Method Article
Automated and High-throughput Microbial Monoclonal Cultivation and Picking Using The Single-cell Microliter-droplet Culture Omics System
In This Article
Summary
This protocol describes how to use the Single-cell Microliter-droplet Culture Omics System (MISS cell) to perform microbial monoclonal isolation, cultivation, and picking. The MISS cell achieves an integrated workflow based on droplet microfluidic technology, which offers excellent droplet monodispersity, high parallel cultivation, and high-throughput biomass detection.
Abstract
Pure bacterial cultures are essential for the study of microbial culturomics. Traditional methods based on solid plates, well plates, and micro-reactors are hindered by cumbersome procedures and low throughput, impeding the rapid progress of microbial culturomics research. To address these challenges, we had successfully developed the Single-cell Microliter-droplet Culture Omics System (MISS cell), an automated high-throughput platform that utilizes droplet microfluidic technology for microbial monoclonal isolation, cultivation, and screening. This system can generate a large number of single-cell droplets and cultivate, screen, and collect monoclonal colonies in a short time, facilitating an integrated process from microbial isolation to picking. In this protocol, we demonstrated its application using the isolation and cultivation of human gut microbiota as an example and compared the microbial isolation efficiency, monoclonal culture performance, and screening throughput using the solid-plate culture method. The experimental workflow was simple, and reagent consumption was very low. Compared to solid-plate culture methods, the MISS cell could cultivate a greater diversity of gut microbiota species, offering significant potential and value for microbial culturomics research.
Introduction
Microbial culturomics has wide applications in researching beneficial microbes in the food industry, the diversity of environmental microbes, screening for new antimicrobial compounds, and the human microbiome in relation to disease1,2,3,4. Traditional methods, primarily based on solid plates, well plates, or micro-reactors to obtain and pick monoclonal colonies, are easy to operate but suffer from low throughput due to their multiple steps. This limitation hinders applications such as microbial mutagenesis screening, microbial culturomics studies, and high-producing colony selection, all of which require extensive monoclonal screening.
Recently, various single-cell detection and dispensing devices have been designed to significantly enhance the processing speed of microbial samples, while reducing labor and minimizing errors from manual handling5. However, these instruments typically address only specific steps within traditional methods, often requiring extensive equipment integration, occupying significant space, and incurring high costs. Therefore, there was a pressing need to develop a low-cost, universally applicable microbial culture and screening platform to compensate for the shortcomings mentioned above.
In our previous work, we successfully developed an automated, high-throughput screening platform, known as the Single-cell Microliter-droplet Culture Omics System (MISS cell, hereafter referred to as "the Omics system")6. This platform utilizes droplet microfluidic technology, which holds promise for achieving automation and integration in microbial isolation, cultivation, and picking7,8,9,10. The Omics system comprises several key modules, including a sampling module, microfluidic chip, droplet detection and collection system, enabling efficient single-cell isolation, cultivation, monoclonal screening, and collection in microbiology research. We have already utilized the Omics system to achieve high-throughput mutagenesis screening of Corynebacterium glutamicum6.
Due to the automation and high-throughput screening capabilities of the Omics system, applying it to microbial culturomics is expected to rapidly obtain a large amount of microbial data. In this protocol, we introduced the detailed operational procedure of the MISS cell, with the isolation and cultivation of human gut microbiota as an example to demonstrate the process of microbial single-cell isolation, cultivation, monoclonal detection, and screening. The operation of the Omics system is simple, and researchers only need to follow the software direction for sequential installation of micro-tubing and droplet generation microfluidic chip, parameter settings, and sample preparation.
In the software operation interface, the Omics system is divided into three main functions-isolation, cultivation, and screening. Researchers can select different stages according to the experiment. Furthermore, during the droplet screening stage, researchers can choose from two detection modes: fluorescent signal or optical density. The software provides real-time visualization of the droplet screening process. Finally, researchers have the flexibility to configure parameters such as culture conditions, detected wavelength, and the number of collection wells based on their specific experimental demands, and they can pause the instrument anytime to carry out other operations. The MISS cell is a microbe-friendly, high-throughput monoclonal screening platform with simple operation and minimal reagent consumption.
Protocol
All study procedures are compliant with all relevant ethical regulations. Procedures were approved by Science and Technology Ethics Committee of Tsinghua University. For studying human gut microbiota, stool samples were collected from a healthy adult with no significant medical conditions, who gave written informed consent.
1. Instrument installation
- Place the Omics system instrument in a clean or sterile environment (such as a sterile room or anaerobic bench). The instrument is a precision device, and when placing it in the facility, consider the following:
- Maintain the instrument under normal pressure and temperature.
- Keep the instrument away from strong electric fields, magnetic fields, and heat radiation sources.
- Ensure that the instrument placement area exceeds dimensions of 2,500 mm (D) x 1,500 mm (W) x 2,000 mm (H).
- Maintain environmental humidity of the instrument below 60%.
2. Preparations
- Sequentially turn on the power for the Omics system, the computer, and the Omics system operating software.
- MISS cell micro-tubing and droplet generation microfluidic chip installation:
- Open the door of the droplet generation and cultivation chamber (Figure 1A) and vertically remove the protective cover for the micro-tubing and droplet generation microfluidic chip. Use a disposable syringe to add 10 mL of sterile distilled water to the humidifier inside the droplet cultivation chamber (Figure 1C) and reinstall the protective cover for the micro-tubing and droplet generation microfluidic chip.
- Open the sterile packaging of the micro-tubing and droplet generation microfluidic chip and vertically place it directly above the cultivation chamber (Figure 1C).
- On the software interface, click on Installation (Figure 2A). At this point, a pop-up window appears with the prompt, Confirm the replacement of micro-tubing and droplet generation microfluidic chip? Click Yes to start the installation.
- Take out the air bubble remover and fix it upside down on the air bubble remover placement in the droplet generation and cultivation chamber. Be careful not to press the droplet inlet and outlet tubes of the air bubble remover (Figure 1E).
- Attach the droplet outlet tube of the air bubble remover to the clamping valve below it and let it pass through the hole, which is directed to the droplet detection and collection chamber (Figure 1B).
- Open the door of droplet detection and collection chamber, vertically insert the detection tube, already connected to the droplet outlet tube, into the detection socket, and ensure that the detection tube is fully inserted (Figure 1D).
NOTE: When inserting the detection tube, insert it vertically without any bends on the tube. - Tighten the screw that secures the detection tube in a clockwise direction. After confirming that the detection tube is fully inserted and secured, close the door of the droplet detection and collection chamber.
- From the micro-tubing and droplet generation microfluidic chip, there are 10 silicone tubes, each labeled with a number (L01-L10). Connect each labeled tube to the corresponding numbered clamp valve (01-10) (Figure 1C).
- Connect the quick connector from the micro-tubing and droplet generation microfluidic chip to the corresponding port on the Omics system: C1 to O1, C2 to O2, C4 to O4, and CF to OF.
NOTE: The installation of the micro-tubing and droplet generation microfluidic chip is complete. C3 does not need to be connected to O3. - After the micro-tubing and droplet generation microfluidic chip installation is completed, a pop-up window appears prompting Droplet tubing clamping valve is opened. Click OK when the micro-tubing and droplet generation microfluidic chip installation is completed. After ensuring all silicone tubes from the micro-tubing and droplet generation microfluidic chip are attached to the corresponding clamp valve, click OK.
- Instrument initialization
- Before performing initialization, click on the Setting interface (Figure 2B) to configure relevant parameters: detection mode (OD-based or fluorescence-based detection; OD here), incubation temperature (37 °C) and time (30 days = 720 h), speed of agitator (20 rpm), the wavelength of OD detection (600 nm), and the excitation and emission wavelength of fluorescence detection.
NOTE: Parameters used for the isolation and cultivation of human gut microbiota in this protocol are given in parentheses. When setting the parameters, the cultivation temperature should be between 5 °C and 50 °C. When selecting the detection mode, the optical fiber must be replaced if the fiber on the droplet detection module is not the same (see step 2.3.2). When configuring parameters, the oil phase reference value (basic spectral) is automatically identified by the software, eliminating the need for manual adjustments. - The replacement of the detected optical fiber
- Remove the detected fiber from the fiber holder (unscrew counterclockwise) and loosen the fiber-fixing screw on the droplet module (Figure 1D).
- If the optical fiber will not be used, remove it from the module, insert it into the fiber holder, and tighten it. Insert the detected fiber into the detection port, and tighten the fiber-fixing screw on the module. The replacement of the optical fiber is finished.
- Turn to the Home interface and click Initialize to let the Omics system perform a self-check of its components, including the injection pump, temperature settings, waste liquid discharge testing, screening module, and droplet detection module.
- During initialization, perform waste liquid discharge testing from the droplet detection module by injecting 1 mL of 75% alcohol into the waste liquid port and observing whether the liquid flows out normally.
- For the screening module test, place a 96-well plate on the plate placement and observe whether the plate movement is normal.
- Before performing initialization, click on the Setting interface (Figure 2B) to configure relevant parameters: detection mode (OD-based or fluorescence-based detection; OD here), incubation temperature (37 °C) and time (30 days = 720 h), speed of agitator (20 rpm), the wavelength of OD detection (600 nm), and the excitation and emission wavelength of fluorescence detection.
3. Droplet generation
- Collection and processing of human gut microbiota samples
- Prepare a chamber pot and stool containers, wash hands, and wear gloves to collect fresh stool samples.
NOTE: When collecting stool samples, avoid urine contamination as much as possible. It is better to urinate beforehand, and place the stool into a clean, dry container. - Aseptically collect the appropriate amount of mid-segment stool and seal it in sterilized cryovials (approximately 3-5 g per vial). Place the vials on ice immediately for subsequent aliquoting and labeling.
NOTE: If the stool sample is large or cannot be collected immediately, it should be collected within 2 h at most. - In the anaerobic bench, use a sterile swab or stool sampling tool to collect a mid-segment sample.
NOTE: The surface layer of stool contains shed intestinal mucosal cells and is prone to external contamination; after exposure to air, some microbial DNA begins to degrade. - Transfer the collected stool samples into 2 mL sterile microcentrifuge tubes or sterile cryovials, with each tube containing 0.5-2.0 g of stool. Prepare two aliquots for freezing per sample.
- Resuspend the fresh stool sample in sterile physiological saline solution, with each 100 mg of the stool diluted in 1 mL of solution. Thoroughly mix the stool until no large particles are visible.
- After natural sedimentation for 10 min, sequentially filter the supernatant through sterile mesh filters with pore sizes of 200 mesh (0.075 mm), 400 mesh (0.038 mm), and 800 mesh (0.018 mm) to remove undigested food and smaller particulate matter. Finally, collect the filtrate in sterile centrifuge tubes.
- Take 10 µL of the filtered fecal suspension and determine the microbial concentration using a hemocytometer and an inverted fluorescence microscope.
NOTE: After filtering the fecal sample supernatant through different mesh sizes, some smaller fecal particles remain. Therefore, when determining the microbial concentration, active particles observed under the microscope are considered as microorganisms, which only allows for an approximate calculation of the microbial concentration. - Transfer the fecal suspension to a 1.5 mL sterile centrifuge tube and label it with the date, microbial concentration, and sample name. Reserve some for subsequent experiments, and store the rest at 4 °C as future use.
NOTE: Promptly record sample information (sample name, collection time) to ensure that samples are collected at the same time (considering mammalian intestinal microbial temporal rhythm changes). The entire sample collection and processing should be carried out in an anaerobic environment.
- Prepare a chamber pot and stool containers, wash hands, and wear gloves to collect fresh stool samples.
- Preparation for the initial fecal suspension
- Prepare Brain Heart Broth (BHI) medium according to the manufacturer's protocol and sterilize it by autoclaving at 121 °C for 15 min.
- Take the fecal suspension from step 3.1.8 and perform serial dilutions with BHI medium to achieve a concentration of ~50 cells/mL.
NOTE: To fill the sample bottle, prepare at least 40 mL of fecal suspension. - Ensuring a small magnetic stirring bar is at the bottom, pour the diluted fecal suspension into the sample bottle up to the sample adding position. Screw the cap and tighten it. Next, insert the quick connector A into quick connector B to complete the sample loading process (Figure 3A).
- Place the sample bottle into the designated position and separate quick connectors A and B from the sample bottle. Connect the quick connector A of the sample bottle to the O3 port on the Omics system, and the C3 connector on the micro-tubing and droplet generation microfluidic chip connect to the quick connector B. Close the door of droplet generation and cultivation chamber (Figure 3B).
- Droplet generation
- Select the desired number of droplet tubings to be generated on the software's home interface (Figure 2A).
NOTE: Each run can produce up to 10 droplet tubings, with each tube generating approximately 5,000 droplets. - Click Produce in the software's home interface to start single-cell droplet generation.
NOTE: Confirm whether the discharge of waste liquid pump is working or not. During the droplet generation, each droplet has a volume of 2.0 µL. See the discussion section for a description of droplet generation. - Wait for the beeper alarm to indicate that the droplet generation is finished. Close the clamp on the C3 connector (Figure 1E) and remove the sample bottle.
- Select the desired number of droplet tubings to be generated on the software's home interface (Figure 2A).
4. Droplet cultivation
- Select the same droplet tubing number as during droplet generation on the software's home interface, click Culture, confirm the cultivation time and temperature, and begin the process. Monitor the progress bar on the home interface that shows the progress of cultivation and remaining time.
- Wait for the beeper alarm to indicate that the droplet cultivation is finished. If the cultivation time must be extended, adjust the time directly on the Setting interface.
5. Droplet screening
- Press the UV button on the Omics system to turn on ultraviolet (UV) light (Figure 1A), irradiate the droplet detection and collection chamber for 30 min, and then turn off the UV light.
NOTE: Before turning on the UV light, ensure the door of droplet detection and collection chamber is closed. - In a super-clean bench, open all 96-well plates used to collect droplets and stack them on top of each other without lids, numbering them sequentially from bottom to top. Ensure that the top well plate is covered with a lid.
NOTE: Each run can accommodate up to ten 96-well plates, and the number of plates depends on the total number of droplets. - Open the door of the droplet detection and collection chamber, place the well plates in the designated positions (Figure 1D), take out the lid from the top well plate, and close the door of the chamber.
- Turn on the UV light on the Omics system for 30 min to perform secondary sterilization.
- Installation of the air bubble remover
- Remove the air bubble remover from its placement position, unscrew the cap, and remove the butterfly-shaped screw from the cap (Figure 3C).
- Pour 200 mL of the air bubble removal oil into the air bubble remover, screw the bottle tightly with the cap of the air bubble remover on the Omics system, and then fix the remover upside down on the air bubble remover placement. The installation of the air bubble remover is complete.
NOTE: When fixing the air bubble remover on the placement, ensure that no oil leaks out. If any leakage occurs, tighten the lid.
- Select the droplet tubing for sorting on the home interface, click Sorting, input the number of well-plates to be collected, and then start the process. After droplet screening begins, observe the process displays area, which shows real-time measurements of droplet optical density (OD) or fluorescence values.
- Analyze approximately 20-30 droplets to check the OD value. For example, if the majority are found to have an OD value of ~0.2, which corresponds to the OD value of empty droplets, based on the Poisson distribution, set the lower OD threshold to 0.5, and the upper OD threshold to 4.0. The droplets within this range will be automatically collected into 96-well plates (Figure 4A).
NOTE: According to Beer-Lambert Law, the OD value of empty droplets is determined by the culture medium composition within the droplets. Typically, the lower OD threshold is set 0.2-0.3 units higher than the OD value of empty droplets to ensure a clear distinction between empty droplets and droplets containing microorganisms. - Wait for the beeper alarm to indicate that the droplet screening and collection is finished. Open the door of the droplet detection and collection chamber, put the well plate lid on the top well plate, and then take all well plates out of the chamber together to conduct the subsequent sequencing and backup.
6. Data export and display of heatmaps
- Click Export data to save the collected droplet signal data (Figure 4A,B).
- Click Heat map, select the droplet collection data file, and observe the OD values of droplets collected in the microplate displayed by the software. Visualize these OD values as a heatmap, where color intensity corresponds to the OD distribution across the wells, providing a clear and intuitive representation of the collected monoclonal OD values (Figure 4A,C).
7. Cleaning of the MISS cell
- After completing the experiment, select the droplet tubing that requires cleaning, and click Clean to start instrument cleaning.
8. Microbial monoclonal backup and sequencing sample preparation
- Sequencing sample preparation
- In the anaerobic bench, add 100 µL of BHI medium to each well of the collected droplet plate, mix well by pipetting, and then take 10 µL from each well and transfer it all to one 15 mL sterile tube. Vortex to obtain a mixed microbial suspension.
- Add 5 mL of phosphate-buffered saline to the mixed microbial suspension, centrifuge at 1,000 × g for 10 min, remove the supernatant, and place the microbial pellet in liquid nitrogen for rapid freezing. Sequencing sample preparation is complete.
- Use 16S rDNA amplicon sequencing methods targeting the V3 and V4 domain of the 16S rDNA. The specific sequencing primers used are as follows: 341F: ACTCCTACGGGAGGCAGCA and 806R: GGACTACHVGGGTWTCTAAT.
- Microbial monoclonal sample cryopreservation:
- After collecting 10 µL of the sample from each well in the droplet plates (from step 8.1.1), add 30 µL of glycerol to each well. Place the plates under -80 °C for microbial strain preservation.
Results
The human gut microbiota, constituting the predominant microbial community, is estimated to harbor approximately 4 × 1013 microorganisms in the gut, showcasing its vast numbers and complex composition11. In this study, we aimed to isolate and culture gut microbiota and used the solid plate method as a control to demonstrate the high-throughput performance of the MISS cell.
First, we used the same fecal suspension to compare the single-cell isolation throughput of both methods. In the MISS cell, we used low microbial concentrations, where the distribution probability of microorganisms in the droplets could be calculated based on Poisson distribution: P(λ,x) = λxe-λ/x!, where λ is the average number of cells over the droplets, which can be calculated by multiplying the microbial concentration and the droplet volume; x is the number of cells encapsulated in the droplets. Here, we utilized an initial microbial suspension concentration of = 0.1 (initial microbial concentration is 50 cells/mL and the droplet volume is 2.0 µL), indicating the probability of empty droplets, single-cell droplets, and multi-cell droplets occurring are 90.5% (x = 0), 9.1% (x = 1), and 0.4% (x ≥ 2), respectively.
In the Omics system, we generated approximately 30,000 droplets at a rate of 5,000 droplets/h and cultivated them in six polytetrafluoroethylene tubes (O.D. 1.67 mm, I.D. 1.07 mm) for 30 days. Ultimately, we screened the droplets through the detection module by using the OD detection mode to collect bacteria-containing droplets in 96-well plates and obtained 1,057 target monoclonal strains. In contrast, through the solid plate culture method, the concentration of the solid agar plate was 3.0 × 103 cells/mL with a total of ten 100 mm Petri dishes (each plate with 100 µL of the initial microbial suspension). After 30 days of cultivation, 536 colonies were picked from the plates. The Omics system yielded 1.97-fold more monoclonal clones than the solid plate method. This indicated that single-cell monoclonal cultivation in microfluidic droplets can effectively isolate microorganisms while eliminating competitive inhibition between colonies.
Next, we performed 16S sequencing analysis on all monoclonal strains and compared the species diversity obtained from the aforementioned two methods. In terms of species diversity at the family level, the same 34 families could be enriched via both methods. Specifically, the Omics system further enriched four families: Bacteroidales unclassified, Bacillales Thermoactinomycetaceae, Burkholderiales Comamonadaceae, and Enterobacterales unclassified (Figure 5B) and easily enriched the lower abundance of Clostridiales Family_XI, Clostridiales Acidaminococcaceae, Desulfovibrionales Desulfovibrionaceae, and Enterobacterales Enterobacteriaceae in the original microbial suspension (Figure 5D).
At the genus level, 74 microbial genera could be enriched by both methods, while the MISS cell method additionally enriched 13 microbial genera: Bacillaceae Bacillus, Bacillaceae Oceanobacillus, Bacillaceae Pseudogracilibacillus, Thermoactinomycetaceae Kroppenstedtia, Peptoniphilaceae Phocea, Clostridiaceae Anaerosalibacter, Peptoniphilaceae Ezakiella, Peptoniphilaceae W5053, XI unclassified, Clostridiaceae Clostridioides, Comamonadaceae Pelomonas, and two unclassified microbial species (Figure 5C). Among them, the genera Enterococcaceae Enterococcus and Acidaminococcaceae Phascolarctobacterium, which were at lower abundance in the original microbial suspension, were easily enriched using the Omics system (Figure 5E). As expected, these two genera belonged to the family of Enterococcaceae and Acidaminococcaceae, respectively, where we observed the same results from the family analysis. Overall, at the family and genus levels, the species enrichment of MISS cell culture method increased by 30.6% and 37.9%, respectively, compared to the solid-plate culture method. These results showed that the MISS cell culture method provided better growth conditions for those strains that were present in low proportions or had poor growth performance in the original microbial suspension.

Figure 1: Structure and essential components of the MISS cell. (A) Exterior of the MISS cell. 1. Droplet generation and cultivation chamber, 2. Droplet detection and collection chamber, 3. Lighting and UV buttons of the droplet collection chamber. (B) Interior of the droplet generation and cultivation chamber. Droplet generation, droplet incubation, and air bubble removal for droplet screening are carried out in this chamber. 4. Droplet cultivation chamber, 5. Clamping valves for clamping silicone tubes from the Micro-tubing and droplet generation microfluidic chip; the clamping valves are numbered sequentially from 1 to 10, left to right, 6. Air bubble remover placement, 7. Ports for droplet generation and screening (O1-O4): the oil, sample, and gas phase are connected to the MISS cell through these ports. 8. Waste port (OF), 9. Clamping valve for clamping the droplet outlet tube of the air bubble remover, 10. The opening leading to the droplet detection and collection chamber, 11. Sample bottle placement; there is a magnetic stirrer underneath the placement, which can be used to control the stirring speed of the sample.(C) Overhead view of the droplet cultivation chamber. Inside the chamber, there is a water inlet port for a humidifier. A syringe is used to add 10 mL of sterile distilled water, the protective cover and the micro-tubing and droplet generation microfluidic chip are installed, and the silicone tubes from the micro-tubing are clamped to the corresponding clamp valves, as shown in the figure on the right. 12. A water inlet port for the humidifier in the droplet cultivation chamber, 13. The micro-tubing and droplet generation microfluidic chip. (D) Interior of the droplet detection and collection chamber. The image on the left shows three well-plate placements and the handling robotic arm moving the well plate between the placements. The image on the right shows the zoomed-in view of the droplet detection and collection module (red rectangle in the image on the left). Placement of 96 well-plates 14. before, 15. during, and 16. after droplet collection. 17. 96 well-plate handling robotic arm. During droplet detection and collection, the robotic arm moves one 96-well plate to the well-plate placement near the droplet detection module. After droplet collection is complete, the well plate is moved to another placement and the same operation is continued until the process is complete. 18. Droplet detection and collection module. 19. The fiber holder for the detection fiber. There are two detection fibers (OD-based and fluorescence-based detection). When one detection fiber is used, the other is inserted into the fiber holder. 20. The detection tube. This tube is already connected to the droplet outlet tube of the air bubble remover. 21. The OD-based and the fluorescence-based detection fiber; the detection fibers are labeled with their respective names. 22. The optical fiber of light source. 23. The detection socket where the detection tube is inserted. 24. The fiber-fixing screw to secure the detection fiber. 25. The screw that secures the detection tube. Tighten the screw after inserting the detection tube into the detection hole properly. 26. The waste liquid port. 27. Waste tube for discharge of droplets outside the collection signal. (E) Installation of the micro-tubing, droplet generation microfluidic chip, and air bubble remover into the corresponding placement. The four tubings (C1, C2, C4, and CF) are respectively connected to the corresponding ports of the MISS cell. (O1, O2, O4, and OF). 28. The protective cover for the micro-tubing and droplet generation microfluidic chip. 29. The air bubble remover. 30. The droplet inlet tube to the air bubble remover. 31. The droplet outlet tube from the air bubble remover. 32. Clamping valve on the C3 connector. Abbreviations: MISS = Single-cell Microliter-droplet Culture Omics System; OD = optical density. Please click here to view a larger version of this figure.
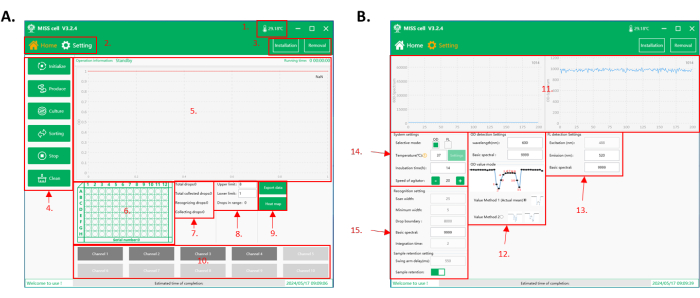
Figure 2: Operation software interface of the MISS cell. (A) The home interface of the software. 1. Temperature in the operation chamber. 2. Function interface. There are two interfaces to operate: home interface and parameter setting interface. 3. Micro-tubing and droplet generation microfluidic chip installation and removal buttons. 4. Function selection: there are six functions to choose from: Initialize, Produce, Culture, Sorting, Stop, and Clean. 5. Process display area: operation information, running time, and collected droplet data are shown in this area. 6. 96-well plate display area: real-time display of the current collection plate number and droplet collection well position. 7. Display area of droplet detection and collection data. 8. Sorting upper and lower limit setting and droplet count display area. The range of the collected droplet is set according to the droplet signal (OD/ fluorescent intensity). The MISS cell system counts the total collected droplet number. 9. Buttons for exporting collection data and displaying heatmaps of plates. 10. Droplet tubing area. Select the number of tubings to be operated for droplet generation, droplet cultivation, droplet screening, and cleaning. (B) The parameter setting interface of the software. 11. Real-time display of spectral values detected during droplet sorting (OD/fluorescent intensity). 12. OD detection setup parameters, including the detection wavelength, the basic spectral value, and the OD mode. There are two OD modes: effective mean value (value method 1), which calculates the mean value of the droplet spectral value; and the minimum value (value method 2), which takes the minimum value of the droplet spectral value as the signal. 13. Fluorescence detection setup parameters, including excitation (device configuration), emission wavelength (350-800 nm), and base spectral value. 14. System setup parameters, including selection of detection mode (OD/fluorescent intensity), incubation temperature, incubation time, and stirring speed of the sample in the reagent bottle. 15. Parameters for droplet identification and sample collection settings. Abbreviations: MISS = Single-cell Microliter-droplet Culture Omics System; OD = optical density. Please click here to view a larger version of this figure.

Figure 3: Installation of the sample bottle and air bubble remover. (A) The MISS cell sample bottle. The microbial suspension is added to the sample bottle to the sample adding position. The lid is tightened immediately and the quick connectors A and B connected. Finally, the sample bottle is placed in the sample bottle placement on the MISS cell. (B) The installation of the sample bottle. The quick connectors A and B are connected to the O3 port on the MISS cell and the C3 connector from the micro-tubing and droplet generation microfluidic chip, respectively. (C) The Installation of the air bubble remover. When installing the air bubble remover, the butterfly-shaped screw is removed from the lid first before installing the bottle containing the air bubble removal oil. Abbreviation: MISS = Single-cell Microliter-droplet Culture Omics System Please click here to view a larger version of this figure.
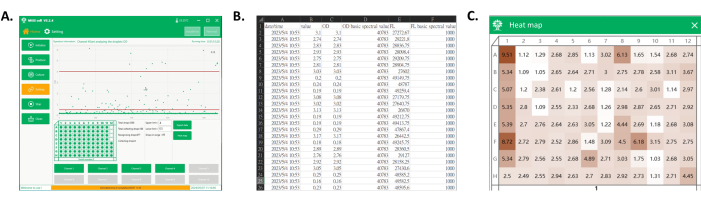
Figure 4: Data export and the heatmap of the collected well-plate. (A) The main interface of the software during droplet detection and collection. In the process display area, each droplet signal is shown, and the droplets in the desired range are collected to the well plate. (B) Screenshot of part of the exported data. The exported data include the spectral signal of collected droplets (columns C and E) and their detection times (column A). (C) Screenshot of the heatmap of the plate. Based on the signals from the collected droplets, the values are normalized to obtain a heatmap of the plate, which could be used later to differentiate the culture performance of monoclonal strains of each clone based on color. Please click here to view a larger version of this figure.
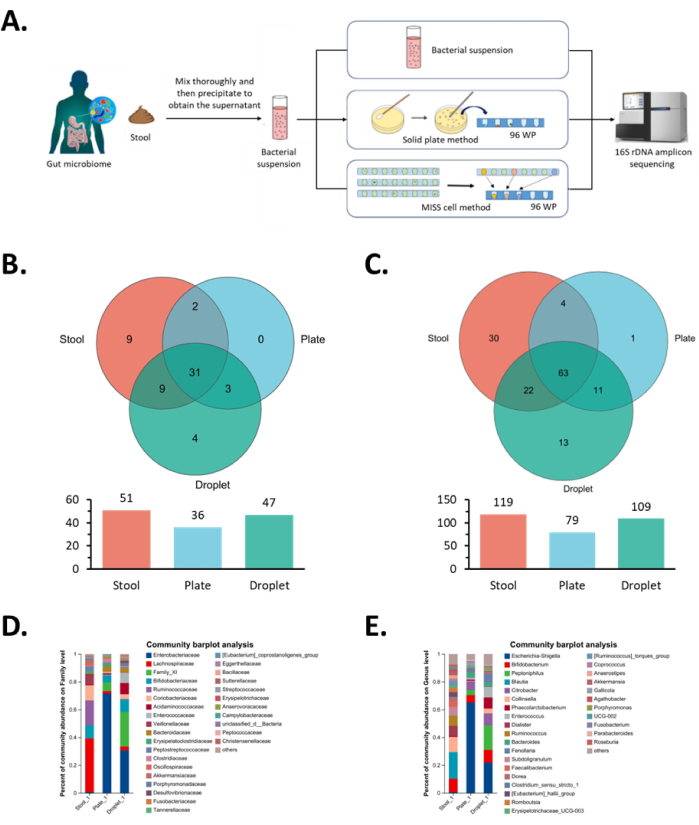
Figure 5: Results of monoclonal isolation and cultivation of intestinal microorganisms in the MISS cell. (A) Workflow diagram for isolation, cultivation, and identification of gut microbiota. The initial microbial suspension, the solid plate method, and the MISS cell method are used to isolate and culture the same gut microbiota followed by sequencing analysis. (B,D) Family-level analysis of monoclonal colonies obtained from the isolation and cultivation of gut microbiota, where B shows the Venn diagram analysis and D shows the community composition analysis. (C,E) Genus-level analysis of monoclonal colonies obtained from the isolation and cultivation of gut microbiota, where C shows the Venn diagram analysis and E shows the community composition analysis. Abbreviation: MISS = Single-cell Microliter-droplet Culture Omics System. Please click here to view a larger version of this figure.
Discussion
This protocol outlines the operation of the MISS cell for automated and high-throughput microbial monoclonal isolation, cultivation, detection, and collection. Compared to traditional methods by which only ~20%-30% of gut microbiota could be isolated and cultured2,12, the number of monoclonal clones obtained using the Omics system was 1.97 fold higher than those obtained from solid plates. This comparison reveals that the MISS cell has advantages in single-cell isolation, monoclonal cultivation, and screening.
For single-cell isolation, the Omics system obtained single-cell droplets according to Poisson distribution, which ensured good monoclonality. Additionally, the Omics system generated droplets at a rate of up to 5,000 droplets/h, enabling high-throughput single-cell isolation while significantly reducing reagent consumption due to the small droplet size. During the droplet generation, a flow-focusing channel on the micro-tubing was used. The Omics system uses the injection pump to push the oil phase, sample solution, and gas into the flow-focusing channel and form droplets. Users can observe whether the droplets are successfully generated through the channel or by looking at the droplet tubing directly. The silicone tubes from the micro-tubing and droplet generation microfluidic chip are connected to the end of the 10 droplet tubings. When enough droplets are generated in one droplet tubing (~5,000 droplets), the clamping valve will automatically clamp the silicone tubes of the tubing and continue to generate the droplet into the next tubing.
For the monoclonal strain cultivation, microorganisms in the droplets benefited from excellent gas exchange performance, high parallel cultivation, and efficient mass transfer, providing optimal growth conditions for low-abundance or difficult-to-grow strains. For the monoclonal strain screening, the Omics system supported fluorescence and OD-based biomass detection due to which the detection could be based on microbial growth performance and the expression level of a specific gene or protein of interest13. Furthermore, the Omics system employed gravity-induced droplet deposition into well plates. By combining this passive screening method with a swing arm, it achieved stable and highly accurate droplet screening, making it a bio-friendly screening platform. Moreover, the Omics system achieved a fully automated process from the isolation of a single microorganism to the final clonal screening, which reduced labor and time costs.
Given the performance of the Omics system, it could be used for microbial culturomics and microbial mutagenesis screening, as well as other applications such as high-producing strain selection and mining of key functional genes6. To obtain accurate experimental results, researchers can optimize the experimental conditions based on the species to be isolated, cultured, and screened. First, the generation of single-cell droplets in the Omics system follows the Poisson distribution, where a low λ value ensures that the generated droplets are predominantly empty or single-cell droplets. In our previous work, we validated the optimal microbial concentration range for generating single-cell droplets in the Omics system to be 16.8-69.7 cells/mL (λ = 0.035-0.145)6. When preparing sample suspensions, it is crucial to dilute within this concentration range to enhance monoclonality accuracy. Second, before using the Omics system, it is necessary to optimize the microbial growth conditions. Based on the microorganism's oxygen requirements, researchers can choose either nitrogen or oxygen as the gas phase for droplet generation. Third, during droplet detection, the absorbance or fluorescence is determined by the intensity of light received after illuminating the droplets. Consequently, the numerical values obtained from the same sample might differ between droplet detection and spectrophotometer measurement due to differences in sample thickness, as observed in our previous work14. Therefore, it is recommended to establish a calibration curve for experimental samples before conducting the experiments.
Moreover, we are also improving the performance of the Omics system to the application of other species such as mammalian cells, including the biocompatibility of oil phase to cells (e.g., fluorinated oil has higher gas solubility and is more suitable for mammalian cell culture15,16), the effect of two-phase flow rate on cellular shear force17,18,19, and the optimization of droplet size13,20. These improvements may significantly facilitate the use of the Omics system in biomedical research such as in cell line development, single-B cell antibody screening, and hybridoma antibody discovery. Based on the three core operational modules of the Omics system-droplet generation, cultivation, and screening, researchers can choose the suitable modules according to the demands of the experiment. Additionally, the droplet injection and droplet splitting module can be added to the system described herein to perform reagent addition or establish strain libraries.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported by the Research and Development projects in key areas of Guangdong Province (2024B1111130002), Research and Development projects of Hebei Province (22375503D), and the Opening Project of Anhui Engineering Laboratory for Industrial Microbiology Molecular Breeding (Grant ELMB-07).
Materials
| Name | Company | Catalog Number | Comments |
| 100 mm Petri dish | Merck KGaA, Darmstadt, Germany | P5731-500EA | For solid plate preparation |
| 30 mL Stool Containers | Boen Healthcare Co., Ltd | 611101 | For collecting the stool samples |
| 37 °C constant temperature incubator | Shanghai Yiheng Technology Co., Ltd. | LRH-150 | Cultivate the solid plate in the incubator |
| 96-well Clear Flat Bottom Polystyrene TC-treated Microplates | Corning | 3599 | For well plate movement detection and droplet collection |
| Agar | Becton, Dickinson and Company | 214010 | For solid plate preparation |
| Air bubble removal oil | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISS cell-S-oil | The oil in the air bubble remover during droplet screening |
| Air bubble remover | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISS cell-S | Exclude the gas phase between droplets before performing droplet detection and collection |
| Anaerobic bench | Argon and Nitrogen Space Equipment Business Department, Haiyu Town, Changshu City | VGB-4CM | For aseptic operation and UV sterilization under anaerobic condition |
| Autoclave | Puhexi Health and Medical Equipment Co., Ltd. | MLS-830L | For autoclaving BHI medium, EP tube, and so on. |
| Brain Heart Infusion (BHI) Broth | Qingdao High-tech Industrial Park Haibo Biotechnology Co., Ltd | HB8297-1 | Components of the BHI medium The ingredient list: 38.5 g/L BHI Broth in distilled water |
| Cell Spreader | Merck KGaA, Darmstadt, Germany | HS8151 | Inoculate the microbial solution onto the solid plate |
| Centrifuge tube, 15 mL | Beijing Xinhengyan Technology Co., Ltd | HB53397 | For microbial solution preparation |
| Computer | Lenovo | E450 | Software installation and MISS cell control |
| Cryovial | Thermo Fisher | 2.0 mL | For stool preservation |
| Distilled water | Beijing Mreda Technology Co., Ltd. | M306444-100ml | Add into humidifier to keep the humidity in droplet cultivation chamber |
| EP tube | Thermo Fisher | 2.0 mL | For collecting the stool samples |
| Fluorescent inverted microscope | Olympus Life Science (LS) | CKX53 | Check and calculate the microbial concentration |
| Glycerol | GENERAL-REAGENT | G66258A | For strain preservation |
| Hemocytometer | Acmec | AYA0810-1ea | Calculate the microbial concentration |
| KCl | Ambeed | A442876 | Components of phosphate buffered saline (PBS solution)The ingredient list: 8 g/L NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 in distilled water |
| KH2PO4 | MACKLIN | P815661 | Components of phosphate buffered saline (PBS solution)The ingredient list: 8 g/L NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 in distilled water |
| Mesh filter | Anping Jiufeng Wire Mesh Manufacturing Co., Ltd | 200 mesh (0.075 mm), 400 mesh (0.038 mm), 800 mesh (0.018 mm) | Remove undigested food and smaller particulate matter from the stool samples |
| Micro-tubing and droplet generation microfluidic chip | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISC-B2 | For droplet generation and droplet incubation |
| MISS cell oil | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISS cell-BOS-B | The oil phase for droplet microfluidics |
| MISS cell software | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISS cell V3.2.4 | Perform experimental operations on the MISS cell instrument |
| Na2HPO4 | Solarbio | D7292 | Components of phosphate buffered saline (PBS solution)The ingredient list: 8 g/L NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4 in distilled water |
| NaCl | GENERAL-REAGENT | G81793J | Components of the physiological saline solution The ingredient list: 9 g/L NaCl in distilled water |
| Pipette | eppendorf | 2.5 μL, 10 μL, 100μL, 1000μL | For liquid handling |
| Polytetrafluoroethylene tube | Shenzhen WOER Heat-shrinkable Material Co., Ltd. | 3401000141 | For droplet incubation. This material was already included in micro-tubing and droplet generation microfluidic chip |
| Sample bottle | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISS cell-bottle | Sampling of microbial solution |
| Single Cell Microliter-droplet Culture Omics System (MISS cell) | Luoyang TMAXTREE Biotechnology Co., Ltd. | MISS cell-G3f | Performing the microbial monoclonal isolation, cultivation, detection and collection |
| Superspeed Centrifuge | Thermo Fisher | Sorvall Lynx 4000 | Prepare the microbial solution for sequencing |
| Syringe | Jiangsu Zhiyu Medical Instructment Co., Ltd | 10 mL | Draw the distilled water and inject it into the humidifier in droplet cultivation chamber |
| Ultra low temperature refrigerator | SANYO Ultra-low | MDF-U4086S | For strain preservation (-80 °C) |
References
- Hahn, M. W., Koll, U., Schmidt, J., Hurst, C. J. Isolation and cultivation of bacteria. The structure and function of aquatic microbial communities. , 313-351 (2019).
- Xu, M. Q., Pan, F., Peng, L. H., Yang, Y. S. Advances in the isolation, cultivation, and identification of gut microbes. Mil Med Res. 11 (1), 34 (2024).
- Lattermann, C., Büchs, J. Microscale and miniscale fermentation and screening. Curr Opin Biotechnol. 35, 1-6 (2015).
- Huang, Y., et al. High-throughput microbial culturomics using automation and machine learning. Nat Biotechnol. 41 (10), 1424-1433 (2023).
- Brehm-Stecher Byron, F., Johnson Eric, A. Single-cell microbiology: Tools, technologies, and applications. Microbiol Mol Biol Rev. 68 (3), 538-559 (2004).
- Jian, X., et al. Single-cell microliter-droplet screening system (miss cell): An integrated platform for automated high-throughput microbial monoclonal cultivation and picking. Biotechnol Bioeng. 120 (3), 778-792 (2023).
- Hu, B., et al. One cell at a time: Droplet-based microbial cultivation, screening and sequencing. Mar Life Sci Technol. 3 (2), 169-188 (2021).
- Leygeber, M., et al. Analyzing microbial population heterogeneity-expanding the toolbox of microfluidic single-cell cultivations. J Mol Biol. 431 (23), 4569-4588 (2019).
- He, Z., Wu, H., Yan, X., Liu, W. Recent advances in droplet microfluidics for microbiology. Chinese Chemical Letters. 33 (4), 1729-1742 (2022).
- Kaminski, T. S., Garstecki, P. Controlled droplet microfluidic systems for multistep chemical and biological assays. Chem Soc Rev. 46 (20), 6210-6226 (2017).
- Sender, R., Fuchs, S., Milo, R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 164 (3), 337-340 (2016).
- Simrén, M., et al. Intestinal microbiota in functional bowel disorders: A rome foundation report. Gut. 62 (1), 159-176 (2013).
- Periyannan Rajeswari, P. K., Joensson, H. N., Andersson-Svahn, H. Droplet size influences division of mammalian cell factories in droplet microfluidic cultivation. Electrophoresis. 38 (2), 305-310 (2017).
- Jian, X., et al. Microbial microdroplet culture system (mmc): An integrated platform for automated, high-throughput microbial cultivation and adaptive evolution. Biotechnol Bioeng. 117 (6), 1724-1737 (2020).
- Baret, J. -. C. Surfactants in droplet-based microfluidics. Lab on a Chip. 12 (3), 422-433 (2012).
- Kubie, L. S. The solubility of O2, CO2, and N2 in mineral oil and the transfer of carbon dioxide from oil to air. J Biol Chem. 72 (2), 545-548 (1927).
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. J Mech Behav Biomed Mater. 126, 105024 (2022).
- Yao, J., Lin, F., Kim, H. S., Park, J. The effect of oil viscosity on droplet generation rate and droplet size in a t-junction microfluidic droplet generator. Micromachines. 10 (12), 808 (2019).
- Venkateshwarlu, A., Bharti, R. P. Effects of capillary number and flow rates on the hydrodynamics of droplet generation in two-phase cross-flow microfluidic systems. Journal of the Taiwan Institute of Chemical Engineers. 129, 64-79 (2021).
- Nekouei, M., Vanapalli, S. A. Volume-of-fluid simulations in microfluidic t-junction devices: Influence of viscosity ratio on droplet size. Physics of Fluids. 29, 032007 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved