA repair pathway choices in CRISPR-Cas9-mediated genome editing. Trends Genet. 37 (7), 639-656 (2021).
- Schnoor, M., et al. Efficient non-viral transfection of THP-1 cells. J Immunol Methods. 344 (2), 109-115 (2009).
- Tang, X., et al. A method for high transfection efficiency in THP-1 suspension cells without PMA treatment. Anal Biochem. 544, 93-97 (2018).
- Ji, W., Zhang, L., Liu, X. Protocol using lentivirus to establish THP-1 suspension cell lines for immunostaining and confocal microscopy. STAR Protoc. 4 (1), 102032 (2023).
- Guo, C., Ma, X., Gao, F., Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol. 11, (2023).
- . Synthego Performance Analysis, ICE Analysis Available from: https://www.synthego.com/help/citing-ice (2019)
- Salamov, A. A., Nishikawa, T., Swindells, M. B. Assessing protein coding region integrity in cDNA sequencing projects. Bioinformatics. 14, 384-390 (1998).
- Pedersen, A. G., Nielsen, H. Neural network prediction of translation initiation sites in eukaryotes: Perspectives for EST and genome analysis. Proc Int Conf Intell Syst Mol Biol. 5, 226-233 (1997).
- Concordet, J. P., Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46 (W1), W242-W245 (2018).
- Laguette, N., et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 474 (7353), 654-657 (2011).
- Hrecka, K., et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 474 (7353), 658-661 (2011).
- Seki, A., Rutz, S. Optimized RNP transfection for highly efficient CRI SPR/Cas9-mediated gene knockout in primary T cells. J Exp Med. 215 (3), 985-997 (2018).
- Batista Napotnik, T., Polajžer, T., Miklavčič, D. Cell death due to electroporation - A review. Bioelectrochemistry. 141, 107871 (2021).
- Perretta-Tejedor, N., Freke, G., Seda, M., Long, D. A., Jenkins, D. Generating mutant renal cell lines using CRISPR technologies. Methods Mol Biol. 2067, 323-340 (2020).
- Sentmanat, M. F., Peters, S. T., Florian, C. P., Connelly, J. P., Pruett-Miller, S. M. A survey of validation strategies for CRISPR-Cas9 editing. Sci Rep. 8 (1), 888 (2018).
- Makino, S., Fukumura, R., Gondo, Y. Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9. Sci Rep. 6, 39608 (2016).
- Bowling, A., et al. Downstream alternate start site allows N-terminal nonsense variants to escape NMD and results in functional recovery by readthrough and modulator combination. J Pers Med. 12 (9), 1448 (2022).
- Inácios, &. #. 1. 9. 4. ;., et al. Nonsense mutations in close proximity to the initiation codon fail to trigger full nonsense-mediated mRNA decay. J Biol Chem. 279 (31), 32170-32180 (2004).
- Li, J., et al. Efficient inversions and duplications of mammalian regulatory DNA elements and gene clusters by CRISPR/Cas9. J Mol Cell Biol. 7 (4), 284-298 (2015).
- Guo, Y., et al. CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell. 162 (4), 900-910 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved