Kinetics of Addition Polymerization to Polydimethylsiloxane
Overview
Source: Kerry M. Dooley and Michael G. Benton, Department of Chemical Engineering, Louisiana State University, Baton Rouge, LA
Polymers are molecules consisting of many repeating monomer units that are chemically bonded into long chains. They exhibit a broad range of physical properties, which are affected by their chemical structure, molecular weight and degree of polymerization. The polymer industry manufactures thousands of raw materials used in a broad variety of commercial products.1,2
The goal of this video is to perform an addition polymerization reaction and then evaluate the resulting product to understand how viscosity can be used to determine polymer molecular weight. Additionally, this experiment will investigate how molecular weight can be related to monomer conversion.
Principles
Many polymers are produced in stirred tank reactors, either batch or continuous. As an example, the polymerization of poly(dimethylsiloxane) (PDMS) is shown in Figure 1. In this reaction, "Me" represents methyl groups and potassium hydroxide is the catalyst. The [Me2SiO]5 is a 5-membered ring which is opened to form the basic building block (the "link") of the polymer. The second product represents finished polymer (it reacts with something called an "endblocker" to stop growth), the first one is a still-growing ("living") polymer. All growth takes place while the chain is attached to the catalyst.
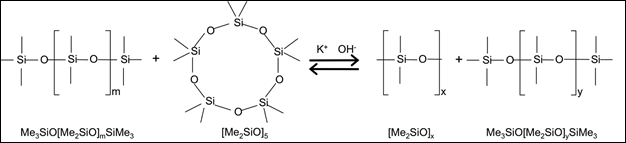
Figure 1: Ring opening polymerization of PDMS.
This is a type of addition polymerization, which is discussed in many kinetics3 and all basic polymer-science textbooks.4 The reaction is mostly thermoneutral and is usually run between 110 - 140 °C and atmospheric pressure. A small amount of molecular weight modifier ("endblocker") is used to stop chain growth, but the catalyst then starts a new chain. Common endblockers are dimethylsiloxanes with trimethylsiloxy end-groups. A "living" chain reacts with the endblocker, forming an end-capped "dead" polysiloxane product with a trimethyl end group.
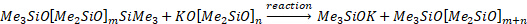
The Me3SiOK reacts with another polysiloxane to create another trimethylsiloxy end-group. The overall effect is not only the endcapping of the polymer, but also control of the chain length. Average chain lengths (m+n) between 43 - 205 are typical for industrial PDMS in which several different grades of product are synthesized. Because the monomer addition rate >> reaction rate with endblocker (otherwise you would never get to a high molecular weight), the endblocker doesn't influence the reaction kinetics, only the molecular weight distribution.
In analyzing polymerization kinetics, the most difficult step is determining the molecular weight from a physical property, such as kinematic viscosity, and calculating the fraction conversion. The viscosity-average molecular weight, which is measured in this demonstration, is an intermediate measurement with a value in between the number-average and weight-average molecular weight of the polymer. The number average molecular weight is the statistical average molecular weight and indicates that 50 % of the polymer chains are below the number average molecular weight, and 50 % are above. The weight average molecular weight is calculated from the weight fractions in which 50 % of the sample weight consists of chains of lower molecular weight and 50 % consists of chains of higher molecular weight.
Dividing the number average MW by the monomer weight gives the number average degree of polymerization, which is related to fraction conversion. The fraction conversions vs. time are used to determine the order of the reaction as learned in Physical Chemistry and Reactor Design classes.
Procedure
The system is controlled by running control sequences PS1-PS5 on a standard industrial distributed control system that is operated from a PC. The sequences open/close/adjust valves in the proper sequence and inform when and how to add components to the reactor.
1. Reactor Set-up
- Open the N2 cylinder connected to the reaction vessel.
- Run the control sequence PS1 to test the equipment.
- Then, close the manual valve to the vacuum pump to check that the system is leak-free.
- Wait 5 min and verify that the pressure does not exceed 600 mm Hg.
- Fill the system with N2. Nitrogen is needed for both safety and kinetics reasons. O2 inhibits many polymerizations, and can lead to explosions.
- Run the control sequence PS3 to add monomer to the reactor.
- When prompted by the sequence, add the catalyst solution and endblocker. through a small funnel called the "adder tank".
2. Polymer Fabrication
- Start the PS4 sequence and monitor the reaction temperature.
- Once the temperature reaches >105 °C, frequently collect liquid samples (at least every 8 min) from the sample draw point (caution: HOT, wear thermal gloves).
- Allow the polymerization reaction to run until near equilibrium. Monitor the power usage of the agitator to confirm the reaction has reached equilibrium. Once power has stopped increasing, then the reaction is close to equilibrium.
- Make sure to collect at least 7 samples.
- When done, open the CO2 tank valve and press "RXN COMPLETE" to neutralize the catalyst with CO2. This will occur as part of the PS4 sequence.
- Begin the PS5 stripping sequence.
- Open the manual valve to the vacuum pump and allow stripping to run for 15 min.
- Select "STRIPPING COMPLETE".
- Collect the low boilers from the reaction into a flask.
- Cool the reactor using the automatic cooldown process. Pumpout will be performed much later.
- Follwong the manufacturer's instructions, measure the collected samples with a rotational (cup and bob) viscometer.
- If the rotational speed is set too high, no viscosity reading will be obtained, and a lower speed is chosen. These viscosity values will be used to determine the molecular-weight distribution of the polymer.
Results
The molecular weight can be determined by empirical relationships, such as Barry's relationship for polydimethylsiloxanes with molecular weights above ~2,500.5
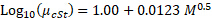
This gives the viscosity-average molecular weight. For molecular weight predictions < 2,500, interpolate the experimental data found in Kuo,6 using the kinematic viscosity of the DC-245 monomer for chain length 1. Divide the viscosity (cP) by the polymer density (g/cm3) to obtain the kinematic viscosity in cSt. Divide the viscosity average MWs by 1.6 (empirical factor for PDMS) to get the number average molecular weight, and divide this value by the monomer molecular weight to get the average chain length, (PN)avg, which includes the unreacted monomer.
To get the fraction conversion (fm), start with the mass balance for the average of PN (polymer only):
 (1)
(1)
The left-hand side is the average of PN (polymer only) up to time t, where f = fm. But the average PN that you measure includes the monomer. To account for monomer in (PN)avg, recall that by definition:3-4
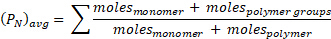
and therefore:
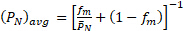 (2)
(2)
The average polymer  and (PN)avg for the entire batch are almost equal at the last batch, where fm approaches 1. Compute fm for the last point using a mass balance and the amount of low boilers that was collected. Solve for
and (PN)avg for the entire batch are almost equal at the last batch, where fm approaches 1. Compute fm for the last point using a mass balance and the amount of low boilers that was collected. Solve for  . For many addition polymerizations,
. For many addition polymerizations,  is constant for the entire batch, which allows fm to be computed at all other times from Equation 2. Also, compute the equilibrium constant K (first-order reversible kinetics model) for the reaction by mass balance.
is constant for the entire batch, which allows fm to be computed at all other times from Equation 2. Also, compute the equilibrium constant K (first-order reversible kinetics model) for the reaction by mass balance.
Once fm has been determined as a function of time, assume irreversible kinetics and determine the reaction order with respect to monomer. Use statistical analysis to determine the quality of the fits and the confidence limit on the rate constant kp. Determine the fit for first-order kinetics (expected from theory),3-4 and test if the two fits actually differ.
At similar conditions, others have reported a first-order rate constant of 10-3 s-1 for the DC-245 monomer, and a K > 60.

Figure 2. Typical polymerization results."DOP" = degree of polymerization. The MW's were computed from available data (see ref. 6) or Barry's equation (>2500).5
The workup of representative raw data is shown in Figure 2. These data are for the polymerization of Dow Corning DC-245 monomer. The reaction conditions were: 0.04 wt% catalyst solution, 12 wt% endblocker (modifier), 130 °C and 1 atm pressure. With a relatively large amount of endblocker used, the final degree of polymerization (DOP) was quite low.
In this experiment, 11.36 L of monomer were reacted, and only 15 mL low boilers were recovered, indicating that the data should follow irreversible kinetics. The fit to first-order (in monomer) kinetics is shown in Figure 3 below. The fraction conversions (f) were determined using Equations 1 and 2 with the assumption that the polymer produced is at constant chain length (PN). The resulting fit is reasonable, but not perfect. Slight deviations from the theoretically expected first-order kinetics can arise for several reasons such as diffusional effects, which is when the viscosity increases and the diffusivities decrease significantly. Two other reasons for deviations are suggested by the raw reaction temperature data (temperature oscillations affect the rate constant) and by small leaks that may be present in the pumps, reactor, and heat exchangers. If there are leaks, some O2 could get into the system and gradually inhibit the reaction.
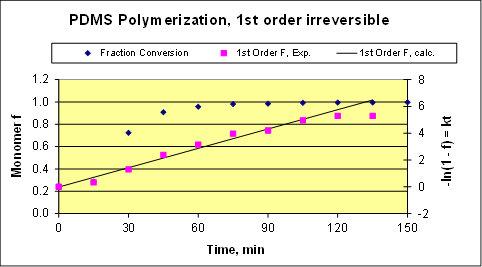
Figure 3. Kinetics analysis. "F" is the 1st order function, the solution of the batch reactor mass balance for a 1st order irreversible reaction.
Application and Summary
Polymer science provides many examples of the basic principles of chemical kinetics and reactor design. Simple rate expressions can describe fairly complex chemical processes, as in this experiment. Reactor system design must find the optimal reactor type (batch, stirred tank, plug flow, or hybrid) considering kinetics, capital costs, and molecular weight distribution. In particular, the last factor is usually the most important, because it largely defines the product. Depending on this factor alone the product can often range from a hard brittle solid to a rubber to a liquid. A bulk (no solvent) polymerization, such as the one performed in this experiment, has the advantage that subsequent processing to obtain a pure polymer is simple - just strip out the low boilers and filter out the neutralized catalyst. However, the disadvantage of bulk polymerization is that if one loses control of temperature (too high), even in a thermoneutral polymerization, other reactions will dominate and lead to "runaway", which is an uncontrolled exothermic reaction that may result in explosion. Polymerizations with higher heats of reaction are reacted either in solution, suspension (a continuous water phase is present, and the monomer is in droplet form), or in the gas phase.
The major takeaways from the experiment are how one can process raw data of an easily measurable physical property (viscosity) to ultimately determine the monomer fraction conversions and the kinetics of the reaction. Many other physical properties, e.g., density and particle light scattering, are used for this purpose in other polymerizations.
Polymers made by ring-opening polymerizations include Nylon-6 from caprolactam, acetal copolymers with ethylene oxide and dioxolane, which are used in everything from fuel tanks to sprinklers, poly(ethyleneimines), which are used in detergents and cosmetics, and many other silicon-backbone polymers.With the exception of Nylon-6, most of these polymers are commercially produced by anionic or cationic polymerizations. Other polymers that are made similarly include copolymers of styrene (especially with isoprene), isobutene-isoprene (butyl) rubber and its halogenated variants, and poly(alkyl vinyl ethers), which are typically used in paints and adhesives. For some such polymerizations, the chain terminations are so controlled that an almost homogeneous molecular-weight distribution is possible. Except for certain specialty grades, it has been found that such narrow distributions present other problems, such as extrusion difficulties.
Many polymers are vacuum-stripped as the first part of their purification to a commercial product. Among these are the poly(vinylidene chloride) copolymers, poly(chloroprene), and many grades of poly(styrene) and its copolymers such as SAN (styrene-acrylonitrile).
Silicone polymers are used in many products, including lubricants, personal care products, medical devices, antifoams, sealants, waterproof coatings, and as components of detergents, electrical insulation, and paints.8 Medical devices composed of very high molecular weight crosslinked silicone may be approved by the FDA for implantation. More common medical uses are consumables such as catheters, tubing, gastric bags, and surgical incision drains. Commercial PDMS is non-hazardous with a flash point higher than 300 °C, minimal toxicological effects, and good resistance to moderately concentrated aqueous alkali and acids.8,9 It does not corrode most common materials. But like many polymers it can oxidatively decompose, in this case above ~150 °C.
Materials List
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Rotational (cup and bob) viscometer | Brookfield | Use to measure the viscosity of polymer samples | |
| Stirred tank reactor | custom | 20 L | |
| Reactor Agitator | McMaster-Carr | 46-460 RPM; 6-blade, flat turbine (Rushton) type, ~4” diameter. | |
| Reagents | |||
| Dimethlysiloxane monomer | Dow Corning | DC-245 | specific gravity = 0.956 at 25 °C; viscosity = 4.2 cSt; m = average number of dimethylsiloxanes = 5 |
| Endblock A | Dow Corning | 10082-147 | specific gravity = 0.88 at 25°C; m = 4.5 (not counting the two end groups) |
| KOH catalyst | VWR | 470302-140 | 45 wt% solution in water |
| Nitrogen | Airgas | UHP grade | Used to blanket the system |
| Carbon dioxide | Airgas | Tech. grade | Used to neutralize the catalyst |
Viscosity and Density Data at Low Molecular Weight
Data originally from: Dow Corning.10
| MW, g/mol | 162 | 410 | 1250 | 28000 |
| Viscosity, cs, 25 °C | 0.65 | 2.0 | 10 | 1000 |
| Specific gravity, 25 °C | 0.760 | 0.872 | 0.935 | 0.970 |
References
- http://www.essentialchemicalindustry.org/polymers/polymers-an-overview.html and http://www.pslc.ws/mactest/maindir.htm (both accessed 8/22/16).
- MatWeb, Material Property Data, http://www.matweb.com/ and Plastics General Polymers Brand Name Listing, http://www.plasticsgeneral.com/BRAND-NAMES-LIST1.htm (both accessed 8/25/16).
- Fogler, F.S., Elements of Chemical Reaction Engineering, 3rd Ed., Prentice-Hall, 2001, pp. 354-382 (sections 7.1.2-7.1.5).
- Odian, G., Principles of Polymerization, 4th Ed., Wiley-VCH, 2004 (ch. 3), or Rodriguez, F., Principles of Polymers Systems, 2nd Ed., McGraw-Hill, 1982 (ch. 4); Fried, J.R., Polymer Science and Technology, Prentice-Hall, 1995 (ch. 2).
- Barry, A.J., Viscometric Investigation of Dimethylsiloxane Polymers, J. Appl. Phys., 1946, 17, 1020-1024.
- Kuo, A.C.M. Poly(dimethylsiloxane), in Polymer Data Handbook, Oxford University Press, 1999, 411.
- Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012 or the Encyclopedia of Polymer Science and Technology, 3rd Ed., Wiley-Interscience, Hoboken, 2003-04.
- http://www.dowcorning.com/content/discover/discoverchem/properties.aspx (accessed 8/25/16)
- Shin-Etsu Silicone Fluid Technical Data, Shin-Etsu Silicones of America, Akron, 2005.
- Dow Corning, Product Information, Silicon Fluids, http://www.dowcorning.com/applications/Product_Finder/Products.aspx (accessed 9/23/16).
Skip to...
Videos from this collection:

Now Playing
Kinetics of Addition Polymerization to Polydimethylsiloxane
Chemical Engineering
16.0K Views

Testing the Heat Transfer Efficiency of a Finned-tube Heat Exchanger
Chemical Engineering
17.9K Views

Using a Tray Dryer to Investigate Convective and Conductive Heat Transfer
Chemical Engineering
43.8K Views

Viscosity of Propylene Glycol Solutions
Chemical Engineering
32.5K Views

Porosimetry of a Silica Alumina Powder
Chemical Engineering
9.6K Views

Demonstration of the Power Law Model Through Extrusion
Chemical Engineering
10.0K Views

Gas Absorber
Chemical Engineering
36.5K Views

Vapor-liquid Equilibrium
Chemical Engineering
88.2K Views

The Effect of Reflux Ratio on Tray Distillation Efficiency
Chemical Engineering
77.5K Views

Efficiency of Liquid-liquid Extraction
Chemical Engineering
48.3K Views

Liquid Phase Reactor: Sucrose Inversion
Chemical Engineering
9.6K Views

Crystallization of Salicylic Acid via Chemical Modification
Chemical Engineering
24.2K Views

Single and Two-phase Flow in a Packed Bed Reactor
Chemical Engineering
18.9K Views

Catalytic Reactor: Hydrogenation of Ethylene
Chemical Engineering
30.2K Views

Evaluating the Heat Transfer of a Spin-and-Chill
Chemical Engineering
7.3K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved