Method Article
扫描浸软组织的电子显微镜可视化的细胞外基质
摘要
Shown here is a method for visualizing extracellular matrix ultrastructure in decellularized cardiac tissues.
摘要
Fibrosis is a component of all forms of heart disease regardless of etiology, and while much progress has been made in the field of cardiac matrix biology, there are still major gaps related to how the matrix is formed, how physiological and pathological remodeling differ, and most importantly how matrix dynamics might be manipulated to promote healing and inhibit fibrosis. There is currently no treatment option for controlling, preventing, or reversing cardiac fibrosis. Part of the reason is likely the sheer complexity of cardiac scar formation, such as occurs after myocardial infarction to immediately replace dead or dying cardiomyocytes. The extracellular matrix itself participates in remodeling by activating resident cells and also by helping to guide infiltrating cells to the defunct lesion. The matrix is also a storage locker of sorts for matricellular proteins that are crucial to normal matrix turnover, as well as fibrotic signaling. The matrix has additionally been demonstrated to play an electromechanical role in cardiac tissue. Most techniques for assessing fibrosis are not qualitative in nature, but rather provide quantitative results that are useful for comparing two groups but that do not provide information related to the underlying matrix structure. Highlighted here is a technique for visualizing cardiac matrix ultrastructure. Scanning electron microscopy of decellularized heart tissue reveals striking differences in structure that might otherwise be missed using traditional quantitative research methods.
引言
Fibrosis disrupts the normal myocardial collagen network, which is critical for normal mechanistic functions of cardiomyocytes 1,2 as well as for inter-cellular communication, intracellular signaling, and cell survival 3. The development of fibrosis is a major determinant of cardiac function, and fibrotic remodeling of the cardiac interstitium arising from a variety of etiologies leads to increased left ventricular stiffness and diastolic dysfunction 4. Myocardial fibrosis may also lead to arrhythmias, and whether the progression of fibrotic remodeling is a general or local phenomenon, it is highly associated with a poor prognosis in patients with ischemic and non-ischemic cardiomyopathy 5. Likewise, the absence of myocardial fibrosis is a strong predictor of ventricular functional recovery and long-term survival 6.
The hallmark of fibrosis is the deposition of excess collagen, which has the tensile strength of steel 7 and can adversely affect cardiomyocyte function on multiple levels. Mechanical forces resulting from an excessively collagenous matrix can lead to cardiomyocyte atrophy 8,9, passive tissue stiffness 10, tonic contraction-induced myocardial stiffness 11-13, and reduced delivery of oxygen to the remaining population of cardiomyocytes. Gap junction coupling of cardiomyocytes and myoFbs can also compromise the heart's electrical characteristics, creating a greater risk for the development of arrhythmias 14-16. Perivascular fibrosis alters vasomotor reactivity of intramural coronary arteries and arterioles 17 and contributes to luminal narrowing that reduces the supply of oxygen and thus the survival of cardiomyocytes 17-22. Pathogenic fibrotic and electrical remodeling, emanating from an initial site of ischemic injury or energy imbalance, inevitably progresses to heart failure.
Cardiomyocyte necrosis initiates the fibrotic response, and subsequent adverse fibrotic remodeling can occur irrespective of etiology. Finding a way to control cardiac fibrosis would be clinically beneficial for the treatment of ischemic and idiopathic cardiomyopathies, hypertensive heart disease, hypertrophic cardiomyopathy, valvular heart disease and dystrophinopathies 23-42. Regardless of how the fibrotic disease process begins, soluble, profibrotic factors can cross the interstitial space and provoke activation of interstitial and adventitial fibroblasts at sites remote to the initial fibrotic scar, creating a cascade effect that ultimately leads to heart failure. The optimum scenario would be to exploit the fibrillogenic process using a targeted therapeutic that can be applied during the compensative hypertrophic stage of cardiomyopathy before it progresses to systolic pump failure, diastolic heart failure, or other end-stage outcomes. The ultimate goal would be to reverse fibrosis so that dead cardiomyocytes can be replaced and heart function restored completely.
The importance of the matrix is widely understood, yet methods to study the matrix are limited mainly to quantitative measurements of major structural components, particularly collagen, and relative levels of different matrix and matricellular proteins. This protocol highlights a rarely used technique that is useful for assessing qualitative differences in the cardiac matrix. This technique has been recently used to compare and contrast fundamental differences in heart matrices from different etiologies of heart disease (in human explants), to examine hearts from post-infarcted pigs treated with the glial growth factor (GGF) isoform of neuregulin-1β, relative to untreated animals 43, and to probe for differences in the matrices of cardiac tissues from mdx mice (a commonly used animal model of Duchenne Muscular Dystrophy) at different ages and compared to wild-type controls. This technique was first introduced by Drs. Caulfield and Borg in 1979 44, but few studies have since employed this powerful technique 45-47, re-introduced here with only slight modification. This methodology is a valuable research tool, because it provides qualitative information about extracellular matrix ultrastructure that might otherwise be overlooked when simply measuring matrix component content and/or level of fibrosis.
研究方案
伦理声明:动物处理的协议是由范德比尔特机构动物护理和使用委员会(IACUC批准,根据AAALAC -国际标准协议数M / 10/117(猪)和M / 10/219(小鼠)和进行。开户人的心脏组织的使用是经范德比尔特大学医学中心IRB(协议号为100887)。
1.样品采集和存储
- 使在0.1M磷酸盐缓冲液(PB)的溶液新鲜的4%戊二醛。
注意:戊二醛是有毒的,戴手套和工作在通风橱。- 使用21.8克Na 2 HPO 4和6.4克的NaH 2 PO 4。 量子SATIS(QS)的PB,pH为7.4的0.2M的原液达1,000ml卫生署2 O.
- 加入400微升50%戊二醛的2.5毫升PB原液和2.1毫升卫生署2 O.
- 沉浸在一个块(不大于2 C米2)的组织的成4%的戊二醛溶液中。注意:小片也可以使用,但应具有足够的尺寸(不小于5 平方毫米),以容易地通过眼睛,以便于以后的协议的步骤可视化的。
- 在室温下孵育1小时,然后在4℃下无限期地存储。
2.心脏组织的脱细胞
- 使用10克NaOH粒料/100毫升卫生署2 O.新鲜的10%NaOH水溶液
注意:NaOH溶液具有腐蚀性,可引起碱烧伤,戴手套。 - 冲洗组织在卫生署2 O.
- 在室温下6孵育在10%NaOH溶液 - 10天(直至从红褐色组织变为灰白色或白色)。
- 在冲洗卫生署2 O,直到组织变得透明。
- 浸泡在1%的单宁酸的组织,在室温下4小时。使用每4毫升卫生署2 O.1毫升5%原液
注意:单宁酸强烈的刺激性,戴手套。 - 在冲洗卫生署2 O过夜。
3. Osmication和心脏组织脱水(在通风橱安全)
- 使用21.4克二甲胂酸钠10.0g二氯化钙和450毫升卫生署2 O的二甲胂酸钠缓冲液中的0.2M的原液混合,然后根据需要将pH调节至7.4 QS添加盐酸。到500毫升的dh 2 O
注意:二甲胂酸钠和盐酸是有毒的,戴手套,工作在通风橱中。 - 使在通风橱安全四氧化锇的2%水溶液原液通过在50毫升的dh 2 O1克四氧化锇晶体溶解
注意:四氧化锇是一个重度吸入性危害;粘膜或眼球蒸汽固定是可能的,因此,只有在带着手套通风柜处理。推荐使用防溅板。 - 在冲洗0.1M的二甲胂酸钠缓冲组织(1混合原液:1与卫生署 2 O)对转子5分钟(或轻柔搅拌)。
- 重复先前的步骤两次,总共三个缓冲器漂洗。
- 浸泡在1%的四氧化锇组织在0.1M二甲胂酸钠缓冲液(混合二甲胂库存钠和库存的四氧化锇1:1)上旋转1小时。
- 冲洗组织在0.1M二甲胂酸钠缓冲液3次,每次在旋转器5分钟。
- 使用的乙醇浓度增加(30%,50%,75%,85%,95%,最后100%)对转子每15分钟冲洗组织。
4.截面表面准备SEM
- 在100%乙醇转移组织以一个浅培养皿还含有100%的乙醇。
- 容纳两个非常尖锐的刀片,使得双方的单位都与彼此和切削刃交叉接触,以形成检体上方的等边三角形的两个方面。要做到这一点,用左手放置一个刀片对样品的最右侧,切向左。在同一时间,用右手放置在第二刀片上最左边的向右样品和切片。因此,刀片会互相从相反的方向滑动,以一个单一的顺利晋级。
- 滑动平刃双方对对方做出最小失真或撕裂力的标本非常干净的切割,最好露出尽可能大的表面积尽可能不破坏样品。
- 重复每个标本暴露清洁的横截面表面在扫描电镜检查。
心脏组织5.临界点干燥(CPD)
- 用刮刀或镊子组织转移到临界点干燥器(CPD)的样品架,以确保组织在任何时候都保持在100%的乙醇。确保该支架被浸入在乙醇中,该传送与暴露于空气中不超过几秒钟的组织来实现。
- 每个操作CPD我们呃手册完成3吹扫和填充循环用液体二氧化碳来代替乙醇。
- 操作每个用户的手册CPD实现样品的临界点干燥,并将它们与二氧化碳的控制排气返回到大气压力。
6.安装心脏组织样本进行扫描电镜
- 制备的SEM样品存根为每个试样,通过粘附碳粘合剂标签,所述铝存根的顶表面。
- 用立体显微镜的帮助下,小心地附着试样到与感兴趣的横截面表面的粘合剂标签朝上(从标签中,可见的距离),并与样品存根表面的平面尽可能接近平行。不要探头或触摸的感兴趣的表面。
- 打破木制涂药棒,实现了锥形刷,非常适合涂料应用。在基座和试样的两侧施加银或碳涂料,以提高粘附到存根。
- 延伸的非常薄的银或碳的线画到感兴趣的表面的边缘,以提供从感兴趣的地面表面的电荷的路径。
- 应用银或碳涂料的2或3小的dAb围绕碳片的周边,以提供从碳片表面到金属短截线的导电路径,从而接地。
- 允许导电性涂料干燥两小时。
- 操作每个用户的手册溅射镀膜机施加相对重的涂层(30目标范围 - 40纳米)金 - 钯合金或金。泵样品室约0.1毫巴;设置定时器为40秒。打开设置阀8:00位置(中等氩气流量)。按Start开始溅射镀膜在30毫安。紫色辉光放电应在试样腔室是可见的。
7.心脏组织样本的扫描电镜检查
- 执行在相对低的加速电压的扫描电子显微镜,以减少成像p与样品中电荷耗散不良有关roblems(充电)。建议初始成像条件是:5千伏加速电压10毫米的工作距离。
- 用一个有经验的操作者的协助下,雇用增加的工作距离,以在需要用于在z方向相当长延伸的多个焦平面纤维,或纤维的焦点成像条件延伸景深。
- 对于这里使用的显微镜(见材料表 ),在用户界面访问在右上角导航选项卡。
- 从舞台菜单进入坐标标签。以增加的工作距离,以毫米为在Z输入一个较大的值的坐标,然后就转到点击到标签到样品台移动到输入工作距离。
- 用一个有经验的操作者的协助下,采用试样倾斜和旋转到兴趣正交的表面定位到电子束。的10至30 DEGR附加倾斜EES从该位置可以改进基体结构的观察和记录。
- 对于这里使用的显微镜(见材料表 ),在用户界面中,单击并按住鼠标右键,然后向左或向右滑动关注感兴趣的准备好的表面平面的外围附近的样本,并指出聚焦工作距离。
- 与手动用户界面摇杆导航到附近的表面和重复焦点的相对边缘移动。如果工作距离不近似等于所述第一位置,倾斜检体来实现在使用Stage菜单的坐标标签(7.2),并进入在T的倾斜坐标值两个位置近似一致。
- 旋转样品九十度(输入值成R坐标场)和直至所有的位置都集中在大致相同的工作距离重复上述过程。
注意:可变压力SEM可以用于改善电荷耗散如果可供使用的话。高真空是用于扫描电子显微镜的标准操作模式。
结果
突出显示的技术应用于心脏组织从一个未使用的人体心脏移植供体( 图1),从移植,从野生型和营养不良的小鼠( 图3)的心外植组织和从猪心肌梗死后心脏的样品中心脏损伤模型( 图2)。 如图1所示,人的心脏基质的横截面中观察时显示一个蜂巢状图案的交联蛋白质的一种复杂的编织。每个'蜂窝'结构是约40微米宽,通常绕过单个肌细胞,考虑图1中的平面图时,当由闰盘相连,几个心肌可以为杆通过"隧道"当隐喻纵向运行设想延伸到立体感。 图1还亮点在切割过程中比在切割过程( 图1,右上)中被"打碎"节中的重要性,以更高的精度得到更多启示地形数据( 图1,左上)。
驻地心肌细胞,血管,和之前的SEM处理循环细胞的去除揭示额外超结构细节,这将是不太明显的由整个心脏组织块的SEM。每个单独的胶原"支柱",例如( 图1,下图)被定期显然对齐并垂直于肌原纤维肌节。这种安排是合适的帮助通过在收缩和舒张周期施加反作用变形,类似纺织品的"经纱和纬纱",有助于保持织物体和形式对拉伸维持心脏结构。
FO:保together.within页="1"> 
图1:细胞外基质的脱细胞左心室组织的三维排列从一个未使用的人类供心获得的代表性扫描电子显微照片的顶两个面板显示在低放大倍数(条= 500微米)的横截面的矩阵,提供正常人体心脏组织的体系结构的鸟瞰图。在更高的放大倍率,可以更好地观察到,为规则间隔肌纤维(中间左侧和右侧面板棒,分别= 100微米和50微米)的机械支撑支持纤维的典型蜂窝状结构。仔细观察,每个"蜂窝"是由被平行地彼此而垂直于驻地心肌(底部左侧和右侧面板棒=分别为10微米和5微米,)组织的纤维。TTPS://www-jove-com.remotexs.ntu.edu.sg/files/ftp_upload/54005/54005fig1large.jpg"目标="_空白">点击此处查看该图的放大版本。
除了提供结构信息,脱细胞的组织的SEM可以允许在响应损伤或心肌病的非伤害形式的细胞外基质的改变有意义,定性评估。例如,这种技术最近用于检查后梗死猪心脏组织43的细胞外基质。在这个大的动物实验的过程中,特别设计的,以评估作为心脏衰竭,其中接受静脉NRG-1β给药后梗塞猪潜在的治疗NRG-1β的GGF2同种型的功效在心脏基质表现出惊人的变化,相比于后梗死动物的未经处理的心脏组织。这些结果随后发表43和德chnique一直用于在该初始,偶然发现构建有价值的工具。 图2包括示例显微照片,该研究的过程中产生的,其突出未处理和NRG-1β处理的矩阵之间急剧矩阵差异。
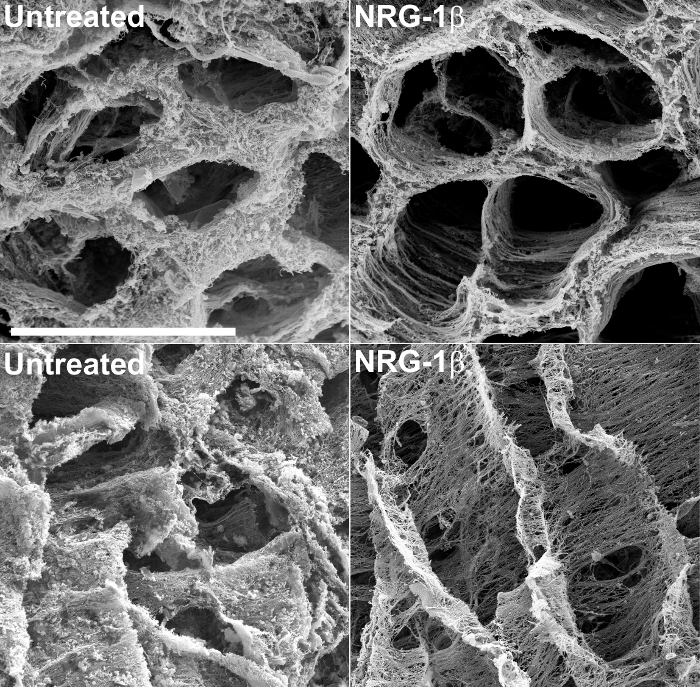
图 2:在未处理和NRG-β -处理的猪 左心室外基质的代表性扫描电子显微照片 ,氢氧化钠浸渍后的截面的基质突出在未处理心肌梗塞后纤维中的常规空间排列(MI后)猪(左上),相比-MI后NRG-1β处理的动物(右上)。当在经度观察,在未经处理的猪的基质表现出厚,雾状外观(左下),而NRG-1β处理的猪的矩阵显示纤维定期空间排列(右下)。白条= 40微米(四个面板)。详细结果和数字都包含在相关稿件43。 请点击此处查看该图的放大版本。
的发生在营养不良心基质变化勘探也取得了定性分析上市杜氏肌营养不良症心肌病(DMD)的动物模型的发展和发展。在mdx小鼠,DMD的一种常用的小鼠模型中,有间的野生型和当用SEM固定和NaOH处理后观看的mdx心显著和年龄依赖性差异。如在图3中所示,矩阵组件相对正常在缺少相对于正常小鼠功能性肌养6周岁的mdx心。更多INTeresting,细胞外基质组织显然破坏或者老年性肌养缺陷型小鼠退化相比年轻mdx小鼠,示出了DMD的心脏中的进步性。没有料到会有这样深刻的分歧,因为mdx鼠是人类DMD病的代表性很差,由于他们表现出更温和型心肌病和死亡率比DMD 48人慢的事实。这表明,在心脏功能甚至微小的变化可使用本手稿呈现的可视化技术被捕获。这种方法也应容易适用于其他器官的细胞外基质,对其中有同样没有当前可用的纤维化靶向疗法。

图3:左法师的代表性扫描电子显微照片心室细胞外基质中的野生型相比 mdx 鼠。在野生型小鼠的左心室心肌基质(顶面板)类似于在其他物种观察。在6周龄mdx鼠矩阵在外观上看起来(中板)比较正常,虽然略显"蓬松"。相反地,较旧的mdx小鼠的心脏基质出现严重退化(底部面板),表明营养不良过程可以在固定,氢氧化钠,浸软组织使用SEM定性捕获。白条= 10微米(全三板)。 请点击此处查看该图的放大版本。
讨论
横截面表面处理是协议中最关键的一步。为了保持精细结构,脱水标本必须在任何时候都保持在100%的乙醇,直到引入到临界点干燥工序。因此样品的切片,以实现EM检查表面必须同时标本在乙醇浸没在浅盘来完成。它也是至关重要的暴露表面不触及或后续处理期间探测。任何重大修改,预计在这一技术到其他组织类型相似矩阵的观测应用程序,不过协议的SEM部分可能需要基本的电子显微镜故障排除任何样本来源。从纤维样品采集到的图像易于由电荷差耗散("充电")介绍了伪像。充电的问题通常可以通过降低加速电压,增加扫描速度(也称为停留时间被最小化)和减小电子束的斑点尺寸。收集几个扫描积分的速度不够快,以避免电荷的工件将产生可比较的信号噪声质量的图像,而不存在于较慢,更高质量的单个扫描图像的电荷工件。
这种技术是固有的定性,因此当沿着定量测量( 例如 ,Masson三色或picoserius红染色,羟脯氨酸含量,质谱和RNA测序测量),以确定可视结构差异如何可以与各种发育或疾病状态被认为补充。然而,尽管有这种限制,该方法是超出心脏纤维化特别显著,由于细胞外基质在体内几乎每个器官的一个基本组成部分。在心脏,心脏矩阵为连续抽特点是复杂的拉伸,扭曲和DEF关键机械支撑ormation,其赋予对发生在人类平均寿命的> 2.5十亿次的氧化和脱氧的血液49最佳条目和流出。定心脏组织的极低再生能力,可以根据上下文需要被改造一个动态矩阵使得逻辑意义。仅与一个想象的轻微弹力,人们可能推断出存在用于操纵基质重塑增强愈合过程的治疗目标,同时限制不利的纤维化。至少在展示技术的应用展示了心脏矩阵的复杂性和美感,这样做进一步强调了其功能的重要性。
而定量测量值对于几乎所有的实验研究的评估的核心原则,该技术此处突出可以用于揭示定性超微结构的变化,不仅补充标准矩阵测量但可能暗示替代路径的调查了解,强调质变的根本生化改变。预期未来这种技术的应用是其在心脏疾病模型和人体组织作为评估矩阵的变化的辅助工具的使用,以及扩大的使用来研究其他器官的量矩阵的变化是疾病过程的一个组成部分。
披露声明
The authors have nothing to disclose.
致谢
This study was funded by grants from the National Institutes of Health (NIH), Heart, Lung, and Blood Institute (NIHLB): K01-HL-121045, K08-HL-094703, 5T32HL007411-35, P20 HL101425, U01 HL100398.
Imaging and tissue processing (after NaOH maceration) were performed through the use of the Vanderbilt University Medical Center (VUMC) Cell Imaging Shared Resource (CISR) (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126). We are especially grateful to the VUMC CISR core directors (Dr. Sam Wells and Dr. W. Gray (Jay) Jerome) for valuable technical advice and also for providing core space and resources for the purposes of filming the technique highlighted in this paper.
We would like to extend our deepest appreciation to Dr. Yan Ru Su and Ms. Kelsey Tomasek in the Cardiology Core Lab for Translational and Clinical Research at Vanderbilt University for providing technical expertise and for collecting human tissue samples used in this study.
材料
| Name | Company | Catalog Number | Comments |
| Calcium Chloride | Electron Microscopy Sciences | 12340 | 100 g |
| Carbon Adhesive | Electron Microscopy Sciences | 12664 | 30 g |
| Carbon Adhesive Tabs | Electron Microscopy Sciences | 77825 | order to fit stubs |
| Double Edge Razor Blades Stainless Steel | Ted Pella, Inc | 121-6 | 250/pkg |
| Ethanol | Electron Microscopy Sciences | 15055 | 450 ml |
| Gluteraldehyde, 50% Solution | Electron Microscopy Sciences | 16310 | EM grade, distillation purified |
| Hydrochloric Acid | Electron Microscopy Sciences | 16760 or 16770 | 100 ml |
| Monosodium Phosphate NaH2PO4 | Sigma-Aldrich | S9251-250G | 250 g |
| Osmium Tetroxide | Electron Microscopy Sciences | 19100 | 1 g |
| Silver Conductive Adhesive | Electron Microscopy Sciences | 12686-15 | 15 g |
| Sodium hydroxide (NaOH) | Sigma-Aldrich | S8045-1KG | 1 kg |
| Sodium Phosphate Dibasic (Na2HPO4) | Sigma-Aldrich | S3264-500G | 500 g |
| Tannic Acid, 5% Aqueous | Electron Microscopy Sciences | 21702-5 | 500 ml |
| Trihydrate Sodium Cacodylate | Electron Microscopy Sciences | 12300 | 100 g |
| Gold-palladium Alloy or Gold | Refining Systems, Inc. | varies | specific to the sputter coater make and model |
| Critical Point Dryer | Electron Microscopy Sciences | 850 | |
| Plain Wooden Applicators | Fisher Scientific | 23-400-102 | |
| Quanta 250 Environmental SEM | FEI | Q250 SEM | |
| Sputter Coater | Cressington Scientific Instruments Ltd. | Model 108 | |
| Alluminum SEM Sample Stubs | Electron Microscopy Sciences | 75220-12 | specific to the miscroscope |
参考文献
- Robinson, T. F., Cohen-Gould, L., Factor, S. M., Eghbali, M., Blumenfeld, O. O. Structure and function of connective tissue in cardiac muscle: collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc. 2, 1005-1015 (1988).
- Robinson, T. F., Geraci, M. A., Sonnenblick, E. H., Factor, S. M. Coiled perimysial fibers of papillary muscle in rat heart: morphology, distribution, and changes in configuration. Circ Res. 63, 577-592 (1988).
- Lunkenheimer, P. P., et al. The myocardium and its fibrous matrix working in concert as a spatially netted mesh: a critical review of the purported tertiary structure of the ventricular mass. Eur J Cardiothorac Surg. 29 Suppl 2, S41-S49 (2006).
- Wu, K. C., et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 51, 2414-2421 (2008).
- Kramer, C. M. The expanding prognostic role of late gadolinium enhanced cardiac magnetic resonance. J Am Coll Cardiol. 48, 1986-1987 (2006).
- Park, S., et al. Delayed hyperenhancement magnetic resonance imaging is useful in predicting functional recovery of nonischemic left ventricular systolic dysfunction. J Card Fail. 12, 93-99 (2006).
- Weber, K. T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 13, 1637-1652 (1989).
- Jalil, J. E., Janicki, J. S., Pick, R., Abrahams, C., Weber, K. T. Fibrosis-induced reduction of endomyocardium in the rat after isoproterenol treatment. Circ Res. 65, 258-264 (1989).
- Fidzianska, A., Bilinska, Z. T., Walczak, E., Witkowski, A., Chojnowska, L. Autophagy in transition from hypertrophic cardiomyopathy to heart failure. J Electron Microsc (Tokyo). 59, 181-183 (2010).
- Lopez, B., Querejeta, R., Gonzalez, A., Larman, M., Diez, J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension. 60, 677-683 (2012).
- Gabbiani, G., Ryan, G. B., Majne, G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 27, 549-550 (1971).
- Lorell, B. H. Diastolic dysfunction in pressure-overload hypertrophy and its modification by angiotensin II: current concepts. Basic Res Cardiol. 87 Suppl 2, 163-172 (1992).
- Friedrich, S. P., et al. Intracardiac angiotensin-converting enzyme inhibition improves diastolic function in patients with left ventricular hypertrophy due to aortic stenosis. Circulation. 90, 2761-2771 (1994).
- Rosker, C., Salvarani, N., Schmutz, S., Grand, T., Rohr, S. Abolishing myofibroblast arrhythmogeneicity by pharmacological ablation of alpha-smooth muscle actin containing stress fibers. Circ Res. 109, 1120-1131 (2011).
- Yue, L., Xie, J., Nattel, S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 89, 744-753 (2011).
- Rohr, S. Myofibroblasts in diseased hearts: new players in cardiac arrhythmias? Heart Rhythm. 6, 848-856 (2009).
- Coen, M., Gabbiani, G., Bochaton-Piallat, M. L. Myofibroblast-mediated adventitial remodeling: an underestimated player in arterial pathology. Arterioscler Thromb Vasc Biol. 31, 2391-2396 (2011).
- Brilla, C. G., Janicki, J. S., Weber, K. T. Cardioreparative effects of lisinopril in rats with genetic hypertension and left ventricular hypertrophy. Circulation. 83, 1771-1779 (1991).
- Youn, H. J., et al. Relation between flow reserve capacity of penetrating intramyocardial coronary arteries and myocardial fibrosis in hypertension: study using transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 19, 373-378 (2006).
- Warnes, C. A., Maron, B. J., Roberts, W. C. Massive cardiac ventricular scarring in first-degree relatives with hypertrophic cardiomyopathy. Am J Cardiol. 54, 1377-1379 (1984).
- Maron, B. J., Wolfson, J. K., Epstein, S. E., Roberts, W. C. Intramural ('small vessel') coronary artery disease in hypertrophic cardiomyopathy. J Am Coll Cardiol. 8, 545-557 (1986).
- Olivotto, I., et al. Microvascular function is selectively impaired in patients with hypertrophic cardiomyopathy and sarcomere myofilament gene mutations. J Am Coll Cardiol. 58, 839-848 (2011).
- Beltrami, C. A., et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 89, 151-163 (1994).
- Factor, S. M., et al. Pathologic fibrosis and matrix connective tissue in the subaortic myocardium of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 17, 1343-1351 (1991).
- Waller, T. A., Hiser, W. L., Capehart, J. E., Roberts, W. C. Comparison of clinical and morphologic cardiac findings in patients having cardiac transplantation for ischemic cardiomyopathy, idiopathic dilated cardiomyopathy, and dilated hypertrophic cardiomyopathy. Am J Cardiol. 81, 884-894 (1998).
- Schaper, J., Lorenz-Meyer, S., Suzuki, K. The role of apoptosis in dilated cardiomyopathy. Herz. 24, 219-224 (1999).
- de Leeuw, N., et al. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 14, 299-306 (2001).
- Yoshikane, H., et al. Collagen in dilated cardiomyopathy--scanning electron microscopic and immunohistochemical observations. Jpn Circ J. 56, 899-910 (1992).
- Marijianowski, M. M., Teeling, P., Mann, J., Becker, A. E. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 25, 1263-1272 (1995).
- Pearlman, E. S., Weber, K. T., Janicki, J. S., Pietra, G. G., Fishman, A. P. Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab Invest. 46, 158-164 (1982).
- Huysman, J. A., Vliegen, H. W., Van der Laarse, A., Eulderink, F. Changes in nonmyocyte tissue composition associated with pressure overload of hypertrophic human hearts. Pathol Res Pract. 184, 577-581 (1989).
- Rossi, M. A. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 16, 1031-1041 (1998).
- Lopez, B., Gonzalez, A., Querejeta, R., Larman, M., Diez, J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol. 48, 89-96 (2006).
- Krayenbuehl, H. P., et al. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 79, 744-755 (1989).
- Schwarz, F., et al. Myocardial structure and function in patients with aortic valve disease and their relation to postoperative results. Am J Cardiol. 41, 661-669 (1978).
- Hein, S., et al. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 107, 984-991 (2003).
- Brooks, W. W., Shen, S. S., Conrad, C. H., Goldstein, R. H., Bing, O. H. Transition from compensated hypertrophy to systolic heart failure in the spontaneously hypertensive rat: Structure, function, and transcript analysis. Genomics. 95, 84-92 (2010).
- O'Hanlon, R., et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 56, 867-874 (2010).
- Green, J. J., Berger, J. S., Kramer, C. M., Salerno, M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 5, 370-377 (2012).
- Frankel, K. A., Rosser, R. J. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 7, 375-386 (1976).
- Otto, R. K., Ferguson, M. R., Friedman, S. D. Cardiac MRI in muscular dystrophy: an overview and future directions. Phys Med Rehabil Clin N Am. 23, 123-132 (2012).
- Finsterer, J., Stollberger, C. The heart in human dystrophinopathies. Cardiology. 99, 1-19 (2003).
- Galindo, C. L., et al. Anti-remodeling and anti-fibrotic effects of the neuregulin-1beta glial growth factor 2 in a large animal model of heart failure. J Am Heart Assoc. 3, e000773(2014).
- Caulfield, J. B., Borg, T. K. The collagen network of the heart. Lab Invest. 40, 364-372 (1979).
- Ohtani, O. Three-dimensional organization of the connective tissue fibers of the human pancreas: a scanning electron microscopic study of NaOH treated-tissues. Arch Histol Jpn. 50, 557-566 (1987).
- Rossi, M. A., Abreu, M. A., Santoro, L. B. Images in cardiovascular medicine. Connective tissue skeleton of the human heart: a demonstration by cell-maceration scanning electron microscope method. Circulation. 97, 934-935 (1998).
- Icardo, J. M., Colvee, E. Collagenous skeleton of the human mitral papillary muscle. Anat Rec. 252, 509-518 (1998).
- McGreevy, J. W., Hakim, C. H., McIntosh, M. A., Duan, D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis Model Mech. 8, 195-213 (2015).
- Buckberg, G., Hoffman, J. I., Mahajan, A., Saleh, S., Coghlan, C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 118, 2571-2587 (2008).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。