Viscosity of Propylene Glycol Solutions
Overview
Source: Michael G. Benton and Kerry M. Dooley, Department of Chemical Engineering, Louisiana State University, Baton Rouge, LA
Viscosity is a measure of a fluid's resistance to flow, and it is a useful parameter in the design of efficient product processing and quality control in a wide range of industries. A variety of viscometers are used to obtain the most accurate readings of experimental materials. The standard method of measuring viscosity is through a glass tube viscometer, which estimates viscosity by measuring the amount of time it takes fluid to flow through a capillary tube made of glass1.
Rotational viscometers operate by applying shearing forces and measuring the time it takes a flowing1. These viscometers make use of the flowing force of the fluid, and they can use either a spring system or a digital encoder system1. Different measuring systems exist as well, with the standard being a cone and plate system, where fluid flows under the cone shape and over the plate, in order to minimize shear stress1. Parallel plate systems use two parallel plates and is ideal for measuring across temperature gradients, allowing a smooth transition1. Couette systems use a cup and filling material, and the fluid flows in between the two1. These systems are best for materials with low viscosity, since this system minimizes shear stress, but the system is also harder to operate routinely due to issues with cleaning and needing larger volumes of fluid1.
In this experiment, a Cannon-Fenske viscometer will be used to measure the viscosities of several propylene glycol solutions to determine the relationship between viscosity and composition.
Principles
Kinematic viscosity is the ratio of dynamic viscosity to density. The ratio of shear stress to the shear rate is the dynamic viscosity of a fluid, which is a measure of the resistance to deformation in laminar flow for a Newtonian fluid. This ratio is unique to kinematic viscosity, which enables the interpolation for an unknown variable by considering two variables, kinematic viscosity and concentration.
Temperature, density, and composition can create changes in the deformation of the flow. Pressure is negligible on liquid-phase fluids, so it does not factor into this experiment. Kinematic viscosity can be measured using a viscometer because of the relationship presented in the Hagen-Poiseuille law, which expresses that the pressure drop is due to gravity, dynamic viscosity, and kinematic viscosity. Kinematic viscosity can also be related to time using a viscometer constant specific to each capillary glass,
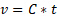
where  is the kinematic viscosity, C is the viscometer constant, and t is time. Viscometer constants are generally measured and provided by the manufacturer of the viscometer. The above equation is used to determine kinematic viscosity, which can be converted to dynamic viscosity by multiplying by the density of the fluid,
is the kinematic viscosity, C is the viscometer constant, and t is time. Viscometer constants are generally measured and provided by the manufacturer of the viscometer. The above equation is used to determine kinematic viscosity, which can be converted to dynamic viscosity by multiplying by the density of the fluid,
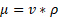
where µ is the dynamic viscosity,  is the kinematic viscosity, and ρ is the density. This information can be used to relate the concentration of solute and dynamic viscosity graphically.
is the kinematic viscosity, and ρ is the density. This information can be used to relate the concentration of solute and dynamic viscosity graphically.
The Cannon-Fenske Viscometer is widely used to measure the viscosity of various solutions. The viscometer is immersed in a large water bath that contains a temperature control coil and fin to maintain constant thermoequilibrium. The capillary constricts the downward flow of the liquid, so that the time required for the liquid to travel through the capillary region is multiplied by the viscometer constant to give the kinematic viscosity. Larger capillary glassware is used to measure solutions with higher viscosities. More viscous solutions take longer to travel through a capillary glass. This information can be used to compare the unknown to known concentrations.
Procedure
1. Preparing the Viscometer
- Prepare seven solutions with varying concentrations of propylene glycol in water (between 0 - 100 mole % polypropylene glycol). Label all solutions. These will be used for calibration. Obtain a sample of the unknown concentration and set it aside
- Check the samples for lint, dust, or other solid material. If needed, filter the sample through a sintered glass filter or fine mesh screen.
- Clean the appropriate viscometer using water, and dry with filtered air to remove the final traces of solvents. Lower viscosity solutions will use size 50 capillary glassware, size 100 will be used for the unknown solution, and higher viscosity solutions will use size 150 capillary glassware.
2. Charging the viscometer
- Pour the sample into the viscometer until it fills at least half of the bigger bulb. Then, wipe the arm clean.
- Place the viscometer into the holder and insert it into the constant temperature bath. Align it vertically with a small plumb bob in the large tube or use a self-aligning holder.
- Allow the sample to equilibrate to the water bath temperature. Allow approximately 10 min for the sample to come to the bath temperature at 40 °C and 15 min at 100 °C.
- Apply suction to the arm and draw the liquid into the tube.
3. Measuring the Efflux
- Use a stopwatch and measure the time required for the meniscus of the sample to flow between the indicated markings. This is the efflux time
- Repeat steps 6 and 7 for duplicate runs. And repeat the entire procedure for each sample.
Results
In this experiment, the viscosity of several concentrations of propylene glycol were measured. As expected, the viscosity was found to increase with propylene glycol concentration. The time for the sample solutions to traverse the viscometer were measured and used to determine the kinematic viscosity. Numerous measurements were collected to minimize random error.
The kinematic viscosity was determined using the time as measured and the viscometer constant:

Then, the kinematic viscosity was multiplied by the density to give the dynamic viscosity:
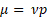
The unknown concentration was calculated and compared to the known sample solutions. Linear interpolation was used to estimate the concentration, and the relationship was best fit to a linear function (Figure 1).
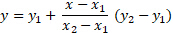
For the above equation, two known data points of kinematic viscosity and concentration were used, and x was set as the measured viscosity of the unknown solution. The solution was solved for y to find the concentration of the unknown sample. The Excel graphing feature can also be used to plot a trendline through the data set and give an equation of best fit.

Figure 1: The relationship between concentration of solution and viscosity demonstrated a linear fit.
This graph shows that the natural logarithm of viscosity and composition follow a linear relationship. As solute composition increases in a solution, viscosity also increases. Knowing this relationship, the concentration of the unknown solution is easily found by measuring its viscosity and relating it to the known relationship between concentration and viscosity. The experimental accuracy can be improved by measuring more known concentrations or using a more precise thermometer.
Application and Summary
The goal of this experiment was to test the relationship of viscosity and composition by using the viscosity of the unknown substance to find its composition. A number of known concentration solutions of propylene glycol and one unknown concentration solution were tested. Relationships between density, dynamic viscosity, and kinematic viscosity were used to compare the solutions. Since solutions become more viscous as they became more concentrated, we were able to narrow the concentration of the unknown solution to a small range. Linear interpolation was used to estimate the concentration, and the relationship was best fit to a linear function. For this experiment, increasing the accuracy of the thermometer could have decreased our uncertainty significantly, since it is the main source of error. More concentrations could also have been tested to increase the precision.
Accurate viscosity testing is important to a variety of fields. In the food processing industry, food must be tested for viscosity throughout its creation as it is transported throughout a facility2. These measurements are used to maximize the efficiency of the process and to establish standards for production2. The viscosity is important to the food industry because it will determine how long transport of food through a pipe or processor will take, how long it will take food to dry, and the time it will take to dispense food into packaging for transportation and retail2. Engineers will use the viscosity to maximize flow of the product through piping in order to save energy and maximize the product, without diminishing the quality of the finished product2. Viscosity is also important to establish safe standards for the force that can be applied to materials and product without damaging it2.
In the petroleum industry, viscosity is an important control for quality assessment3. When purchasing or processing crude oil, companies must measure the viscosity to determine the appropriate treatment3. The viscosity provides important information about the composition of the crude oil3. Oil of different compositions is used to create different products3. Some refineries can only process oils of a certain viscosity, so accurate testing is important to determine which materials they can use for refining3. In oil refining, the viscosity of the oil is used to plan the most efficient methods for extraction, transportation, and refining method3. Temperature can also have an impact on the viscosity of oil, so controls must be put in place to have the oil at an appropriate temperature for its viscosity3. Additionally, the viscosity of an oil will determine the way it is cleaned up in the case of a leak3.
References
- Basic Introduction to Viscometry." A Basic Introduction to Viscometry. N.p., n.d. Web. 7 Jan. 2017.
- Scientific. "What is Viscosity, and Why is Measuring Viscosity Important?" What is Viscosity, and Why is Measuring Viscosity Important? N.p., n.d. Web. 7 Jan. 2017.
- Applications." Anton Paar. N.p., n.d. Web. 13 Jan. 2017.
Tags
Skip to...
Videos from this collection:

Now Playing
Viscosity of Propylene Glycol Solutions
Chemical Engineering
32.6K Views

Testing the Heat Transfer Efficiency of a Finned-tube Heat Exchanger
Chemical Engineering
17.9K Views

Using a Tray Dryer to Investigate Convective and Conductive Heat Transfer
Chemical Engineering
43.8K Views

Porosimetry of a Silica Alumina Powder
Chemical Engineering
9.6K Views

Demonstration of the Power Law Model Through Extrusion
Chemical Engineering
10.0K Views

Gas Absorber
Chemical Engineering
36.5K Views

Vapor-liquid Equilibrium
Chemical Engineering
88.2K Views

The Effect of Reflux Ratio on Tray Distillation Efficiency
Chemical Engineering
77.5K Views

Efficiency of Liquid-liquid Extraction
Chemical Engineering
48.3K Views

Liquid Phase Reactor: Sucrose Inversion
Chemical Engineering
9.6K Views

Crystallization of Salicylic Acid via Chemical Modification
Chemical Engineering
24.2K Views

Single and Two-phase Flow in a Packed Bed Reactor
Chemical Engineering
18.9K Views

Kinetics of Addition Polymerization to Polydimethylsiloxane
Chemical Engineering
16.1K Views

Catalytic Reactor: Hydrogenation of Ethylene
Chemical Engineering
30.2K Views

Evaluating the Heat Transfer of a Spin-and-Chill
Chemical Engineering
7.3K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved