Method Article
A Modified Mouse Model of Intracranial Aneurysm Based on Hemodynamic Change and Hypertension
* These authors contributed equally
In This Article
Summary
Intracranial aneurysm (IA) was constructed in mice using the risk factors of hypertension and hemodynamic changes. Hemodynamic changes were induced by ligating branches of the carotid artery, while hypertension was achieved by ligating the posterior branches of the renal artery. IA formation was detected through magnetic resonance angiography, stereomicroscopy, and pathological analysis.
Abstract
Intracranial aneurysm (IA) poses a significant health risk due to morbidity and mortality associated with aneurysm rupture. However, the molecular mechanisms underlying IA development remain unclear, and a suitable mouse model is required. A mouse model of IA was established by ligating the pterygopalatine artery (PPA) to induce additive hemodynamic changes, combined with hypertension induction. In C57BL/6 male mice, vessels, including the right PPA, external carotid artery (ECA), occipital artery (OcA), and the left contralateral common carotid artery (CCA), were ligated to induce hemodynamic changes. One week later, the bilateral posterior branches of the renal artery (pRA) were ligated, and an 8% salt diet was introduced to induce hypertension. Magnetic resonance angiography (MRA), stereomicroscopy, and immunohistochemical (IHC) staining were performed to evaluate the morphological and pathological changes in IA three months after induction. In the experimental group, four mice died after the initial induction. IA at different locations was detected in five of the eleven remaining mice. Both microscopic and MRA examinations confirmed IA formation. Pathological and IHC analyses revealed disruption of the internal elastic lamina, disconnection of collagen fibers, and infiltration of CD86-positive M1 macrophages, findings consistent with those observed in human IA. This mouse model of IA replicates the pathological changes observed in human samples and may serve as a valuable tool for investigating the molecular mechanisms of IA formation and progression.
Introduction
The prevalence of intracranial aneurysm (IA) is estimated to be 3.2% of the general population1. IA poses a significant health risk due to its high associated morbidity and mortality. IA is a complex and multidimensional pathological condition influenced by hemodynamic changes, inflammation, and vascular remodeling2,3. Hemodynamic changes and hypertension are implicated in the formation and progression of aneurysms4,5. IA frequently occurs at cerebral bifurcations with elevated hemodynamic shear stress6, and bifurcations with narrow angles are identified as risk factors for IA development in humans7. Despite advancements in endovascular treatments and surgical strategies, subarachnoid hemorrhage caused by IA rupture remains catastrophic. Therefore, exploring pharmacological treatments is a promising approach for preventing aneurysm rupture8. However, the mechanisms underlying the pathological formation and progression of IA remain unclear. Developing a suitable mouse model for IA formation and progression, based on human risk factors, is crucial to uncovering the underlying mechanisms and identifying potential therapeutic targets. This study aims to construct a model of IA formation without rupture in mice that mimic human IA characteristics.
The circle of Willis (CW) connects and communicates the right internal carotid artery (ICA), left ICA, and bilateral vertebrobasilar arteries. The CW serves as a compensatory mechanism in cases of occlusion or stenosis of the ICA or vertebral artery9. The pterygopalatine artery (PPA) is a branch of the ICA that supplies blood to the external part of the brain10. Based on the compensatory function of the CW, PPA occlusion increases blood flow in the ICA. Combining ligation of the left common carotid artery (CCA), right external carotid artery (ECA), and occipital artery (OcA) results in increased blood flow in the CW, particularly at narrowed angles, leading to hemodynamic changes. In this model, the blood supply to the brain is supported by the vertebrobasilar artery and the right ICA. PPA ligation did not contribute to mortality in the mice11.
To induce an IA model based on elastase injection, hypertension was induced by angiotensin-II (Ang-II) release via an Alzet pump or deoxycorticosterone acetate (DOCA)-salt12,13. The high cost of Alzet and DOCA should be considered in experiments involving a large number of animals. The achieved levels of hypertension were not significantly different between ligation of the posterior and inferior branches of the bilateral renal arteries or only the posterior branches of the bilateral renal arteries. However, the former approach resulted in greater renal dysfunction14. Therefore, ligation of the bilateral posterior renal arteries (pRA) is considered a rational method for most investigators.
Elastase was injected into the cerebrospinal fluid at the right basal cistern via a single stereotaxic injection12. The elastase injection-based IA model caused 60%-80% IA rupture three weeks after injection15,16, which is too short to study IA formation and development. Furthermore, there is no evidence to suggest elevated elastase levels in humans during IA formation. Additionally, stereotaxic injection into the right cistern is associated with high mortality and disability in mice, posing significant challenges for novices.
In this study, a mouse model of IA without rupture within three months was constructed based on human risk factors. This model eliminates the high cost associated with DOCA and Alzet. Moreover, it can be performed using only a stereomicroscope and can be easily mastered by novices.
Protocol
All operational procedures in mice adhered to the criteria of the Ethical Review Committee and were approved by the Institutional Animal Care and Use Committee of Shanghai Jiaotong University. C57BL/6 male mice (8 weeks old, 20-25 g) were housed at a temperature of 22 °C with a 12 h/12 h light/dark cycle. The operational process is shown in Figure 1A. Briefly, in anesthetized animals, the left common carotid artery (CCA), right external carotid artery (ECA), occipital artery (OcA), and pterygopalatine artery (PPA) were ligated to induce hemodynamic changes. Hypertension was subsequently induced by ligating the bilateral renal arteries (bRA) one week after the initiation of hemodynamic changes, and the animals were fed a diet containing 8% salt. Hemodynamic changes and IA formation are depicted in Figure 1B. Details of the reagents and equipment used in this study are provided in the Table of Materials.
1. Establishment of mouse UIA based on hemodynamic changes and hypertension
NOTE: Mice were fasted for 12 h before the operation. Surgical instruments were sterilized by soaking them in 70% alcohol for at least 30 min.
- Administer anesthesia (following institutionally approved protocols) using a small animal anesthesia machine with 2% isoflurane inhalation in a mixture of O2 (1 L/min) on a heated pad maintained at 37 °C.
- Ligate the left CCA
- Make a 1 cm linear incision along the cervical midline. Dissect subcutaneous tissue and platysma to expose the trachea.
- Pull the jugular vein away and locate the carotid sheath along the trachea (Figure 2A i,ii). Separate the left CCA from the vagus nerve. Ligate the left CCA with a 6-0 silk suture (Figure 2A iii,iv).
- Ligate the right ECA and OcA
- Expose the anatomical structure of the CCA, ICA, ECA, OcA, vagus nerve, and hypoglossal nerve on the right side. Ligate the right ECA with a 6-0 silk suture (Figure 2A iii,iv).
- Isolate the OcA and ligate it with an 8-0 silk suture (Figure 2A v - viii). Disconnect the OcA using two straight micro forceps.
NOTE: The OcA, arising proximally from the ECA, lies below the hypoglossal nerve (Figure 2A v - viii). Dissecting the OcA helps to expose the PPA anatomy effectively.
- Ligate the right PPA
- Isolate the hypoglossal nerve from the ICA. Place surgical cotton between the hypoglossal nerve and ICA to protect the nerve from procedure-related injury.
- Dissect the perivascular tissue around the PPA using microforceps. Clamp the PPA and temporarily pull it up lightly. Place an 8-0 silk suture between the PPA and ICA to ligate the PPA (Figure 2A ix,x).
- Ligate the PPA and maintain sufficient distance between the ligation site and the origin of the PPA to ensure blood flow in the circle of Willis (Figure 2A x).
NOTE: The primary challenge in this step is ligating the PPA within an extremely tight operating space without impairing peripheral nerves and vascular structures. Avoid compressing the trachea during PPA exposure.
- Finalize the operation
- Sterilize the operational field with povidone-iodine and suture the wound. Monitor mice until they recover from anesthesia.
- Induce hypertension
- Make a 2 cm long midline incision at the 12th vertebral level on the back. Cut the dorsal muscles to expose the kidney. Clamp the adipose tissue around the kidney using forceps and fix the kidney inside the muscle incision (Figure 2B i,ii).
- Isolate the adipose tissue around the renal pedicle. Identify the pRA in close contact with the renal vein (Figure 2B iii,iv). Clamp the pRA and pull it up using straight micro forceps. Dissect the fascia between the pRA and renal vein using another micro forceps (Figure 2B v,vi).
NOTE: Exercise caution to prevent damage to the renal vein, which could lead to catastrophic bleeding. - Ligate the pRA with a 6-0 silk suture (Figure 2B vii,viii). Immediately after ligation, observe ischemic foci forming in the upper part of the kidney (Figure 2B ix,x). Suture the muscle and skin incisions.
2. IA Examination via MRA and stereomicroscope
- Perform time-of-flight (TOF) 7.0 T magnetic resonance angiography (MRA) three months after aneurysm induction to evaluate IA formation.
- Euthanize the mice via exsanguination and cervical dislocation after isoflurane anesthesia (following institutionally approved protocols). Detect IA under a stereomicroscope as previously reported17.
- Infuse the samples with cooled PBS, followed by 4% paraformaldehyde, and then Microfil to visualize vessels and aneurysms.
- Define an aneurysm as outward bulging 1.5 times larger than the parent artery, as observed by two independent neurosurgeons8,18.
3. Histological and immunohistochemistry analyses
- Isolate the circles of Willis under a microscope. Fix the specimens with 4% paraformaldehyde at 4 °C for 24 h19.
- Process the specimens into paraffin and frozen sections for histological and immunofluorescence staining. Stain paraffin sections with EVG and Masson stains according to the manufacturers' instructions19.
- Incubate the paraffin sections with a blocking solution to minimize nonspecific binding.
- Incubate sections with primary antibodies against CD86 overnight at 4 °C. After incubation with a secondary antibody, develop a brown color by reacting the sections with DAB20.
- Perform counterstaining with hematoxylin and mount the sections with an aqueous mounting solution20.
Results
Rate of IA formation
In the experimental group (n = 15), 2 mice died within the first week after the initial procedure for unknown reasons. One mouse died from an infection in the back wound on the third day after the second procedure, and another mouse died on day 38 for unknown reasons, with no aneurysms detected. In the control group (n = 5), all 5 mice survived until sacrifice. Among the surviving mice in the experimental group (n = 11), the systolic blood pressure at three months post-induction was significantly higher than before induction (90 ± 1.39 mm Hg vs. 126.63 ± 1.83 mm Hg; P < 0.0001). In the control group (n = 5), blood pressure remained consistent before induction and at sacrifice (88 ± 2.34 mm Hg vs. 91.8 ± 1.2 mm Hg; P > 0.05).
IA evaluation and findings
In the experimental group, aneurysms at different locations were detected in 5 of the 11 surviving mice using MRA and light microscopy. Two aneurysms were located at the anterior cerebral artery-olfactory artery bifurcations, two at the anterior communicating artery, and one at the posterior cerebral artery (Figure 3). Additionally, 2 of the 11 mice exhibited tortuosity in the anterior cerebral artery and middle cerebral artery. None of the aneurysms ruptured within three months of induction. No aneurysmal changes were observed in 4 of the 11 mice in the experimental group or in any of the 5 mice in the control group.
Histological and immunofluorescence examination
In human cerebral aneurysms, the vascular wall is characterized by disruption of the elastic lamina, decellularization of the media, disconnection of collagen fibers, and infiltration of M1 macrophages21. Compared to control blood vessels, EVG-Verhoeff staining revealed significant disruption of the inner elastic layer in IA tissue (Figure 4A). Masson staining showed considerable disconnection of collagen fibers in IA walls (Figure 4B). Immunohistochemical analysis indicated significant infiltration of CD86-positive macrophages in the aneurysm wall (Figure 4C,D).
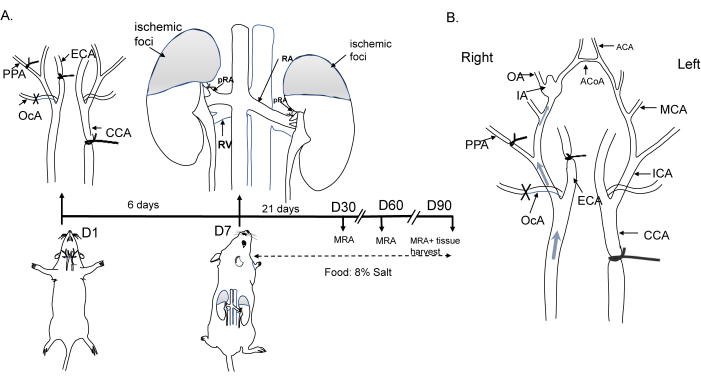
Figure 1: Diagram of the induction of the mouse aneurysm model. (A) Schematic representation of the ligation of the left common carotid artery, the right external carotid artery, the pterygopalatine artery, and the bilateral posterior branches of the renal artery, along with an 8% NaCl diet. (B) Hemodynamic changes leading to flow alterations in the circle of Willis and aneurysm formation. Abbreviations: ACA, anterior cerebral artery; ACoA, anterior communicating artery; CCA, common carotid artery; ECA, external carotid artery; IA, intracranial aneurysm; MCA, middle cerebral artery; MRA, magnetic resonance angiography; OA, olfactory artery; OcA, occipital artery; PPA, pterygopalatine artery. Please click here to view a larger version of this figure.

Figure 2: Anatomical and schematic diagrams of artery ligation. (A) Exposure and ligation of arteries in the neck. i-iv, ligation of the left CCA; v-vi, ligation of the right ECA; vii-viii, ligation of the right OcA; ix-x, ligation of the right PPA. (B) Exposure and ligation of the posterior branches of the renal artery (pRA). i-iv, exposure of the right pRA; v-vi, separation of the pRA; vii-viii, ligation of the pRA; ix-x, return of the kidneys to their original position. Please click here to view a larger version of this figure.

Figure 3: Morphological changes in intracranial aneurysms. (A) The normal bifurcation of the olfactory artery and anterior cerebral artery in the control group. (B) Typical aneurysmal changes at the bifurcation of the olfactory artery and anterior cerebral artery observed on MRA and under a microscope (red arrow). Magnification: 6x. Please click here to view a larger version of this figure.

Figure 4: Pathological analysis of intracranial aneurysms. (A) EVG staining: The elastic layer in the normal group is intact and smooth, while the elastic layer in the aneurysm group is disrupted. (B) Masson staining: Collagen fibers are intact in the normal group, whereas a large number of collagen fibers are interrupted in the aneurysm group. (C) IHC staining for CD86: CD86-positive cells are absent in the normal group but are significantly present in the aneurysm wall. Scale bars = 200 µm (for non-magnified images); 50 µm (for magnified images), ***P < 0.001. Abbreviation: IA, intracranial aneurysm. Please click here to view a larger version of this figure.
Discussion
This study presents a modified approach to constructing a mouse model of IA through ligation of the PPA to induce additive hemodynamic changes in combination with hypertension. MRA imaging and microscopic analysis demonstrated significant aneurysmal changes in the circle of Willis. The pathological alterations observed in this model are consistent with those found in human samples. This mouse model may serve as a valuable tool for investigating the molecular mechanisms underlying IA formation and development.
IA was induced in mice by ligating the left CCA, ECA, OcA, PPA, and bilateral posterior renal arteries (pRA), along with a high-salt diet. Cai et al. employed a similar procedure to establish a rat aneurysm model with normal blood pressure and identified cerebral aneurysms and the expansion of anterior cerebral arteries22. Miyamoto et al. reported that, compared with ligating only the left CCA, additional ligation of the right PPA and right ECA significantly increased IA formation in rats. Ligation of the ECA and PPA may amplify hemodynamic changes by increasing blood flow volume18. In humans, carotid artery occlusion has been linked to the formation of intracranial aneurysms23. Therefore, this modified mouse model of IA may prove beneficial for exploring the formation and development of IA.
The choice of animal species in research studies is crucial and serves distinct research purposes. Rat models, which have been used for decades, exhibit specific pathological features such as fragmentation of the internal elastic lamina and thinning of the smooth muscle cell layer. Rat models are employed mostly to investigate the pathogenesis of aneurysms24,25. Rabbit models, on the other hand, are particularly suitable for preclinical studies related to endovascular treatment26,27,28. Compared to these models, mice are preferred for genetic analyses due to their shorter life cycles and transgenic manipulability. Additionally, mice exhibit significant similarity in the expression and function of orthologous genes with those of humans29. Thus, mouse models represent an optimal choice for laboratory investigations.
In previous studies, intracranial aneurysms (IAs) were induced in mice using elastase to degrade the elastic lamina30. Although elastase-based aneurysms exhibit morphological and histological changes similar to those observed in humans31,32,33, they are associated with a high rupture rate, making them advantageous for studying IA rupture. However, elastase-induced vessel weakness does not accurately represent the natural process of IA formation. The current model, which replicates the pathological characteristics of human IA, was developed based on two independent risk factors for IA formation and did not result in rupture during the three-month observation period.
There are several limitations to this study. While the mouse model was induced through additive hemodynamic changes and hypertension, the incidence of cerebral aneurysms in a small sample size was not determined, nor was it compared with the incidence of aneurysms induced solely by ligation of the common carotid artery or bilateral ligation of the posterior renal arteries34. The observation window was limited to three months, which partially simulates the natural process of IA formation but does not eliminate the possibility of rupture. Replicating the complex and realistic hemodynamics of humans remains a challenge. Additionally, the hemodynamic changes caused by the additional ligation of the PPA were not assessed, and blood flow changes were not accurately measured. Nonetheless, IAs in mice were detected using MRA, and the pathological changes observed were consistent with those found in human IAs. This mouse model of IA is a valuable tool for investigating the process of IA formation.
Disclosures
The manuscript has been read and approved by all named authors, and there are no other persons who satisfied the criteria for authorship but are not listed. The authors have no conflicts of interest associated with the manuscript, and there has been no significant financial support for this work that could have influenced its outcome. The funders were not involved in the data collection, data analysis, or paper writing. The manuscript has not been previously published online or in print, including journals, websites, or blogs.
Acknowledgements
This study was supported by the National Facility for Translational Medicine (Shanghai TMSK-2021-147), the Shanghai Renji Hospital Research Project (RJTJ-QT-007), and the China Postdoctoral Science Foundation (Certificate Number: 2024M760658).
Materials
| Name | Company | Catalog Number | Comments |
| 7.0 T magnetic resonance angiography | Bruker | BioSpec 70/20 | |
| C57BL/6 mice | Charles River Laboratories | sex: male | |
| CD86 antibody | CST | 91882 | |
| Elastic Van Gieson (EVG)stain | Solarbio | G1597 | |
| Masson | Solarbio | G1340 | |
| Micro forcep | Shanghai Jinzhong Instrument Company | ||
| Microfil | Flow Tech Inc. | MV-120 | |
| Small animal anesthesia machine | RWD | R500 | |
| Stereo microscope | Shanghai Optical Instrument Company | XYH-6B | |
| Suture | Shanghai Jinhuan Medical Company | 6-0, 8-0 |
References
- Vlak, M. H., Algra, A., Brandenburg, R., Rinkel, G. J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 10 (7), 626-636 (2011).
- Turjman, A. S., Turjman, F., Edelman, E. R. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation. 129 (3), 373-382 (2014).
- Shikata, F., et al. Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension. 73 (2), 491-496 (2019).
- Penn, D. L., Komotar, R. J., Sander Connolly, E. Hemodynamic mechanisms underlying cerebral aneurysm pathogenesis. J Clin Neurosci. 18 (11), 1435-1438 (2011).
- Vlak, M. H., Rinkel, G. J., Greebe, P., Algra, A. Independent risk factors for intracranial aneurysms and their joint effect: A case-control study. Stroke. 44 (4), 984-987 (2013).
- Alfano, J., et al. Intracranial aneurysms occur more frequently at bifurcation sites that typically experience higher hemodynamic stresses. Neurosurgery. 73 (3), 497-505 (2013).
- Idil Soylu, A., Ozturk, M., Akan, H. Can vessel diameters, diameter ratios, and vessel angles predict the development of anterior communicating artery aneurysms: A morphological analysis. J Clin Neurosci. 68, 250-255 (2019).
- Starke, R. M., et al. Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 11, 77 (2014).
- Vrselja, Z., Brkic, H., Mrdenovic, S., Radic, R., Curic, G. Function of circle of Willis. J Cereb Blood Flow Metab. 34 (4), 578-584 (2014).
- Azedi, F., et al. Intra-arterial drug delivery to the ischemic brain in rat middle cerebral artery occlusion model. Bio Protoc. 9 (23), e3438 (2019).
- Yoshimura, S., et al. Reliable infarction of the middle cerebral artery territory in C57BL/6 mice using pterygopalatine artery ligation and filament optimization - The PURE-MCAo model. J Cereb Blood Flow Metab. , (2024).
- Nuki, Y., et al. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 54 (6), 1337-1344 (2009).
- Tada, Y., et al. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 45 (2), 579-586 (2014).
- Eldawoody, H., et al. Simplified experimental cerebral aneurysm model in rats: Comprehensive evaluation of induced aneurysms and arterial changes in the circle of Willis. Brain Res. 1300, 159-168 (2009).
- Hosaka, K., Downes, D. P., Nowicki, K. W., Hoh, B. L. Modified murine intracranial aneurysm model: aneurysm formation and rupture by elastase and hypertension. J Neurointerv Surg. 6 (6), 474-479 (2014).
- Furukawa, H., et al. Mast cell promotes the development of intracranial aneurysm rupture. Stroke. 51 (11), 3332-3339 (2020).
- Dungan, C. M., et al. Deletion of SA beta-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell. 21 (1), e13528 (2022).
- Miyamoto, T., et al. Site-specific elevation of interleukin-1beta and matrix metalloproteinase-9 in the Willis circle by hemodynamic changes is associated with rupture in a novel rat cerebral aneurysm model. J Cereb Blood Flow Metab. 37 (8), 2795-2805 (2017).
- Xu, C., et al. CD147 monoclonal antibody attenuates abdominal aortic aneurysm formation in angiotensin II-Infused apoE(-/-) mice. Int Immunopharmacol. 122, 110526 (2023).
- Taube, J. M., et al. Multi-institutional TSA-amplified multiplexed immunofluorescence reproducibility evaluation (MITRE) study. J Immunother Cancer. 9 (7), e002197 (2021).
- Frosen, J., et al. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 123 (6), 773-786 (2012).
- Cai, J., He, C., Yuan, F., Chen, L., Ling, F. A novel haemodynamic cerebral aneurysm model of rats with normal blood pressure. J Clin Neurosci. 19 (1), 135-138 (2012).
- Arnaout, O. M., et al. De novo large fusiform posterior circulation intracranial aneurysm presenting with subarachnoid hemorrhage 7 years after therapeutic internal carotid artery occlusion: case report and review of the literature. Neurosurgery. 71 (3), E764-E771 (2012).
- Nagata, I., Handa, H., Hashimoto, N., Hazama, F. Experimentally induced cerebral aneurysms in rats: Part VI. Hypertension. Surg Neurol. 14 (6), 477-479 (1980).
- Aoki, T., Kataoka, H., Morimoto, M., Nozaki, K., Hashimoto, N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 38 (1), 162-169 (2007).
- Grunwald, I. Q., et al. An experimental aneurysm model: a training model for neurointerventionalists. Interv Neuroradiol. 12 (1), 17-24 (2006).
- Brinjikji, W., Ding, Y. H., Kallmes, D. F., Kadirvel, R. From bench to bedside: Utility of the rabbit elastase aneurysm model in preclinical studies of intracranial aneurysm treatment. J Neurointerv Surg. 8 (5), 521-525 (2016).
- Adibi, A., Eesa, M., Wong, J. H., Sen, A., Mitha, A. P. Combined endovascular coiling and intra-aneurysmal allogeneic mesenchymal stromal cell therapy for intracranial aneurysms in a rabbit model: A proof-of-concept study. J Neurointerv Surg. 9 (7), 707-712 (2017).
- Liao, B. Y., Zhang, J. Evolutionary conservation of expression profiles between human and mouse orthologous genes. Mol Biol Evol. 23 (3), 530-540 (2006).
- Han, Y., et al. Axl promotes intracranial aneurysm rupture by regulating macrophage polarization toward M1 via STAT1/HIF-1alpha. Front Immunol. 14, 1158758 (2023).
- Wang, S., et al. Rabbit aneurysm models mimic histologic wall types identified in human intracranial aneurysms. J Neurointerv Surg. 10 (4), 411-415 (2018).
- Nowicki, K. W., Hosaka, K., Walch, F. J., Scott, E. W., Hoh, B. L. M1 macrophages are required for murine cerebral aneurysm formation. J Neurointerv Surg. 10 (1), 93-97 (2018).
- Shi, Y., et al. Nrf-2 signaling inhibits intracranial aneurysm formation and progression by modulating vascular smooth muscle cell phenotype and function. J Neuroinflammation. 16 (1), 185 (2019).
- Morimoto, M., et al. Mouse model of cerebral aneurysm: experimental induction by renal hypertension and local hemodynamic changes. Stroke. 33 (7), 1911-1915 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved