Method Article
In Vitro Thrombosis Test for Ventricular Assist Devices
In This Article
Summary
We present a benchtop protocol to induce thrombosis in ventricular assist devices (VAD) within a recirculating test platform. This method serves to identify thrombogenic hotspots in the blood flow path and can help improve thromboresistance ahead of preclinical testing in animal models.
Abstract
The risk of thrombosis remains a significant concern in the development and clinical use of ventricular assist devices (VADs). Traditional assessments of VAD thrombogenicity, primarily through animal studies, are costly and time-consuming, raise ethical concerns, and ultimately may not accurately reflect human outcomes. To address these limitations, we developed an aggressive in vitro testing protocol designed to provoke thrombosis and identify potential high-risk areas within the blood flow path. This protocol, motivated by the work of Maruyama et al., employs a modified anticoagulation strategy and utilizes readily available components, making it accessible to most laboratories conducting in vitro blood testing of VADs. We demonstrated the utility of this method through iterative testing and refinement of a miniature magnetically levitated pediatric VAD (PediaFlow PF5). The method has been effective in identifying thrombogenic hotspots caused by design and manufacturing flaws in early VAD prototypes, enabling targeted improvements before advancing to animal studies. Despite its limitations, including the absence of pulsatile flow and the influence of donor blood characteristics, this protocol serves as a practical tool for early-stage VAD development and risk mitigation.
Introduction
Ventricular assist devices (VADs) have become a standard of care in the management of patients with advanced heart failure, yet the risk of thrombosis and stroke remains a significant challenge1,2. Thrombosis within VADs is typically assessed during preclinical animal studies, which, while valuable, present substantial costs and logistical challenges. These studies are resource-intensive, time-consuming, and are susceptible to a single defect compromising the entire test and necessitating additional trials. This not only increases the financial burden but also raises ethical concerns due to the need for repeated animal testing.
Although there exist many numerical models for predicting platelet deposition and thrombosis3,4,5,6, only a few are suitable for simulating thrombus formation in macro-scale devices such as VADs7,8,9. Moreover, existing models inevitably assume idealized surfaces and simplified "watertight" geometries, which do not accurately reflect the complexities and imperfections of real-world pump assemblies. When platelet-surface interactions are considered, these macro-scale models generally employ uniformly prescribed material properties (typically modeled as a coefficient in the surface-flux boundary conditions)10,11,12. Consequently, numerical models cannot completely substitute for experimental testing with blood.
Both material choice and surface finish play critical roles in platelet adhesion on VAD surfaces13,14,15,16,17. Imperfections such as rough spots or irregularities can promote platelet adhesion and thrombus formation. Additionally, crevices between components in the flow path can serve as a nidus for thrombosis, providing protected environments where clots can form and grow18,19. The use of grease, lubricants, or sealants during assembly can also pose a risk, as these substances may seep into the flow path and interact with the blood, further increasing the risk of complications.
There is, therefore, a need for a well-defined in vitro testing protocol that can reliably assess the thromboresistance of VADs before they are subjected to animal testing or clinical use. While there is a widely adopted ASTM standard for the assessment of hemolysis20, no such standard exists for thrombogenicity testing of VADs under clinically relevant operating conditions21. Despite seminal studies dating back three decades demonstrating the feasibility of in vitro thrombosis testing for blood pumps22,23,24,25, animal testing has persisted as the de facto practice for evaluating thrombosis to date26. The hindrance to wider adoption of in vitro methods has likely been the complex nature of coagulation, with the multitude of confounding factors that can influence test results, making it challenging to differentiate intrinsic pump thrombogenicity from artifacts arising due to methodological limitations and procedural errors.
This motivated us to share a detailed protocol as a guide for experimentalists to avoid pitfalls, hence promoting the use of in vitro testing and mitigating the reliance on animal studies. The protocol described herein, derived from Maruyama et al.27, was refined and validated during the design of the 5th generation PediaFlow (PF5) pediatric VAD28,29. This testing method proved instrumental in systematically identifying and addressing potential thrombogenic risks in the VAD prototypes ahead of animal testing.
Protocol
Ovine whole blood used in this study was obtained from a commercial vendor and, therefore, did not require a review by Cornell University's Institutional Animal Care and Use Committee.
1. Construction of the test flow loop
NOTE: See the Table of Materials for a detailed list of loop components and all other materials used in this protocol.
- Modify the intravenous (IV) bag.
- Use a plastic heat sealer to modify an IV bag to adjust its volume and shape, as depicted in Figure 1.
NOTE: The shape of the bag facilitates de-airing and allows the bag to be clamped with a hemostat across the fluid line30,31. The location of the sealing line is chosen based on the priming volume of the VAD and the tubing to achieve a total priming volume of 150 mL. - Introduce an air vent by making a 4-mm incision at the top of the bag. Insert a barbed female luer lock into the incision and seal the site with RTV silicone rubber adhesive. Attach a one-way stopcock to the luer lock.
- Use a plastic heat sealer to modify an IV bag to adjust its volume and shape, as depicted in Figure 1.
- Assemble the test loop using the modified IV bag, polyvinyl chloride (PVC) tubing, polycarbonate barbed connectors, and 3-way and 1-way stopcocks, as shown in Figure 1.
- Connect the pressure manometer.
- Attach 6-inch extension lines to the inlet and outlet-side pressure ports. Attach a one-way stopcock to the other end of the extension line.
NOTE: The stopcock allows disconnecting the manometer tubing to prime the extension line with fluid before starting the pump, as described in step 4.3. - Connect one end of each 1/8" inner diameter (ID) tubing to a manometer barb and insert a male luer fitting to the other end.
- Connect male luer fittings to the one-way stopcocks. Open the stopcocks to enable pressure readings.
- Attach 6-inch extension lines to the inlet and outlet-side pressure ports. Attach a one-way stopcock to the other end of the extension line.
- Apply a clamp-on ultrasonic flow probe to measure the flow rate.
- Place a Hoffman clamp distal to the outlet pressure port to regulate flow resistance.
2. Preparation of the calcium chloride (CaCl2) solution
- Dissolve powdered CaCl2 in 1x TRIS-buffered saline (pH 7.4) to prepare a 1 M solution. Avoid phosphate-containing buffers (e.g., PBS or DPBS) since calcium and phosphate react to form an insoluble precipitate.
- If preparing CaCl2 in large quantities, add the CaCl2 powder incrementally in several steps, as its dissolution is an exothermic process.
- Titrate the pH of the solution to 6-8 using hydrochloric acid (HCl) or sodium hydroxide (NaOH) if necessary.
3. Preparation of blood
NOTE: The ovine blood used in this study was obtained from a commercial vendor listed in the Table of Materials. The blood was collected using a 14-G needle, with the animal restrained in a standard agricultural standing position. The collection process took 10-12 min from needle insertion to completion. The blood was anticoagulated with 14 parts CPD to 86 parts blood (CPD formulation: 26.3 g/L Na-Citrate, 25.2 g of dextrose, 3 g/L citric acid, and 2.2 g/L Na Phosphate in deionized [DI] water). The blood bag was shipped overnight in an insulated container with ice packs and was used for the experiment within 24 h of collection.
- Prepare the donor blood for use.
- Place the blood bag on a tilting platform for 15-30 min to mix the blood gently and allow it to reach room temperature (RT).
- Place a magnetic stir bar into a polycarbonate beaker or container designated for the blood transfer.
- Transfer the blood into the beaker through a transfusion filter to remove macroaggregates. Handle the blood gently to prevent damage to cellular components. Pour the blood along the container walls to avoid free fall and minimize cellular trauma.
NOTE: Discard the blood if large thrombi are present or if the filter clogs. - Place the beaker or container on a magnetic stirrer and adjust the speed until the surface of the blood begins to spin gently without forming a large vortex. Stir continuously for at least 5 min.
- Measure the hematocrit and platelet count to ensure the values are within the normal range for the species.
- Perform titrations to determine the target CaCl2 concentration.
NOTE: Citrated blood is recalcified to enable coagulation and thrombosis testing, with heparin added to prevent runaway coagulation. The target activated clotting time (ACT) for the start of the test is 300 s. Please note that the reference ACT values provided in this section were obtained using donor sheep blood and may vary depending on the species and the level of anticoagulation used during collection. Additionally, ACT measurements can vary between instruments32,33,34. Some trial and error may be required to establish the optimal ACT range for a specific laboratory setup.- Transfer 2 mL of the prepared 1 M CaCl2 solution and 0.5 mL of heparin sodium (1000 U/mL) from the manufacturer's packaging into microcentrifuge tubes to avoid contamination of the stock solutions.
- Transfer 10 mL of blood into 14-mL polypropylene test tubes. Prepare four such tubes.
- To achieve 0.5 U/mL heparin concentration in blood, add 5 µL of heparin sodium to the 10 mL blood sample.
- To achieve a calcium concentration of 5 mM in blood, add 50 µL of 1 M CaCl2 solution to the 10 mL blood sample. While dispensing, swirl the pipette tip to distribute the CaCl2 over a wider area.
- Immediately, invert the tube 10 times, roll it horizontally (on a flat surface or between palms), then invert 10 more times to ensure thorough mixing.
- Measure the ACT using a point-of-care whole blood coagulation system following the manufacturer's instructions. The initial ACT will typically range between 400-500 s.
- While keeping the heparin concentration constant, increase the CaCl2 concentration (to approximately 7-8 mM) to achieve an ACT of 300 s.
- Verify the final concentration and the resulting ACT in a separate tube of blood.
4. Pre-test procedures
NOTE: All the steps described in this section apply to Sections 5 and 6. Perform these steps before operating the pump with bovine serum albumin (BSA) or blood in the loop. Transfer blood between vessels using gravity-feeding to minimize mechanical stress. Avoid using the syringe plunger to drive blood, as this can create excessive pressure. Additionally, avoid throttling blood through narrow openings to prevent damage to cellular components.
- Filling and draining the test loop
- Attach a 30-inch extension line to the injection port and connect a 60-mL syringe barrel as a funnel. Open the reservoir stopcock to act as an air vent.
- Pour fluid into the funnel, raising it as needed to speed filing. Fill the loop to 1-2 cm below the air vent at the top of the bag. Close the air vent and injection port stopcocks.
- To drain, open the air vent and injection port stopcocks and lower the funnel below bench level. Raise the pump above the drainage port to evacuate the remaining fluid.
- De-airing the test loop
NOTE: Perform this procedure when filling the loop with BSA or blood prior to starting the pump.- Ensure that there is no air trapped anywhere in the loop. Dislodge air bubbles on surfaces by gently tapping and squeezing the tubing and reservoir.
- If evaluating an axial flow blood pump, rotate it into a vertical position to release any air via buoyancy. If using a centrifugal pump, flip it upside down and rotate it to ensure no air is trapped in secondary flow paths.
- Closely inspect horizontal sections of tubing and the junctions between tubing and connectors to ensure no air bubbles are present.
- Squeeze the IV bag to bring the fluid level close to the top narrow portion and clamp the bag with a hemostat across the fluid line to eliminate the fluid-air interface. Close the air vent stopcock.
- Priming the pressure lines and the sampling port
- Close the stopcock at the end of the pressure lines, remove the manometer tubing, and attach a 3-mL syringe. Open the stopcock and draw 1-2 mL of fluid into the extension line (approximately 4 cm).
- Close the stopcock, detach the syringe, and reconnect the manometer tubing. Open the stopcock to enable pressure readings.
- Prime the sampling port using a syringe.
- Cleaning the injection and sampling ports
- Cut or tear a lint-free wipe into three equal fragments. Twist the corner of one fragment to form a tip and insert it into the port to absorb any residual blood.
- Twist the second fragment, moisten it with saline, and insert the wet tip into the port. Twist it around the port to remove all remaining blood.
- Use the third fragment to absorb any residual saline in the port.
NOTE: Perform this cleaning procedure immediately after drawing a blood sample or whenever residual blood is present in the ports.
5. Passivating the blood-contacting surfaces
- Fill the loop with 1% BSA solution and operate the VAD to circulate it for at least 30 min.
- Inspect the loop for leaks and reassemble if necessary.
- Drain the BSA solution completely to avoid hemodilution.
6. Thrombosis testing
- Prepare the test loop.
- Fill the loop with 150 mL of blood.
- De-air the loop and prime the pressure lines and sampling port as described in steps 4.2-4.4.
- Start the pump at a low speed and operate it for about 5 s to dislodge any trapped air bubbles within the pump, then stop the pump.
- If any bubbles appear in the bag, repeat step 4.2 to perform de-airing again.
- Restart the pump at a low speed to circulate the blood in the loop.
- Add heparin to the blood in the loop.
- Transfer 1 mL of heparin sodium (1000 U/mL) and 1.5 mL of ethylenediaminetetraacetic acid (EDTA; 0.5 M) from manufacturer's packaging into separate tubes for ease of handling during the test.
- Use the transverse port of the 3-way stopcock to administer substances into the loop using a syringe. Rotate the male luer lock on the stopcock so the injection port faces upward to trap air bubbles at the top.
- Achieve a heparin concentration of 0.5 U/mL in the loop by adding 75 U of heparin sodium to 150 mL of blood. Aspirate 75 U of heparin (75 µL if using 1000 U/mL concentration) into a micropipette and dispense it into a 3-mL syringe by simultaneously dispensing the pipette and drawing the syringe plunger. Ensure all liquid is transferred without spilling.
- Attach the syringe to the injection port via the luer lock, with the port facing up and the syringe tip pointing vertically down. Draw the syringe plunger to fill it with blood and mix the heparin with the blood while aspirating any air into the syringe. Allow the air bubble to rise to the top of the syringe. Inject the mixture into the loop, ensuring no air enters.
- Shuttle blood in and out of the syringe 4-5 times to ensure that the heparinized blood does not stay confined to the space in the port. Ensure that no air enters the loop.
- Titrate the ACT of blood in the loop.
- Achieve an initial calcium concentration of 5 mM in the loop by adding 750 µL of 1 M CaCl2 solution to 150 mL of blood using the same procedure as heparin.
- Blood exposed to high calcium concentrations may start coagulating in the syringe. Swiftly inject the fluid after removing residual air. Optionally, dilute the CaCl2 in the syringe with 1 ml of TRIS-buffered saline to reduce the risk of premature coagulation.
- Allow the injected CaCl2 to circulate in the loop for at least 2 min before drawing a blood sample to measure the initial ACT.
- Attach a 1-mL syringe to the sampling port. Draw and discard 0.5 mL of waste blood to clear stagnant blood from the port.
- Attach a new 1-mL syringe, draw 0.5 mL of blood for analysis, and measure the ACT. Clean the sampling port using the procedure in step 4.4.
- Refer to the titration values in step 3.2 to inform the target CaCl2 concentration. Incrementally increase the calcium concentration to achieve an ACT of 300 s. Avoid adding the full amount of CaCl2 at once to prevent rapid clotting.
NOTE: In the testing used in this study, the initial calcium concentration of 5 mM resulted in ACT of 450 ± 50 s, and the final concentration of 7-8 mM yielded ACT of 300 ± 20 s. While this response may vary, aim to bring the ACT to or below 300 s for the start of the test.
- Achieve an initial calcium concentration of 5 mM in the loop by adding 750 µL of 1 M CaCl2 solution to 150 mL of blood using the same procedure as heparin.
- Commence the test.
- Once the ACT of 300 s is achieved in the loop, begin the 1-h test. Adjust the pump to the desired flow rate and pressure by modifying the rotor speed and regulating the resistance using the Hoffman clamp.
- Measure the ACT every 15 min following steps 6.3.3-6.3.4.
- If the ACT drops below 200 s, inject an additional 25 U of heparin sodium into the loop.
- End the test.
- At the end of 1 h, inject EDTA into the loop to inhibit further coagulation, targeting a final concentration of 5 mM in blood. (Inject 1.5 mL if using a 0.5 M EDTA solution.)
- Allow the EDTA to circulate and mix for 2 min, then stop the pump.
- Disconnect and flush the pump.
- Clamp the tubing connected to the pump inlet and outlet using hemostats, positioning the clamps 3-4 cm away from the inlet and outlet barbs.
- Carefully disconnect the tubing to release the pump.
- Drain the blood from the pump and flow loop into a container.
- Wash any residual blood from the pump by gravity-feeding or pipetting saline through the pump inlet and outlet.
NOTE: Steps 6.7 and 6.8 are performed concurrently.
- Inspect the pump flow path.
- Capture images of the pump inlet and outlet using an endoscope/borescope camera.
- Disassemble the pump (if applicable). Wash components in a saline bath and inspect for thrombi using a dissecting scope or macro lens.
- Photograph blood-contacting surfaces for documentation.
NOTE: Consistent inspection and thorough documentation are critical for comparative testing.
- Filter the used blood.
- Cut a piece of 100-µm nylon mesh fabric large enough to cover the opening of a catch container.
- Position the mesh over the container with a slight drape to allow blood to flow through it effectively. Secure the edges of the mesh to prevent it from slipping during filtration.
- Pass the used blood through nylon mesh. Dispose of the blood according to the institution's biological waste handling guidelines.
- Examine the mesh for captured thrombi and document the findings.
- Prepare for histological analysis.
- If histological analysis is planned, fix any thrombi found in the formalin solution following appropriate safety procedures.
CAUTION: Always use a fume hood when handling formaldehyde.
- If histological analysis is planned, fix any thrombi found in the formalin solution following appropriate safety procedures.
7. Cleaning procedure
- Disassemble the flow loop. Discard the PVC blood bag and tubing.
- Soak all other blood-contacting components (connectors, stopcocks, luer locks, etc.) in a 1% enzyme-active powdered detergent solution overnight. Rinse with warm tap water, followed by DI water, and air-dry.
- If clots remain, sonicate components in the detergent solution. Rinse thoroughly and air dry.
- Clean the pump.
- If the pump can be disassembled, soak and sonicate the blood-contacting components with a 1% all-purpose cleaner/degreaser solution, followed by a 1% enzyme-active powdered detergent solution.
- If the pump cannot be disassembled, connect it to a new flow loop filled with cleaning solutions and operate it at high speed for at least 30 min. Repeat as necessary.
- Rinse the pump thoroughly with warm tap water, followed by DI water, and air-dry.
Results
Successful execution of this protocol enables the identification of localized areas of platelet deposition, revealing problematic spots within the pump's flow path. Consistent application of this protocol allows for incremental improvements by addressing these identified "hotspots".
For example, during the development of the PediaFlow PF5 VAD, we encountered challenges in manually polishing the pressure side of the stator vanes due to the miniature size of the components. In vitro thrombosis tests highlighted this issue, as platelet deposition was consistently observed along the roots of the blades, as seen in Figure 2A. A similar deposition pattern was later confirmed in an acute animal study, where the pump was connected extracorporeally to a sheep's circulation and operated for 3 h (not detailed in this paper). To address this, we employed an electropolishing technique to achieve a mirror finish on these titanium components, which successfully eliminated thrombus formation in these areas (Figure 2B). This pump was later employed in a 7-day in vivo study in sheep, which also demonstrated freedom from thrombosis.
Another problematic feature identified in our pump prototypes was the junction between the fore and aft housing components. Imperfect coaptation at this junction could create a crevice where blood might seep in and coagulate, as seen in Figure 3A. We tested several lapping and polishing methods to improve the fitment of these components and used the in vitro thrombosis testing method to evaluate the outcomes. The improvements were assessed by reapplying the protocol, which confirmed the reduction in thrombus formation at the junction, as demonstrated in Figure 3B.
It is crucial to distinguish thrombosis caused by experimental error or artifact from actual clot formation within the device. Several factors can trigger excessive clotting. When administering the CaCl2 solution, a small volume of blood is drawn into the syringe to aspirate any air trapped in the port, as described in step 6.3.1. If this blood remains exposed to a high concentration of CaCl2 for too long, it may begin to coagulate prematurely. When subsequently injected into the loop, this coagulated blood can act as a nidus for further clot growth, potentially being ingested into the VAD. Figure 4 shows a large thrombus that was presumably ingested into a centrifugal maglev pump, occluding the flow path.
Injecting a large volume of CaCl2 at once can trigger runaway coagulation. To avoid this, it is recommended to first increase the CaCl2 concentration in the loop to 5 mM, check the ACT, and then proceed with a second dose to achieve the target ACT.
Spherical, loosely adhered clots observed on surfaces are often the result of air bubbles encapsulated within clots, examples of which are shown in Figure 5A,B. These circulating clots can impinge on the leading edges of impeller and stator blades, creating deposits that resemble bug smears on a car windshield. This emphasizes the importance of thoroughly de-airing the loop as a critical step in the protocol. Similarly, any foreign material and debris circulating in the loop might be encapsulated in thrombi and adhere to pump surfaces, such as shown in Figure 5C,D.
Conversely, the presence of ring thrombi at tubing-connector junctions, as shown in Figure 6, typically indicates that the ACT was within an optimal range that supported localized clotting without escalating into runaway coagulation. The tubing-connectors junctions are known to be the most thrombogenic region in the ECMO circuits35. In our experience, this optimal ACT range is between 200 s and 300 s. If the ACT during the experiments falls below 200 s, an additional heparin bolus of 25 U can be injected into the loop.
Avoiding the pitfalls described above and ensuring consistent execution of the protocol will maximize its utility in identifying potential thrombogenic risks early in the VAD development process, allowing for targeted improvements before advancing to animal studies.

Figure 1: Schematic of the test flow loop. (A) Test flow loop for a pump with 1/4" inflow and outflow barbs and (B) for a pump with 3/8" inflow and outflow barbs. Please click here to view a larger version of this figure.
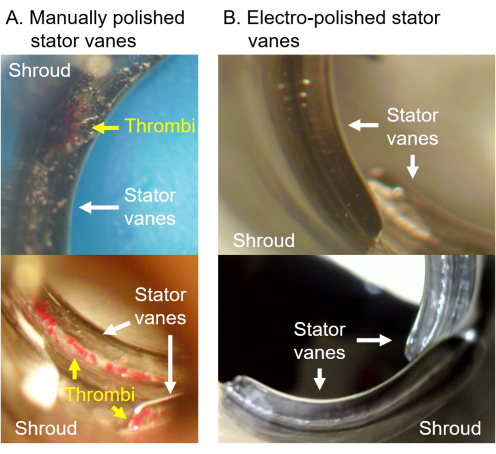
Figure 2: Iterative improvement and assessment of VAD prototypes using the in vitro thrombosis protocol. (A) Platelet deposition was consistently observed along the roots of the stator vanes in the manually polished version of the pump. (B) This issue was resolved in the electropolished version. In both tests, ACT was maintained between 250 s and 300 s, and the pump was operated at 2.5 L/min for 1 h. Please click here to view a larger version of this figure.

Figure 3: The in vitro thrombosis protocol was used to troubleshoot the issue of imperfect fitment between mating components of the pump housing. (A) The baseline design suffered from imperfect coaptation at this junction, leading to blood pooling and coagulating in that space. Inset: the resulting clot, extracted and stretched between two cotton swabs. (B) A revised design with improved fitment eliminated this issue. Please click here to view a larger version of this figure.

Figure 4: A large thrombus occluding the flow path of a centrifugal maglev pump, presumably resulting from an experimental error during the injection of CaCl2 into the flow loop. Please click here to view a larger version of this figure.

Figure 5: Loosely adhered clots, foreign material, and debris. (A,B) Spherical, loosely adhered clots observed on surfaces are often the result of air bubbles encapsulated within clots. (C,D) Any foreign material and debris circulating in the loop might become encapsulated in thrombi that adhere to pump surfaces or impinge on the leading edges (LE) of impeller and stator blades. Please click here to view a larger version of this figure.

Figure 6: Ring thrombus. (A) A ring thrombus formed at the tubing-connector junction. (B) The ring thrombus laid out next to a stainless steel razor blade. Please click here to view a larger version of this figure.
Discussion
First-in-human trial of a new pump is always a precarious endeavor, as preclinical studies cannot reliably predict the thrombogenicity of VADs in humans26. Notably, some VADs that demonstrated freedom from thrombosis in animal trials have later exhibited significant thrombogenicity in clinical use36. An aggressive in vitro testing regimen specifically designed to provoke thrombosis provides a valuable opportunity to identify potential design or manufacturing flaws early in the development process. Although this protocol cannot offer a definitive prediction of the future in vivo performance of a VAD, this proactive approach can reveal potential thrombosis hotspots that may emerge under procoagulant conditions.
Existing in vitro methods for VAD thrombosis vary widely in test duration and anticoagulation strategies. Hastings et al. conducted tests with highly heparinized porcine blood over a 48-hour period, successfully replicating thrombosis patterns observed clinically in ECMO pumps37. In contrast, pioneering studies by Schima et al. used protamine to lower the ACT of heparinized blood to 3x its baseline value at the start of the test, terminating the experiment when the ACT dropped to 1.5x baseline23. This innovative approach compressed typical 2 to 3-day in vivo studies into just 1 h to 3 h of in vitro testing.
Maruyama et al. systematically compared the use of citrate-calcium and heparin-protamine complexes for thrombosis testing, where calcium chloride is added to reverse the action of citrate, and protamine sulfate is used to neutralize the heparin27. They concluded that recalcifying citrated blood provided a more gradual and monotonic ACT response. By employing the citrate-calcium complex, Maruyama and colleagues achieved precise ACT control, maintaining it at 200 s throughout a 2-h test. Their in vitro study comparing multiple pump prototypes effectively replicated the thrombosis patterns observed in animal studies.
While the protocol described here is largely based on Maruyama's method, we sought to address two key barriers to its broader accessibility. First, Maruyama's study utilized bioactive heparin-coated flow loop components, which can be expensive and challenging to procure. We simplified the approach by directly adding a fixed amount of heparin to the blood and using readily available test loop components from common vendors. Second, their method required multiple injections of calcium chloride and trisodium citrate throughout the test to maintain a constant ACT. In contrast, this protocol initiates testing at a higher ACT and avoids the need for continuous adjustments, thereby reducing complexity and the potential for procedural errors. These adaptations make the protocol in this study more accessible to most laboratories conducting in vitro blood testing of VADs.
While this protocol addresses key technical barriers, its success and ultimate utility still hinge on the careful and consistent execution of each experimental step. A critical aspect of this protocol is the thorough de-airing of the loop and the careful method of injecting substances into the circuit without generating air emboli. Failure in either of these areas could introduce artifacts that might confound the results by inducing excessive thrombosis.
It is critical that no air bubbles and blood-air interfaces are present in the loop before starting the test. Any air bubbles circulating in the loop might become encapsulated in clots and will most likely be deposited on the leading edges of the impeller and stator blades. The inlet-side pressure line poses the greatest risk of introducing air due to the low pressure created upstream of the pump inlet. When starting the pump and increasing its speed, the air column in the pressure line will be drawn into the flow. To avoid this, the pressure lines are pre-primed with BSA or blood, as outlined in step 4.3. While de-airing is particularly important when working with blood, we also recommend de-airing before circulating BSA. This precaution is necessary because the ingestion and break-up of large air emboli can produce foam that may persist after draining the BSA solution and potentially interfere with subsequent testing.
The method of injecting heparin and CaCl2 solutions demands precise technique and careful execution, as repeated flushing of the syringe increases the risk of introducing air emboli. Incorporating a motorized syringe programmed to dispense precise volumes of substances could be considered to mitigate this risk. Such an apparatus would also enable continuous heparin injection during the test, calibrated to maintain the ACT at a constant level, thereby reducing variability caused by changes in coagulation dynamics over time.
There are limitations to this method, particularly the steady-state conditions in vitro, which differ from the pulsatile flow conditions in vivo. Although pulsatile in vitro loops are available, they require a greater number of components, adding complexity that can potentially introduce artifacts.
Another notable aspect of this protocol is that the tests were conducted at room temperature rather than at the physiological temperature of 37 °C. While physiological temperature would better mimic in vivo conditions, placing the flow loop in a heated chamber and maintaining 37 °C consistently was challenging due to the need for frequent access to the flow loop during the experiments. Submerging the blood reservoir in a heated water bath was also considered; however, this approach was incompatible with the current vertical loop setup, which minimizes experimental artifacts by facilitating the transport of air bubbles to the top of the blood reservoir.
Furthermore, the study by Patel et al. evaluated the impact of temperature on in vitro thrombosis testing with ovine and bovine blood38. Their findings suggest that room-temperature testing offers better sensitivity compared to 37 °C testing and allows for shorter test durations (1 h versus 2 h). Considering the added benefit of eliminating cumbersome heating equipment, they propose that testing at room temperature is a viable and practical option for in vitro thrombogenicity assessment of biomaterials.
This protocol relies on visual detection of thrombus formation, which makes it particularly suited for VADs with transparent or removable housings. For devices with permanent, opaque housings, direct inspection for thrombus deposition is more challenging without compromising the structural integrity of the device. In such cases, the use of flexible fiber optic cameras may provide a partial solution, enabling limited internal inspection.
Finally, factors such as the choice of donor species, collection method, the type of anticoagulant used, and the age of the blood can all influence the thrombogenic response. The heparin and calcium concentrations provided in this protocol are reference values specific to the setup used in this study. Additionally, ACT measurements can vary between different instruments32,33,34. As such, this protocol may require fine-tuning adjustments through trial and error to determine the optimal ACT range for a given laboratory setup.
To further improve consistency across experiments, future studies could incorporate elements of the ASTM F2888-19 assay as a control measure, utilizing the recalcified blood from the titration stage for concurrent thrombogenicity assessment in reference materials39,40. This approach would reduce the reliance on achieving a standardized ACT, as this metric may not fully account for variations in coagulability arising from differences in blood properties such as hematocrit, platelet count, and total protein concentration.
Despite these limitations, this testing method remains a valuable tool for the early identification of thrombogenic risks during VAD development and offers a practical and accessible approach for iteratively improving device safety before advancing to animal studies.
Disclosures
S.E.O. currently serves as a consultant for Magenta Medical and was previously a consultant at Boston Scientific. No other authors have any relevant financial disclosures or conflicts of interest to report.
Acknowledgements
This work was supported by the National Institutes of Health grant R01HL089456 and the U.S. Army Medical Research Acquisition Activity Project Number W81XWH2010387.
Materials
| Name | Company | Catalog Number | Comments |
| 14-mL test tubes | Falcon | 352059 | Round bottom polypropylene test tubes with snap-cap |
| 1-way stopcock | Qosina | 99759 | Female Luer Lock, Male Luer with Spin Lock |
| 3-way stopcock | Qosina | 99771 | 2 Female Luer Locks, Rotating Male Luer Lock |
| ACT+ cuvettes for Hemochron | Werfen | 000JACT+ | 45/Box |
| All-purpose cleaner/degreaser | Simple Green | 2710200613005 | Simple Green Cleaner and Degreaser. Use 1% solution. |
| Barbed connectors | Qosina | 73311 | Material: polycarbonate; ¼” x ¼” straight connector |
| Barbed connectors w/ luer lock | Qosina | 73316 | Material: polycarbonate; ¼” x ¼” straight connector with luer lock |
| Bovine Serum Albumin (BSA) | Thermo Scientific Chemicals | AAJ6465522 | Or equivalent |
| Calcium chloride, CaCl2 | Thermo Scientific Chemicals | AA89866-30 | Anhydrous, ≥96.0% ACS |
| Dissecting scope (recommended) | Olympus | https://www.olympus-lifescience.com/en/technology/museum/micro/1984/ | Olympus SZH10 (continuous zoom magnification 7x - 70x) or similar |
| DPBS (w/o calcium and magnesium) | Gibco | 14200075 | Dulbecco's phosphate-buffered saline, no calcium, no magnesium, 10X (must be diluted to 1X before use) |
| EDTA | Quality Biological | 351-027-721EA | 0.5 M, pH 7.0–8.0 (Ethylenediaminetetraacetic acid) |
| Endoscope/borescope/otoscope camera (optional) | Bebird | https://bebird.com/products/earsight-pro-ear-wax-removers | 3–4 mm probe diameter |
| Enzyme-active powdered detergent | Alconox | 1304-1 | Alconox Tergazyme. Use 1% solution. |
| Extension Line, 30" | Qosina | 36218 | 30" length, female luer lock to male luer lock |
| Extension Line, 6" | Qosina | 36212 | 6" length, female luer lock to male luer lock |
| Female luer lock, barbed | Qosina | 11548 | Fits 1/8 inch ID Tubing; material: polycarbonate; |
| Flow meter | Transonic | https://www.transonic.com/t402-t403-consoles | Transonic TS410 module |
| Hemostat | Fisherbrand | 13-820-004 | Locking hemostat with at least 5 cm tip length |
| Heparin Sodium | McKesson Packaging Services | 949513 | 1000 U/mL concentration |
| Hoffman clamp | Humboldt | H8720 | Fine-threaded clamp |
| IV bag (compliant blood reservoir) | Qosina | 51494 | Material: PVC, 2 Tube ports 0.258” ID. The 100-ml bag is modified using a heat sealer |
| Lint-free wipes | Kimberly-Clark Professional | 34120 | Kimtech Science Wipers |
| Magnetic stirrer | INTLLAB | MS-500 | Or similar |
| Male luer lock, barbed | Qosina | 11549 | Fits 1/8 inch ID Tubing; material: polycarbonate; |
| Manometer (digital) | Sper Scientific | 840081 | SPER-840081 or similar |
| Nylon filtering mesh | McMaster-Carr | 9318T21 | 100-μm (0.0039") opening size |
| Ovine blood | Lampire | 7209004 | Donor whole blood, anticoagulated with ACD 14:86, shipped overnight |
| Plastic bag heat sealer | Uline | H-190 | Uline H-190 or similar (without cutter) |
| Silicone rubber adhesive | Smooth-On | B00IRC1YI0 | Sil-Poxy or similar |
| Syringe w/ luer lock, 1 mL | Fisher Scientific | 14-955-646 | Fisherbrand manual syringe without needle for research purposes |
| Syringe w/ luer lock, 3 mL | Fisher Scientific | 14-955-457 | Fisherbrand manual syringe without needle for research purposes |
| Syringe w/ luer lock, 60 mL | Fisher Scientific | 14-955-461 | Fisherbrand manual syringe without needle for research purposes |
| Transfusion filter | Haemonetics Corporation | SQ40S/SQ40NS | Haemonetics Corporation SQ40S pall blood transfusion filter |
| TRIS Buffered Saline | Thermo Scientific Chemicals | AAJ62938K2 | TBS 10x (must be diluted to 1X before use), pH 7.4 |
| Tubing | Tygon | ADF00017 | Tygon ND-100-65 tubing (medical grade) |
| Ultrasonic flow sensor | Transonic | https://www.transonic.com/hqxl-flowsensors | Select appropriate flow sensor model for the tubing size used. ME6PXL clamp-on sensor fits the 3/8” OD tubing. The sensor is calibrated by Transonic for the test fluid (e.g., blood at 24C) and tubing grade (e.g. Tygon ND-100-65) |
| Ultrasonic sonicator (optional) | Branson Ultrasonics | CPX952238R | Branson CPX2800H or similar |
| VAD system | PediaFlow | PF5 | The VAD system to be tested; includes the pump and the controller |
| Whole Blood Coagulation System | Werfen | https://www.werfen.com/na/en/point-of-care-testing-devices/ACT-machine-hemochron-signature-elite | Hemochron Signature Elite or Signature Jr |
References
- Kirklin, J. K., et al. Eighth annual INTERMACS report Special focus on framing the impact of adverse events. J Heart Lung Transplant. 36 (10), 1080-1086 (2017).
- Kormos, R. L., et al. The Society of Thoracic Surgeons Intermacs Database Annual Report: Evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg. 107 (2), 341-353 (2019).
- Leiderman, K., Fogelson, A. An overview of mathematical modeling of thrombus formation under flow. Thromb Res. 133 (SUPPL. 1), S12-S14 (2014).
- Anand, M., Rajagopal, K. A short review of advances in the modelling of blood rheology and clot formation. Fluids. 2 (3), 35 (2017).
- Belyaev, A. V., Dunster, J. L., Gibbins, J. M., Panteleev, M. A., Volpert, V. Modeling thrombosis in silico: Frontiers, challenges, unresolved problems and milestones. Phys Life Rev. 26- 27, 57-95 (2018).
- Manning, K. B., Nicoud, F., Shea, S. M. Mathematical and computational modeling of device-induced thrombosis. Curr Opin Biomed Eng. 20, 100349 (2021).
- Wu, W. T., Yang, F., Wu, J., Aubry, N., Massoudi, M., Antaki, J. F. High fidelity computational simulation of thrombus formation in Thoratec HeartMate II continuous flow ventricular assist device. Sci Rep. 6 (Decemeber), 1-11 (2016).
- Méndez Rojano, R., Lai, A., Zhussupbekov, M., Burgreen, G. W., Cook, K., Antaki, J. F. A fibrin enhanced thrombosis model for medical devices operating at low shear regimes or large surface areas. PLoS Comput Biol. 18 (10), e1010277 (2022).
- Taylor, J. O., Meyer, R. S., Deutsch, S., Manning, K. B. Development of a computational model for macroscopic predictions of device-induced thrombosis. Biomech Model Mechanobiol. 15 (6), 1713-1731 (2016).
- Strong, A. B., Stubley, G. D., Chang, G., Absolom, D. R. Theoretical and experimental analysis of cellular adhesion to polymer surfaces. J Biomed Mater Res. 21 (8), 1039-1055 (1987).
- Sorensen, E. N., Burgreen, G. W., Wagner, W. R., Antaki, J. F. Computational simulation of platelet deposition and activation: I. Model development and properties. Ann Biomed Eng. 27 (4), 436-448 (1999).
- Wu, W. -. T., Jamiolkowski, M. A., Wagner, W. R., Aubry, N., Massoudi, M., Antaki, J. F. Multi-constituent simulation of thrombus deposition. Sci Rep. 7 (1), 42720 (2017).
- Yamane, T. How Do We Select Materials. Mechanism of Artificial Heart. , (2016).
- Sin, D., Kei, H., Miao, X. Surface coatings for ventricular assist devices. Expert Rev Med Devices. 6 (1), 51-60 (2009).
- Zhang, M., Tansley, G. D., Dargusch, M. S., Fraser, J. F., Pauls, J. P. Surface coatings for rotary ventricular assist devices: A systematic review. ASAIO J. 68 (5), 623-632 (2022).
- Linneweber, J., Dohmen, P. M., Kerzscher, U., Affeld, K., Nosé, Y., Konertz, W. The effect of surface roughness on activation of the coagulation system and platelet adhesion in rotary blood pumps. Artif Organs. 31 (5), 345-351 (2007).
- Jayaraman, A., Kang, J., Antaki, J. F., Kirby, B. J. The roles of sub-micron and microscale roughness on shear-driven thrombosis on titanium alloy surfaces. Artif Organs. 47 (3), 490-501 (2023).
- Jamiolkowski, M. A., Pedersen, D. D., Wu, W., Antaki, J. F., Wagner, W. R. Visualization and analysis of biomaterial-centered thrombus formation within a defined crevice under flow. Biomaterials. 96, 72-83 (2016).
- Zhussupbekov, M., Wu, W. -. T., Jamiolkowski, M. A., Massoudi, M., Antaki, J. F. Influence of shear rate and surface chemistry on thrombus formation in micro-crevice. J Biomech. 121, 110397 (2021).
- . ASTM F1841-19: Standard practice for assessment of hemolysis in continuous flow blood pumps Available from: https://www.astm.org/f1841-19.html (2019)
- Sarode, D. N., Roy, S. In vitro models for thrombogenicity testing of blood-recirculating medical devices. Expert Rev Med Devices. 16 (7), 603-616 (2019).
- Swier, P., Bos, W. J., Mohammad, S. F., Olsen, D. B., Kolff, W. J. An in vitro test model to study the performance and thrombogenecity of cardiovascular devices. ASAIO Trans. 35 (3), 683-686 (1989).
- Schima, H., et al. In vitro investigation of thrombogenesis in rotary blood pumps. Artif Organs. 17 (7), 605-608 (1993).
- Tayama, E., et al. In vitro thrombogenic evaluation of centrifugal pumps. Artif organs. 21 (5), 418-420 (1997).
- Paul, R., et al. In vitro thrombogenicity testing of artificial organs. Int J Artif Organs. 21 (9), 548-552 (1998).
- Jamiolkowski, M. A., Snyder, T. A., Perkins, I. L., Malinauskas, R. A., Lu, Q. Preclinical device thrombogenicity assessments: Key messages from the 2018 FDA, industry, and academia forum. ASAIO J. 67 (2), 214-219 (2021).
- Maruyama, O., Tomari, Y., Suciyama, D., Nishida, M., Tsutsui, T., Yamane, T. Simple in vitro testing method for antithrombogenic evaluation of centrifugal blood pumps. ASAIO J. 55 (4), 314-322 (2009).
- Olia, S. E., et al. Preclinical performance of a pediatric mechanical circulatory support device: The PediaFlow ventricular assist device. J Thorac Cardiovasc Surg. 156 (4), 1643-1651.e7 (2018).
- Borovetz, H. S., Olia, S. E., Antaki, J. F. Toward the Development of the PediaFlowTM Pediatric Ventricular Assist Device: Past, Present, Future. Appl Eng Sci. 11, 100113 (2022).
- Herbertson, L. H., et al. Multilaboratory study of flow-induced hemolysis using the FDA benchmark nozzle model. Artif Organs. 39 (3), 237-248 (2015).
- Olia, S. E., Herbertson, L. H., Malinauskas, R. A., Kameneva, M. V. A reusable, compliant, small volume blood reservoir for in vitro hemolysis testing. Artif Organs. 41 (2), 175-178 (2017).
- Doherty, T. M., Shavelle, R. M., French, W. J. Reproducibility and variability of activated clotting time measurements in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 65 (3), 330-337 (2005).
- Chia, S., Van Cott, E. M., Raffel, C. O., Jang, I. -. K. Comparison of activated clotting times obtained using Hemochron and Medtronic analysers in patients receiving anti-thrombin therapy during cardiac catheterisation. Thromb Haemost. 101 (03), 535-540 (2009).
- Li, H., Serrick, C., Rao, V., Yip, P. M. A comparative analysis of four activated clotting time measurement devices in cardiac surgery with cardiopulmonary bypass. Perfusion. 36 (6), 610-619 (2021).
- Hastings, S. M., Ku, D. N., Wagoner, S., Maher, K. O., Deshpande, S. Sources of circuit thrombosis in pediatric extracorporeal membrane oxygenation. ASAIO J. 63 (1), 86-92 (2017).
- Starling, R. C., et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 370 (1), 33-40 (2014).
- Hastings, S. M., Deshpande, S. R., Wagoner, S., Maher, K., Ku, D. N. Thrombosis in centrifugal pumps: Location and composition in clinical and in vitro circuits. Int J Artif Organs. 39 (4), 200-204 (2016).
- Patel, M., Jamiolkowski, M. A., Vejendla, A., Bentley, V., Malinauskas, R. A., Lu, Q. Effect of temperature on thrombogenicity testing of biomaterials in an in vitro dynamic flow loop system. ASAIO J. 69 (6), 576-582 (2023).
- . ASTM F2888-19: Standard practice for platelet leukocyte count-An in-vitro measure for hemocompatibility assessment of cardiovascular materials Available from: https://www.astm.org/f2888-19.html (2019)
- Patel, M., Serna, C., Parrish, A., Gupta, A., Jamiolkowski, M., Lu, Q. Alternative anticoagulant strategy to improve the test sensitivity of ASTM F2888-19 standard for platelet and leukocyte count assay. J Biomed Mater Res B Appl Biomater. 112 (12), e35514 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved