Method Article
Comparative Proteomic Analysis of Whole Kidney, Medulla, and Cortical Tubules in Diabetic Pathogenesis of Kidney Injury in Mice
In This Article
Summary
Here, we present a detailed protocol for proteomic analysis of the whole kidney, isolated cortical tubule, and medullary proteomes. The study also compares regional proteomes in a diabetic mouse model and non-diabetic mice.
Abstract
Defining the sequence of events in renal disease is the cornerstone of clinical practice in the nephrologist toolkit. Tissue proteomic analyses are a significant approach to understanding the fundamental physiological and molecular processes of renal pathophysiology. The methods and protocols we present here will allow for the molecular dissection of the kidney in each specific region of interest related to disease sequelae. To determine the effects of disease on specific kidney regions and structures with unique functions, the goals of this protocol are to demonstrate simplified mouse kidney compartmentalization and renal cortical tubule isolation techniques in tandem with streamlined label-free quantitative proteomic workflows. Combining these methods will assist in the identification of perturbed molecular patterns in the whole kidney, medullary compartments, and cortical tubule structures of kidneys, with the ultimate and eventual goal of single-cell proteomics in pathological contexts. Applying these methods in virtually any disease model will be helpful in delineating mechanisms of pathology related to kidney dysfunction.
Introduction
Chronic kidney disease (CKD) is a major concern in modern medicine, amounting to over $86 billion in healthcare expenditures in the United States alone1. Worldwide, the incidence of CKD is increasing with the prevalence of diabetes and associated renal comorbidities. Close to 14 % of the US population has CKD (USRDS 2024 annual report). Furthermore, diabetic nephropathy is a form of CKD and the leading cause of end-stage renal disease (ESRD), and 60% of ESRD patients have diabetes1,2,3. Diabetes affects all kidney structures and cell types of nephrons, the functional unit of kidneys. As shown in Figure 1, different portions of nephrons are contained in the kidney cortex and medulla regions. The majority of the kidney is composed of tubules. Renal tubule dysfunction and structural lesions significantly contribute to the development of diabetic nephropathy (DN), and these changes correlate well with the rate of renal function decline4,5,6,7,8. Early in diabetes, in response to increased glucose and associated sodium uptake and membrane transport protein demands in all tubule segments, tubules undergo hypertrophy. With increased microvascular injury later in diabetes, tubules exhibit atrophy and dilation, while there is fibrosis and expansion of the interstitium9. Previous studies from our lab have found altered proteomes and an abundance of stress response proteins in cortical tubules of diabetic mice10,11. The medulla is important for regulating urine concentration, and dysfunction during kidney disease is associated with oxidative stress, with diabetes leading to decreased oxygen tension in this region of the kidney due to increased oxygen consumption from metabolic activities, increased activity of membrane transport proteins, and microvascular injury12,13,14.
Understanding the detailed mechanisms of development and progression of DN is an ongoing effort that will require novel and integrated approaches invoking modeling of disease and molecular profiling of signaling processes, adaptive changes in cellular protein dynamics, and precise definition of renal cell and tissue components affected by injury in chronic conditions. Proteomics, metabolomics, and transcriptomics offer the possibility to analytically probe the molecular mechanisms of kidney diseases. Omics is a relatively new field that utilizes systems biology approaches to gain a more global understanding of biological systems. Proteomics has been a powerful Omics tool in nephrology in recent decades. Biomarker research has expanded within the past 20 years, as indicated in the growth in publications, though extensive work is necessary to fully implement these findings into the clinic15. With the widely different tubule cell populations and respective functional roles within the renal cortex and medulla, proteomic analysis of whole kidneys can mask unique changes associated with specific structures within these different regions. Therefore, the goals of this study are to demonstrate the separation of the renal cortex and medulla, as well as the separation of cortical tubules from glomeruli, followed by detailed protocols for the preparation of protein extracts from isolated structures for state-of-the-art mass spectrometry and bioinformatics analyses. Mouse models of diabetic nephropathy are instrumental in defining mechanisms of disease progression. For this study, we used the OVE26 transgenic mouse, which develops early-onset type I diabetes and shows features of early and late-stage DN in humans, including 1) an early rise and later decline in glomerular filtration rate, 2) renal hypertrophy, 3) glomerular basement membrane thickening and mesangial expansion, 4) severe proteinuria, and 5) tubulointerstitial fibrosis9,16,17. Two months old mice were chosen to demonstrate proteomic changes in tubule compartments prior to overt structural lesions. As previously reported and shown in Figure 2, 2-month-old OVE26 mice exhibit glomerular mesangial matrix expansion (Figure 2, green arrow) and severe proteinuria17, without overt histologic changes to proximal tubules (Figure 2, yellow arrow) in young diabetic mice. Here we present a combined quantitative proteomics approach for the characterization of the whole kidney, medulla, and cortical tubules to elucidate and illustrate the differences in the proteome in each compartment with diabetes.
Protocol
Studies with OVE26 and FVB mice were approved by the University of Louisville Institutional Animal Care and Use Committee (IACUC) guidelines. Transgenic female OVE26 (diabetic; Strain #:005564) and FVB (non-diabetic; background strain; Strain #:001800) mice were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were maintained on a 12 h light/dark cycle at 25 °C, and given free access to water and food. All studies were conducted on 2-month old mice.
1. Animal model
- Kidney isolation
- Anesthetize mice by intraperitoneally administering 80 mg/kg Ketamine and 12 mg/kg Xylazine.
- Make a midline abdominal incision with small surgical scissors and gently move the intestines out and to the side of the mouse using swabs to expose the vena cava.
- Make a small cut in the vena cava above the right kidney.
- Perfuse the mouse through the left ventricle with 20 mL of cold phosphate-buffered saline (PBS: pH 7.4; 210 mg/L KH2PO4, 9 g/L NaCl, 726 mg/L Na2HPO4·7H2O) using a peristaltic pump, at a rate of 10 mL/min.
NOTE: This step is not required if the inclusion of blood proteins and cells in kidneys is not a critical factor in downstream analyses. - Remove kidneys using small surgical scissors. Remove any excess fat from the outside of the kidneys. Weigh the kidneys and place them in cold PBS on ice.
- Remove the kidney capsule.
- Place kidneys on a glass plate or Petri dish set on ice. Make several transverse cuts through the kidneys using a razor blade (Figure 3) to end up with 3-4 slices between the superior and inferior poles of the kidneys.
- Reserve 1-2 middle slices and the two poles (circled kidney slices in Figure 3) from each kidney aside to serve as "whole kidney" samples. Place a portion into 1.5 mL centrifuge tubes and add ice-cold homogenization buffer (10% glycerol, 50 mM HEPES, 100 mM KCl, 2 mM EDTA, 0.1% NP40, 10 mM NaF, 0.25 mM NaVO3, 1x HALTS protease inhibitor) at approximately 10 µL/mg of tissue sample (based on estimated mass of kidney slices, derived from acquired kidney weight). Leave the tube on ice.
- For the remainder of the middle transverse slices, lay the slice flat and carefully slice away the cortex (outer 1 mm) from each slice using a razor blade or scalpel (Figure 3, schematic workflow). Keep the cortex separated from medulla regions for cortical tubule isolation protocol (below, step 1.3).
- Place a portion of the dissected medulla into 1.5 mL centrifuge tubes, add ice-cold homogenization buffer as in step 1.1.8, and leave the tube on ice.
- Snap freeze in liquid nitrogen all remaining whole kidney and medulla pieces not used for homogenization and store at -80 °C.
- Alternatively, freeze all separated whole kidney and medulla compartments and store them as described in step 1.1.11. Remove them from storage at a later time for the addition of homogenization buffer as in step 1.1.8.
- Cortical tubule isolation
- Mince the dissected cortex into 1-3 mm pieces on a glass plate or Petri dish with a razor blade, forming a paste.
- Digest the minced cortex in 1 mL of type IA collagenase (1 mg/mL) for 30 min, at 37 °C, in a rocking water bath.
- Place a 100 µm cell strainer on top of a 50 mL conical tube on ice.
- Place the digested cortical suspension onto the 100 µm cell strainer and gently press through the strainer using the plunger of a 10 mL syringe. Wash the top of the strainer with 1 mL of PBS and the underside of the strainer with 1 mL of PBS.
- Pass the filtrate in the 50 mL tube through an additional 100 µm strainer, then wash the top of the strainer with 1 mL of PBS.
- Pass the filtrate through a 70 µm cell strainer placed over a 50 mL conical tube on ice. Tubules will pass through this strainer into the filtrate (Glomeruli will be retained on the 70 µm strainer). Wash the strainer with 1 mL of PBS.
- Spin the final filtrate at 120 × g for 2 min at 4 °C. Discard excess PBS from the tubule pellet.
- Check the purity of the cortical tubule pellet by visualization under a microscope, using a 10x objective. If more than 10% of the fraction contains glomeruli, re-suspend the filtrate with 1 mL of PBS and pass through a clean 70 µm cell strainer without any additional washing of the strainer.
- Distribute enriched tubule fraction into 1.5 mL centrifuge tubes and spin at 2000 × g for 2 min at 4 °C. Discard the supernatant.
- Add homogenization buffer to the tubule pellet as with the whole kidney and medulla (steps 1.1.8 and 1.1.10).
- Tissue homogenization/Protein extraction
- Homogenize each sample type in 1.5 mL microcentrifuge tubes using a plastic pestle specifically for 1.5 mL tubes. If the homogenized suspension is too thick, add additional homogenization buffer as needed.
- Leave homogenized suspension on ice for 15 min followed by sonication for 5 min in a bath sonicator (on/off switch only, no adjustable settings on unit), with water in the bath at 25 °C. Leave the samples on ice for 10 min.
- Briefly vortex (1000-1500 RPM) homogenate, then triturate 20 times with a pipette and leave on ice for another 15 min.
- Triturate the samples several times prior to centrifugation at 13,000 × g for 20 min at 4 °C.
- Transfer cleared protein extracts to clean tubes.
- Aliquot 30 µL of cleared extract for protein estimation and sample preparation for mass spectrometry analysis. Store the remainder of the samples at -80 °C.
2. Suspension-Trap mini spin column digestion protocol
- Proteolytic digestion of proteins into tryptic fragments for proteomic analyses
- Dilute ~40-50 µg of sample (at ~2 µg/ µL) into sample lysis buffer (10% w/v sodium dodecyl sulfate [SDS] in 100 mM triethylammonium bicarbonate (TEA-BC) pH 8.5, 40 mM tris (2-carboxyethyl)phosphine [TCEP]) to a final volume of 46 µL.
- Reduction and alkylation of proteins
- Incubate at 65 °C for 30 min (the final concentration of TCEP is 20 mM).
- Add 4 µL of 0.5 M iodoacetamide in liquid chromatography-mass spectrometry (LC-MS) grade water and incubate at room temperature (RT) in the dark for 30 min (the final concentration of iodoacetamide is 40 mM).
- Add 5 µL of 12% w/w phosphoric acid in LC-MS grade water (dilute 141 µL of 85% phosphoric acid into 859 µL of water for 12% solution).
- Alternative to phosphoric acid, use trifluoroacetic acid or formic acid if performing phosphopeptide enrichment18.
- Add 350 µL of suspension-trap binding buffer (100 mM TEA-BC in 90% v/v methanol/10% v/v water pH 7.55).
- Add sample to suspension-trap column. Centrifuge at 4,000 × g for 30 s at RT in a fixed-angle rotor to pass each volume through the column.
- Wash with 4x 400 µL of suspension-trap binding buffer. Centrifuge at 4,000 x g for 30 s at RT in a fixed-angle rotor to pass each wash through the column.
NOTE: To aid in the removal of SDS, rotate the column in the rotor 180° after each wash. - Centrifuge at 4,000 g for 1 min at RT to completely remove binding buffer (prevents dripping during digestion).
- Transfer the suspension-trap column to a clean collection tube.
- Add 2.5-5 µg of trypsin in 125 µL of 50 mM TEA-BC pH 8.5 in LC-MS grade water (Trypsin Protease, MS-Grade). The final ratio of trypsin to the sample is 1:20 up to 1:10.
- Incubate at 47 °C for 2 h (do not tighten the cap; a thermal mixer set at 0 RPM is preferred). Poking a hole in the cap with a needle may prevent pressure from building in the upper chamber of the suspension trap (increased pressure will cause trypsin solution to drip through the column).
- Add 80 µL of 50 mM TEA-BC pH 8.5 in LC-MS grade water. Centrifuge at 4,000 g at RT for 1 min in a fixed-angle rotor to pass each digest through the column.
- Add 80 µL of 0.2% v/v formic acid in LC-MS grade water. Centrifuge at 4,000 g for 1 min at RT in a fixed-angle rotor to collect with digest from step 2.11.
- Add 80 µL of 50% v/v acetonitrile in LC-MS grade water. Centrifuge at 4,000 g at RT for 1 min in a fixed-angle rotor to collect with digest from step 2.11.
- Store the suspension trap eluate at -80 °C.
- Dry the sample in a vacuum concentrator.
- Store the dried sample at -80 °C.
3. Cleanup with hydrophilic-lipophilic balance (HLB) column
- Make solutions A (2% v/v acetonitrile/0.1% v/v formic acid) and B (80% v/v acetonitrile/0.1% v/v formic acid).
- Dissolve the sample in 500 µL of solution A.
- Place the column in a vacuum manifold and apply gas pressure at ~1-2 mL/min.
- Add 500 µL of solution B to the HLB column and evacuate at 1-2 mL/min; repeat twice.
- Add 750 µL of solution A to the HLB column and evacuate at 1-2 mL/min; repeat twice.
- Place a clean 2 mL microtube in the rack below the HLB column, load the sample in 500 µL of solution A, and gas pressure as above; pass the flow-through into the column a second time.
- Place the HLB column above the waste tube, add 500 µL of solution A, and evacuate as above; repeat twice.
- Place the column above a clean 2 mL microtube, add 500 µL of solution B, and evacuate as above; repeat twice.
- Freeze the eluate at -80 °C, then dry in a vacuum concentrator. Store the dried residue at -80 °C.
- Dissolve the residue in 20 µL of 2% v/v acetonitrile/0.1% v/v formic acid prior to MS analysis. Use a spectrophotometer to estimate peptide concentration at an absorbance of 205 nm.
4. Mass spectrometry analysis
- One-dimensional reverse phase liquid chromatography (LC)-MS/MS
- Inject an equal mass of peptides (~600 ng) onto 1-dimensional nano-LC and fractionate it on reversed phase C18 columns.
- Elute the peptides directly into the MS at a spray voltage of 1.8 kV, with an ion transfer tube maintained at 250 °C.
- Acquire the spectra in data-dependent top 20 ion mode where the most intense MS/MS fragment is removed from the analysis queue to improve the depth of ion mapping on lower abundance ions.
5. Data analysis and bioinformatics
- Data handling procedures
- Following spectral assignment and peptide and protein identification in PEAKS 12.0 (LC-MS/MS data analysis software), submit the lists of proteins for analysis in MetaboAnalyst 6.0 (metabolomics data analysis platform) using the single-factor statistical analysis approach (https://www.metaboanalyst.ca/).
- Use LC-MS/MS data analysis software to search and filter spectra using strict false discovery rate (FDR) criteria (1% protein and peptide) against the Mouse Reviewed FASTA database.
NOTE: Alternative search programs that are readily available include Maxquant, Proteome Discoverer, among other widely used options. - Specify carbamidomethylation of cysteine as a fixed modification. Specify oxidation of methionine as a variable modification.
- Convert the lists to comma separated values (CSV) format and group/sample labels assigned to each column and row. This format is flexible in the metabolomics data analysis platform.
- Impute the missing values using the 1/5 minimum value rule for missing variables.
- Filter the data to exclude highly variable values using the interquartile range, where 40% variance is used for the cutoff.
- Normalize the values to median intensity, log10 transformed, and mean centered for scaling. Use other normalization approaches, keeping in mind that the results may vary.
- Perform T-tests in an unpaired manner with a p-value cutoff < 0.05. Perform multiple testing corrections at this stage.
NOTE: This study did not perform this correction for the representative dataset for data presentation purposes. - Indicate volcano plots for 1.5 fold change (FC) and p < 0.05.
- Generate heatmaps and perform partial least squares discriminant analysis (PLSDA) and statistical (variable of importance of projection [VIP]) analysis of the sample groups.
- Potential candidates for follow-up are either robust enough to eclipse 1.5FC and p-val < 0.05 or VIP > 1.0, as indicated in PLSDA analysis.
- Generate Venn diagrams to illustrate differences in the proteome of the whole kidney, tubule, and medulla in OVE26 mice.
Results
Overall, total protein identifications from each sample type were 1) whole kidney (1438) 2) medulla (2145), and cortical tubules (1859). Following data processing in MetaboAnalyst 6.0, data filtering, and imputation, finally analyzed protein identifications for each kidney compartment were: whole kidney (455), medulla (997), and cortical tubules (896). Figure 4 shows global proteomic changes in the OVE26 diabetic mouse kidney. Label-free quantitative (LFQ) analysis allows for high-depth proteome coverage while introducing more variability in the total dataset as compared to reporter ion labeling (TMT, iTRAQ etc.). One approach to overcome LFQ variability is to increase the number of samples analyzed for each condition. Here, we utilized an intensity-based quantification approach from the PEAKS search algorithms with a 1% false-discovery rate (FDR) at the peptide and protein assignment level to allow for robust dataset generation in our paired comparison of two animals in each condition with technical replicates included. Figure 4A illustrates the log2FC (fold change) plotted against the unadjusted p-value (p-value < 0.05). Typically, the p-value will be corrected for multiple comparisons testing when the intent is to validate robust candidate proteins for orthogonal follow-up. Here, we show the entire unadjusted proteome comparison between OVE26 and FVB in the volcano plot for presentation purposes. As an initial overview of the quantitative analysis, volcano plots depict a snapshot preview of potentially interesting candidates with biological implications. Figure 4A shows the ratio of OVE26 protein quantification vs. the control FVB strain in whole kidney proteomes. Notably, GAPDH is robustly upregulated in the OVE26 kidney along with ALDH6A1, suggesting metabolic responses are mounted in early diabetic kidneys. Individual violin plots are provided in Figure 4B to illustrate variability and the effects of normalization of the data using MetaboAnalyst 6.0. Note the robust downregulation of a matrix metalloendopeptidase (MME) and cathepsin A (CTSA) in OVE26 mouse whole kidney. To illustrate overall proteome differences in the whole kidney, the filtered proteome dataset can be submitted for dimensional reduction and supervised multivariate analyses (PLSDA, sPLSDA, oPLSDA) to show group-level differences and to find variables of the importance of the projection (VIP). For this, we performed PLSDA on the OVE26 and FVB kidney proteome, as shown in Figure 4C, and the VIPs are shown in Figure 4D. PLSDA analyses show robust separation of the OVE26 proteome (green) when compared to the control FVB kidney proteome (red). VIP scores > 1 are typically good candidate variables, proteins in this case, for further analyses in pathway ontology enrichment using Gprofiler (https://biit.cs.ut.ee/gprofiler/gost) shown in the plots in Supplemental Figures (Supplementary Figure 1). Notably, many of the protein VIPs are found in the volcano plot analyses in Figure 4A. Identification of candidate lists for further analyses should include proteins with large fold change, statistically robust and important proteins involved in group stratification (VIPs>1). Further illustration of differences in proteomes at the group level and individual protein level can be visualized in hierarchical heatmaps with group and feature clustering, as shown in Figure 4E.
For comparative purposes, we performed a 1-dimensional LC-MS/MS quantitative proteomics analysis on isolated cortical tubules (Figure 5) and medulla (Figure 6) from OVE26 and FVB mice to illustrate the ability to drill into the proteome of specific areas of the kidney with effective depth of detection in the absence of higher abundance components. Finally, overlap in the proteome can be illustrated by a comparison of total significant identifications and overlaying the most statistically significant proteins in each condition (whole kidney, tubules, and medulla) as illustrated in a Venn Diagram (Figure 7). Notably, we utilize a combination approach of t-test and VIP (variable of importance of projection) from PLSDA dimensional reduction statistical approaches (Figure 4C,D, Figure 5C,D, and Figure 6C,D) to determine potential follow-up candidate proteins. Note that the t-stat (T-test) criteria (Figure 7A) was most amenable to identifying a larger number of proteins above the threshold in the whole kidney and, to a lesser extent, in the medulla and tubules. Using another statistical threshold (VIP > 1) in Figure 7B to expand the candidate list gave more evenly distributed and novel candidate proteins in the medulla and cortical tubule proteomic analyses. Notable is the lack of overlap in each of the 3 proteomes, as either 0 or 2 candidates were shared using either approach. Multiple approaches can be applied to enable drilling deeper into the kidney proteome. Isolation and fractionation of the proteome at the tissue and tissue compartment level is an initial approach that can yield robust results, as indicated by the Venn diagram comparison (Figure 7) in the t-test and VIP sorting approaches. All raw files and peak lists will be deposited into MASSIVE (massive.ucsd.edu) according to HUPO standards and publicly shared as a partial submission upon publication.
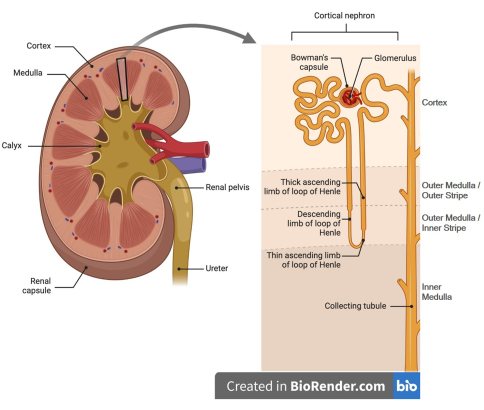
Figure 1: Cartoon rendering of the kidney nephron. (Left panel) Kidneys have cortex (outer) and medulla (inner) regions. (Right panel) Kidney cortex contains glomeruli and respective Bowman's capsule, proximal convoluted tubules and the majority of its straight segment, portions of thick ascending loops of Henle, distal convoluted tubules, cortical segments of the collecting ducts, and peritubular capillaries (not shown). The medulla is comprised of descending and ascending loops of Henle, the medullary portion of the thick ascending loop of Henle and collecting ducts, and vasa recta capillaries surrounding these tubule segments (not shown). The figure was generated using Biorender. Please click here to view a larger version of this figure.

Figure 2: Representative PAS (periodic acid Schiff) staining to demonstrate histology in 2-month old FVB (non-diabetic) and OVE26 (diabetic) mice. Glomeruli (green arrow) from diabetic mice show increased pink PAS staining, demonstrating mesangial expansion, while overt histologic changes to the tubulointerstitium are not yet evident in young diabetic mice. Proximal tubules show slight/modest enlargement/hypertrophy (yellow Arrow). Scale bars: 30 µm. Original magnification 100x. Please click here to view a larger version of this figure.
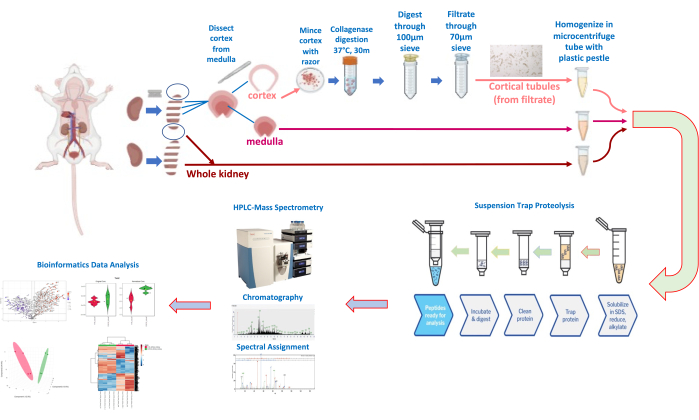
Figure 3: Kidney dissection and tubule isolation and quantitative proteomics workflow. Please click here to view a larger version of this figure.
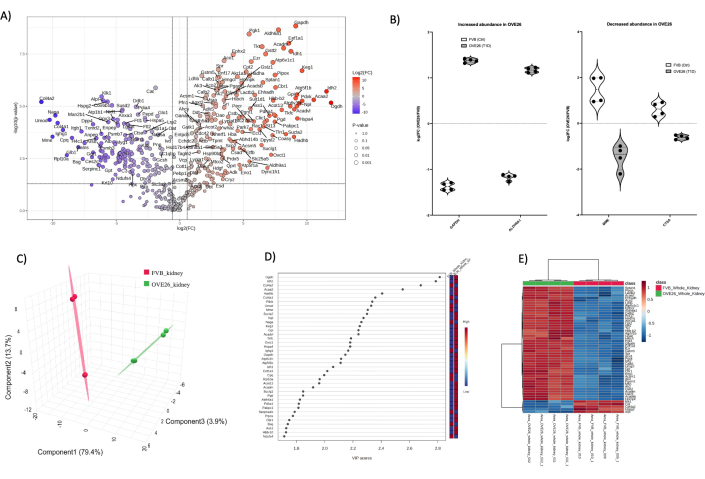
Figure 4: Global changes in the OVE26 diabetic whole mouse kidney proteome. (A) Shows the log2FC (fold change of OVE26/FVB) plotted against the unadjusted p-value (-log10 p-value) in a volcano plot with 1.5-fold negative (blue) or positive (red) change and p < 0.05 cutoffs indicated by the vertical and horizontal lines, all significant proteins quantifications are labeled at the data point. (B) Violin plots showing the variability of each replicate quantification for select proteins GAPDH and ALDH6A1 increasing in OVE26 and MME and CTSA decreasing in OVE26 whole kidney. (C) The dimensional reduction approach using partial least squares discriminant analysis shows group separation of OVE26 (green) and FVB (red). (D) Variables of importance of projection plot show the contribution of individual proteins in the PLSDA analyses by VIP score. (E) Hierarchical clustering heatmap of the top modulated proteins indicating group differences in intensity of protein quantification. Please click here to view a larger version of this figure.
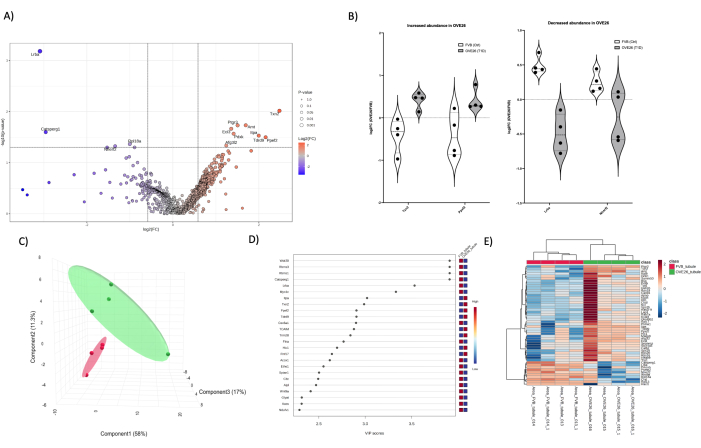
Figure 5: Changes in the OVE26 diabetic cortical tubule proteome. (A) Shows the log2FC (fold change of OVE26/FVB) plotted against the unadjusted p-value (-log10 p-value) in a volcano plot with 1.5-fold negative (blue) or positive (red) change and p < 0.05 cutoffs indicated by the vertical and horizontal lines, all significant proteins quantifications are labeled at the data point. (B) Violin plots showing variability of each replicate quantification for select proteins TXN2 and PPEF2 increasing in OVE26 and LRBA and NHERF2 decreasing in OVE26 tubular proteome. (C) The dimensional reduction approach using partial least squares discriminant analysis shows group separation of OVE26 (green) and FVB (red). (D) Variables of importance of projection plot shows the contribution of individual proteins in the PLSDA analyses by VIP score. (E) Hierarchical clustering heatmap of the top modulated proteins indicating group differences in intensity of protein quantification. Please click here to view a larger version of this figure.
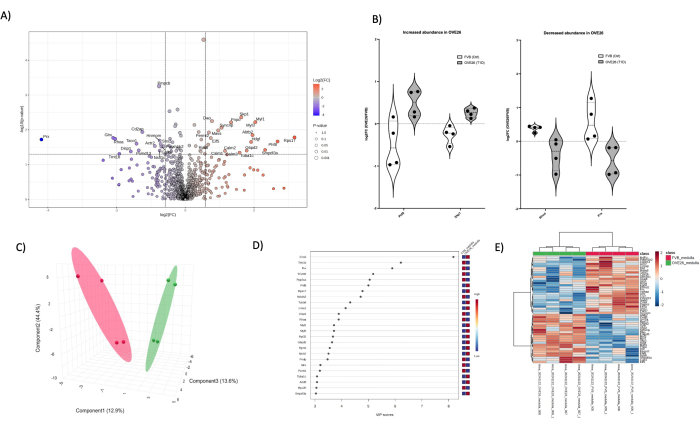
Figure 6: Changes in the OVE26 diabetic medulla proteome. (A) Shows the log2FC (fold change of OVE26/FVB) plotted against the unadjusted p-value (-log10 p-value) in a volcano plot with 1.5-fold negative (blue) or positive (red) change and p < 0.05 cutoffs indicated by the vertical and horizontal lines, all significant proteins quantifications are labeled at the data point. (B) Violin plots showing the variability of each replicate quantification for select proteins PHF8 and SKP1 increasing in OVE26 and RHOA and PRX decreasing in the OVE26 medullary proteome. (C) The dimensional reduction approach using partial least squares discriminant analysis shows group separation of OVE26 (green) and FVB (red). (D) Variables of importance of projection plot shows the contribution of individual proteins in the PLSDA analyses by VIP score. (E) Hierarchical clustering heatmap of the top modulated proteins indicating group differences in intensity of protein quantification. Please click here to view a larger version of this figure.

Figure 7: Venn diagram of the differentially abundant proteins in whole kidney, tubule, and medullary proteomes. Kidney proteomes were compared following a t-test (p-val < 0.05) and fold change threshold of +/- 1.5FC and lists of VIP > 1.0. Please click here to view a larger version of this figure.
Supplementary Figure 1: Whole kidney proteome pathways analysis using Gprofiler generated by submitting VIP protein lists (VIP > 1) in panel 4D. Gprofiler was implemented to analyze pathway differences for proteins with VIP scores greater than 1 from PLSDA analysis in Figure 4. (A) Shows graphical pathways enrichment with p-value cutoffs for molecular functions (MF), cellular component (CC), and biological process (BP). (B) Gene ontology pathway enrichment table for each specific process, function, or cellular compartment. (C) Specific protein enrichment plot for whole kidney proteome molecular functions. Please click here to download this File.
Discussion
The methods presented in this technical approach are designed to illustrate comparative proteome analysis of different areas of the kidney. Here, we utilized methods for isolating medulla and cortical tubules in diabetic (OVE26) and control (FVB) mice and performed 1-dimensional LC-MS/MS and bioinformatics analysis to illustrate basic differences in the proteome in each part of the kidney, in addition to the proteome of whole kidneys.
Isolation of the kidney compartments requires careful dissection of the cortex away from medulla. With initial transverse slicing of kidneys, thin slices will allow more reliable dissection of the cortex. Preparation of the cortical tubules using a sieving approach can result in filtration of glomeruli through 70 µm sieves. It is critical to check the purity of tubules under a microscope to ensure enrichment but avoid loss of tubule mass with each successive wash step. Avoiding excessive pressing of the digested cortex through initial sieving steps can decrease contamination in the final tubule prep.
Suspension trapping approaches offer a large degree of flexibility in the solubilization and protein extraction steps without ionic interference in the mass spectrometry elution steps. Critically important in step 2.10 is the ratio of trypsin (1:20 or 1:10 appear optimal for most applications) to peptide concentration. Efficient proteolysis of protein but minimization of non-specific cleavages will yield more consistent results. Acidification steps following alkylation in step 2.4.1 can utilize formic or trifluoroacetic acid if enrichment of phosphopeptides will be performed. Phosphoric acid can compete with phosphopeptide binding to metal affinity chromatography resins.
Pathways analysis can be utilized to indicate pathway differences and changes related to diabetic injury in the OVE26 kidney as shown in a representative analysis (Supplementary Figure 1). Notable differences in pathways analysis indicated by ontological annotation demonstrate the heterogeneity of metabolism in renal nephrons. The whole kidney proteome was markedly enriched for proteins involved in amino acid metabolic and catabolic processes, fatty acid oxidation, and overall energy management themes (Supplementary Figure 1). The cortical tubule proteome had increased structural proteins and ion transporters, as expected in this compartment, as shown in Figure 5. The medulla is enriched in carbohydrate metabolism proteins (Figure 6) as this site is enriched in glycolytic enzymes, and glucose is a fuel source in the outer medulla, as compared to the renal cortex, which is the site of gluconeogenesis and glucose is a poor fuel for respiration19,20. Furthermore, enrichment of kinase signaling pathways in the medulla is likely reflective of interstitial osmolality and cell size fluctuations in this region21 and metabolic disturbances and oxidative stress associated with this region during diabetes22. Using the volcano plots, VIP plots, and heatmap results combined with a pathways enrichment approach like Gprofiler can help researchers better understand the effects of injury or disease on changes in the different renal compartments. Additional analysis software options include DAVID (https://davidbioinformatics.nih.gov/), Express Analyst (https://www.expressanalyst.ca/ExpressAnalyst/) and PANTHERDB (https://www.pantherdb.org/) for ontology and pathways characterization.
In conclusion, proteomic comparison of the total whole kidney proteome (Figure 4) and that of the cortical tubule (Figure 5) and medulla (Figure 6) compartments is a useful approach to illustrate heterogeneity in whole kidney extracts when compared to isolated sections of the kidney. Additionally, isolated proteomes allow for drilling deeper into the proteome of specific structures in the absence of high abundance (whole tissue) proteins, thus unmasking medium abundance (tissue compartments) and then finally low abundance (single cell) proteins. Ultimately, the ideal isolation would be at single-cell levels to allow for cell dynamics to be measured and linked to a specific pathological condition such as diabetes or chronic kidney diseases.
Disclosures
The authors have no disclosures.
Acknowledgements
Work for this project was partially supported with funds for MTB (NIH K01DK080951) and TDC (NephCure-Pediatric Nephrology Research Consortium NKI-2023-04) and the University of Louisville Kidney Disease Program and the Proteomics Technology Center (TDC, MTB).
Materials
| Name | Company | Catalog Number | Comments |
| Collagenase type 1A | Millipore Signal | C9891 | |
| Exploris 480 Orbitrap | Thermofisher | https://www.thermofisher.com/order/catalog/product/BRE725539 | MS |
| Falcon Cell Strainer, 100 µm | VWR | 21008-950 | |
| Falcon Cell Strainer, 70 µm | VWR | 21008-952 | |
| Gibco PBS pH 7.4 | Thermo | 1001023 | |
| Halt Protease and Phosphatase Inhibitor Cocktail | VWR | PI78440 | |
| Iodoacetamide | Sigma Aldrich | I1149 | |
| MetaboAnalyst 6.0 | MetaboAnalyst 6.0 | https://www.metaboanalyst.ca/ | metabolomics data analysis platform |
| NanoDrop 2000 | Thermofisher | https://www.thermofisher.com/order/catalog/product/ND-2000 | |
| Oasis HLB column | Waters | 186002034 | |
| PEAKS 12.0 | Bioinformatics Solutions Inc | LC-MS/MS data analysis software | |
| Pestle for 1.5 mL Microtube | Fisher Scientific | NC0782485 | |
| Suspension Trap (S-trap) | Protifi | C02-micro-10 | |
| TCEP | ThermoFisher Scientific | 20490 | |
| TEABC | Sigma Aldrich | T7408 | |
| Trypsin Protease, MS-Grade | ThermoFisher Scientific | 90057 | |
| Ultrasonic Cleaner | Cole-Parmer | Model 0884900 |
References
- Ding, X., et al. Epidemiological patterns of chronic kidney disease attributed to type 2 diabetes from 1990-2019. Front Endocrinol. 15, 1383777 (2024).
- Liu, W., et al. Global trends in the burden of chronic kidney disease attributable to type 2 diabetes: an age-period-cohort analysis. Diabetes Obes Metab. 26 (2), 602-610 (2024).
- Xie, D., et al. Global burden and influencing factors of chronic kidney disease due to type 2 diabetes in adults aged 20-59 years, 1990-2019. Sci Rep. 13 (1), 20234 (2023).
- Giunti, S., Barit, D., Cooper, M. E. Mechanisms of diabetic nephropathy: role of hypertension. Hypertension. 48 (4), 519-526 (2006).
- Thomas, M. C., Burns, W. C., Cooper, M. E. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 12 (2), 177-186 (2005).
- Thomas, M. C., et al. Diabetic kidney disease. Nat Rev Dis Primers. 1, 15018 (2015).
- Nath, K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 20 (1), 1-17 (1992).
- Bonventre, J. V. Can we target tubular damage to prevent renal function decline in diabetes. Semin Nephrol. 32 (5), 452-462 (2012).
- Powell, D. W., et al. Renal tubulointerstitial fibrosis in OVE26 type 1 diabetic mice. Nephron Exp Nephrol. 111 (1), e11-e19 (2008).
- Cummins, T. D., et al. Quantitative mass spectrometry of diabetic kidney tubules identifies GRAP as a novel regulator of TGF-beta signaling. Biochim Biophys Acta. (4), 653-661 (2010).
- Barati, M. T., et al. Differential expression of endoplasmic reticulumstress-response proteins in different renal tubule subtypes of OVE26 diabetic mice. Cell Stress Chaperones. 21 (1), 155-166 (2016).
- Zheleznova, N. N., et al. Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics. 44 (17), 829-842 (2012).
- Nordquist, L., Palm, F. Diabetes-induced alterations in renal medullary microcirculation and metabolism. Curr Diabetes Rev. 3 (1), 53-65 (2007).
- dos Santos, E. A., Li, L. -. P., Ji, L., Prasad, P. V. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol. 42 (3), 157-162 (2007).
- Cummins, T. D., et al. Quantitative Mass Spectrometry Normalization in UrineBiomarker Analysis in Nephrotic Syndrome. Glomerular Dis. (3), 121-131 (2022).
- Carlson, E. C., Audette, J. L., Klevay, L. M., Nguyen, H., Epstein, P. N. Ultrastructural and functional analyses of nephropathy in calmodulin-induced diabetic transgenic mice. Anat Rec. 247 (1), 9-19 (1997).
- Zheng, S., et al. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 53 (12), 3248-3257 (2004).
- Wang, F., Veth, T., Kuipers, M., Altelaar, M., Stecker, K. E. Optimized suspension trapping method for phosphoproteomics sample preparation. Anal Chem. 95 (25), 9471-9479 (2023).
- Mather, A., Pollock, C. Glucose handling by the kidney. Kidney Int Suppl. 120, S1-S6 (2011).
- Ross, B. D., Espinal, J., Silva, P. Glucose metabolism in renal tubular function. Kidney Int. 29 (1), 54-67 (1986).
- Roger, F., Martin, P. Y., Rousselot, M., Favre, H., Féraille, E. Cell shrinkage triggers the activation of mitogen-activated protein kinases by hypertonicity in the rat kidney medullary thick ascending limb of the Henle's loop: requirement of p38 kinase for the regulatory volume increase response. J Biol Chem. 274 (48), 34103-34110 (1999).
- Yang, J., Lane, P. H., Pollock, J. S., Carmines, P. K. PKC-dependent superoxide production by the renal medullary thick ascending limb from diabetic rats. Am J Physiol Renal Physiol. 297 (5), F1220-F1228 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved