Entropy
Source: Ketron Mitchell-Wynne, PhD, Asantha Cooray, PhD, Department of Physics & Astronomy, School of Physical Sciences, University of California, Irvine, CA
The second law of thermodynamics is a fundamental law of nature. It states that the entropy of a system always increases over time or remains constant in ideal cases when a system is in a steady state or undergoing a "reversible process." If the system is undergoing an irreversible process, the entropy of the system will always increase. This means that the change in entropy, ΔS, is always greater than or equal to zero. The entropy of a system is a measure of the number of microscopic configurations the system can attain. For example, gas in a container with known volume, pressure, and temperature can have an enormous number of possible configurations of the individual gas molecules. If the container is opened, the gas molecules escape and the number of configurations increases dramatically, essentially approaching infinity. When the container is opened, the entropy is said to increase. Therefore, entropy can be considered a measure of the "disorder" of a system.
Entropy is a "state property," which is a quantity that depends only upon the current state of the system. Quantities that are state properties do not depend on the path by which the system arrived at its present state. Therefore, the most useful way to quantify a state property is to measure its change.
The change in entropy S is defined as:
ΔS = Q / T, (Equation 1)
where Q is the heat supplied to the system and T is the temperature of the system. In the context of thermodynamics, heat, like work, is defined as a transfer of energy. Heat is energy transferred from one object to another because of a difference in temperature. Consider a bath of ice and water at 0 °C. If one supplies heat to the ice/water bath, some of the ice will melt, and the number of states available to the water molecules will increase by a large amount, proportional to the amount of heat that was added to the system. The entropy will then increase proportionally to this amount. The relationship between two objects at different temperatures was first described by Newton.
Newton's law of cooling states that the rate of change of the temperature of some object is proportional to the difference between its own temperature and the temperature of its surroundings. For an object at temperature T placed in a closed system at temperature Tf, this change in temperature as a function of time t is described by the differential equation:
dT/dt = -k(T - Tf ), (Equation 2)
where k is a constant that depends upon the characteristics of the object and its surroundings. Equation 1 is equivalently written as:
-k dt = dT / (T - Tf ). (Equation 3)
Integrating both sides gives:
-k t = log(T - Tf ) + log C. (Equation 4)
Applying the exponential function to both sides of the equation and then rearranging gives:
T - Tf = C e-kt . (Equation 5)
If the object in question is at an initial temperature Ti at time t = 0:
Ti- Tf = C. (Equation 6)
It follows that the temperature as a function of time is:
T(t) =Tf + (Ti- Tf ) e-kt .(Equation 7)
Therefore, when a hot object is placed in a cooler closed system, its temperature will decrease at an exponential rate. In this closed system, the heat from the hot object Q will increase the temperature of the cooler surroundings and thus increase the number of available states. Thus, the change in entropy, ΔS, is positive and nonzero.
1. Setup.
- Obtain a heating element and stand, a thermometer, a stopwatch, a few paper towels, water, and a large beaker.
- Fill the beaker with enough water so that the sample will not cool down too rapidly (i.e., at least 500 mL).
- Place the beaker full of water on the stand below the heating element and turn it on.
- Once the beaker of water reaches a boil, insert the thermometer and turn off the heating element.
- Carefully remove the beaker from the heating stand and place it on the table, on top of the paper towels. These will act as insulation from the table.
2. Recording data.
- Begin the stopwatch and record the temperature and time.
- For the first 20 min, take a measurement about every 1 min.
- For the next 20 min, take a measurement about every 3-5 min.
- Record these values in Table 1.
- Plot the data points that were collected in Table 1 in a graph of temperature versus time.
- Using the initial temperature of the water and any two data points for the time and temperature, solve Equation 7 for the cooling constant k.
- Using this value for k, plot Equation 7 as a continuous function of t. Compare the function with the data points that were collected.
Representative results for 680 mL of water are shown in Table 1. The cooling constant k was found using the data points in the table and solving Equation 7. After 35 min, T(35) = 50.6. The initial temperature was 100 °C, and data collection ceased at 28.5 °C. Using these variables gives the following equation to obtain k:
50.6 = 28.5 + (100 - 28.5) e-k 35. (Equation 8)
Solving for k gives a value k = 0.034. The curve with this cooling constant is shown as a dashed gray line in Figure 1, along with the data points from the experiment. The functional form of Equation 6 matches the experimental results very closely.
As the water cools, the entropy decreases, since the number of states available to the water molecules decreases. The entropy of the ambient air in the room increases because the water beaker transfers heat to the air molecules surrounding it; the overall entropy of the water + air system increases. The number of states that the now-hotter air molecules can occupy is much higher than before the hot water was introduced to the room.
In differential form, the heat dQ added to or removed from the water can be calculated using the relationship between mass, specific heat c, and temperature change:
dQ = mc dT, (Equation 9)
where c is known to be 4.18 J/(gK) for water. The change in entropy of the water is then:
ΔSwater = 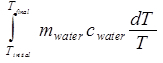
= mwater cwater ln(Tfinal / Tinitial). (Equation 10)
Using the conversion to Kelvin as K = °C + 273.15, the change in the entropy of the water is calculated as:
ΔSwater = 680 g * 4.18 J/(g K) * ln[(28.5 + 273.15)/(100 + 273.15)]
= -604 J/K.
The ambient air temperature is constant at 20.4 °C, so this is an isothermal process. The entropy change of the air is then:
ΔSair = 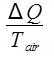 ,
,
where Q is the heat released by the water, which is given by Equation 9. The change in entropy of the air is then calculated as:
ΔSair = 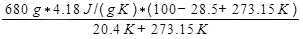
= 3337 J/K.
The total change in the entropy of the water + air system, ΔStot, is the sum of the individual changes in entropy of the water and ambient air:
ΔStot = ΔSwater +ΔSair (Equation 11)
= -604 J/k + 3337 J/K
= 2733 J/K.
Table 1. Temperatures recorded during the experiment.
| Time (min s) | Water Temperature (°C) |
| 0 0 | 99.6 |
| 1 10 | 97.1 |
| 1 50 | 94.2 |
| 2 30 | 91.8 |
| 3 22 | 89 |
| 4 05 | 87.2 |
| 5 08 | 82.7 |
| 6 05 | 82.4 |
| 8 25 | 78 |
| 9 15 | 76.5 |
| 10 15 | 74.6 |
| 11 38 | 72.7 |
| 12 58 | 70.7 |
| 13 58 | 69.2 |
| 15 15 | 67.7 |
| 16 55 | 65.8 |
| 18 38 | 64 |
| 20 25 | 62.3 |
| 24 02 | 58.8 |
| 25 45 | 57.3 |
| 34 45 | 50.6 |
| 40 50 | 47.4 |
| 44 30 | 45.9 |
| 49 59 | 43.6 |
| 53 42 | 42.4 |
| 60 01 | 40.2 |
| 64 20 | 39.5 |
| 76 37 | 37 |
| 103 50 | 32.1 |
| 116 41 | 30.3 |
| 122 46 | 29.6 |
| 134 11 | 28.5 |

Figure 1. Plot of temperature versus time. The blue dots indicate the experimental data, and the dashed line represents theoretical data based on Newton's law of cooling.
A pair of headphones kept in a bag always tends to become knotted-this is an increase in entropy caused by carrying the bag around. It is necessary to do work on the headphones to un-knot them and decrease the entropy (this can be thought of as a "reversible process"). The most efficient heat engine cycle allowed by physical laws is the Carnot cycle. The second law states that not all heat supplied to a heat engine can be used to do work. The Carnot efficiency sets the limiting value on the fraction of heat that can be used. The cycle consists of two isothermal processes followed by two adiabatic processes. A refrigerator, which is essentially just a heat pump, is also a classic example of the second law. Refrigerators move heat from one location at a lower temperature (the "source") to another location at a higher temperature (the "heat sink") using mechanical work. According to the second law, heat cannot spontaneously flow from a colder location to a hotter one; thus, work (energy) is required for refrigeration.
Newton's law of cooling was demonstrated by a beaker full of water at 100 °C cooling down to room temperature, which led to an increase in the entropy of the water-air system. By measuring the temperature of the water as a function of time over a period of 135 min, it was possible to confirm that the cooling of the water was exponential in form. The cooling constant of the water sample was found by solving the cooling equation using collected data.
Skip to...
Videos from this collection:

Now Playing
Entropy
Physics I
17.6K Views

Newton's Laws of Motion
Physics I
75.7K Views

Force and Acceleration
Physics I
79.1K Views

Vectors in Multiple Directions
Physics I
182.3K Views

Kinematics and Projectile Motion
Physics I
72.6K Views

Newton's Law of Universal Gravitation
Physics I
190.8K Views

Conservation of Momentum
Physics I
43.3K Views

Friction
Physics I
52.9K Views

Hooke's Law and Simple Harmonic Motion
Physics I
61.3K Views

Equilibrium and Free-body Diagrams
Physics I
37.3K Views

Torque
Physics I
24.3K Views

Rotational Inertia
Physics I
43.5K Views

Angular Momentum
Physics I
36.2K Views

Energy and Work
Physics I
49.7K Views

Enthalpy
Physics I
60.4K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved