Method Article
Picrosirius Red Staining for Semiquantitative Histopathologic Evaluation of Collagen Deposition in Murine Models of Chronic Lung Allograft Rejection
* These authors contributed equally
In This Article
Summary
Picrosirius Red staining is a semiquantitative method for objectively assessing collagen deposition in murine pulmonary fibrotic remodeling in a spatially resolved manner. Apart from evaluating total collagen deposition, Picrosirius Red staining allows for differentiating collagen fibers of different thicknesses.
Abstract
Fibrosis is the pathophysiologic hallmark of chronic rejection after lung transplantation and the foremost hurdle to long-term recipient survival. Several murine lung transplantation models are available for the study of chronic rejection. However, they display heterogeneous results regarding fibrotic changes of the graft, and the histologic extent of fibrosis is mostly reported qualitatively. Therefore, a spatially resolved approach that allows for statistical analysis can aid in evaluating fibrosis in these models. This study presents Picrosirius Red staining for a semiquantitative evaluation of collagen organization in murine lung allografts and compares it to standard Hematoxylin and Eosin, Masson's Trichrome, and Herovici's stains. Staining was performed on sections from two different murine transplantation models based on minor and major histocompatibility complex (MHC) mismatches. The method was established for semiquantitative analysis of collagen organization in whole lung sections. Thus, it can serve as a tool for murine experimental models of fibrotic lung diseases.
Introduction
Lung transplantation is the definite therapeutic option for patients suffering from end-stage lung disease. However, long-term survival is hampered by chronic rejection, affecting 50% of recipients within the first five postoperative years1. Fibrotic remodeling of the small airways and the lung parenchyma is the histologic hallmark underlying the progressive loss of pulmonary function in chronic lung allograft rejection2. Experimentally, chronic lung allograft rejection can be modeled with murine orthotopic lung transplantation between MHC-mismatched mouse strains. Different strain combinations have been proposed to achieve the phenotype of chronic rejection3. Among them, the transplant combination of the minor MHC mismatched C57BL/10 donor and C57BL/6 recipient is frequently used4. Alternatively, BALB/c donor lungs can be transplanted to C57BL/6 recipients receiving daily immunosuppressive treatment5. These models result in histologically different degrees of fibrotic changes6. These changes are often reported qualitatively using the standard Hematoxylin and Eosin, and Masson's Trichrome staining.
The standard histological staining with Hematoxylin and Eosin allows for an overview of the specimen as it stains nuclei blue, while cytoplasm and collagen fibers appear red. With Masson's Trichrome staining, nuclei are stained black, collagen fibers are stained green to blue, and the background, including cytoplasm, fibrin, and muscles, are red. Because Masson's Trichrome stain may lead to underestimated values, using Picrosirius Red stain is considered to be beneficial7. Picrosirius Red is a linear anionic dye that associates with long cationic collagen fibers and provides contrast-rich red staining. Furthermore, Picrosirius Red staining enhances the natural birefringence of collagen fibers under cross-polarized light8. This way, collagen fiber thickness and packing can be evaluated. Using a polarizing filter, the background turns dark, and distinguishing the thickness of deposited collagen becomes possible. While thick collagen fibers appear red, thin collagen fibers emerge green under polarization. A direct association between birefringence and collagen subtype is frequently described in the literature, with red birefringence assigned to collagen I and green birefringence to collagen III9.
This protocol describes the use of Picrosirius Red staining to evaluate fibrosis in murine lung allografts. In addition to conventional histological staining, it allows for a semiquantitative evaluation of fibrotic change in murine models of chronic rejection, provides a means of distinction between thick and thin collagen fibers in only one staining, is cost-efficient, and is easy to perform. This method can equally be applied to other murine experimental models characterized by fibrotic remodeling of the lung parenchyma.
Protocol
All animal protocols comply with the ethical principles of the 3Rs for humane animal research and were approved by the local veterinary ethical committee (Veterinäramt Kanton Zürich, Switzerland, Study number 45/2014). Likewise, readers should obtain permission from the relevant institutions before performing any procedures on laboratory animals.
1. Sample acquisition
- Conduct animal experiments according to individual needs and respective ethical approval. A single microsurgeon performs all surgeries without any additional prolonged ischemia times.
NOTE: For a reproduction of our representative results, obtain specific pathogen-free adult male C57BL/6J, C57BL/10J, and BALB/c mice weighing 27-30 g from Charles River Laboratories. For isografts, use C57BL/6J animals as both donors and recipients. In the minor mismatched model, transplant C57BL/10J donor lungs to C57BL/6J recipients. In the major mismatched model, transplant BALB/c donor lungs to C57BL/6J recipients and postoperatively inject cyclosporine (10 mg/kg/day) and methylprednisolone (1.6 mg/kg/day) subcutaneously daily5. Use saline solution for perfusion and retrieve lungs after 8 weeks (56 days). - For euthanasia, intubate the animal with a 20G catheter and ventilate it with O2 supplemented with 2%-3% isoflurane. Confirm sufficient depth of anesthesia by the absence of a response to a paw pinch.
- Perform a median laparosternotomy by incising the abdominal wall with surgical scissors and cutting the length of the sternum to expose the heart. Identify the inferior vena cava and the left atrial appendage by their anatomical location.
- In quick succession, cut the inferior vena cava and the left atrial appendage with surgical scissors and perfuse the animal with 3-5 mL of 0.9% NaCl through the root of the pulmonary artery with a syringe to remove all blood from the pulmonary circulation.
NOTE: Euthanasia should be performed according to the respective ethical approval. Other methods can be equally applied. It is recommended that blood from the pulmonary circulation be thoroughly removed, which can also be performed by perfusion after circulatory arrest. - Remove the perfused lungs using scissors.
- For conservation, incubate the samples in 4% formalin. Inject the formalin solution directly into the bronchus and pulmonary vasculature. Submerge the samples in formalin and let them incubate for at least 6 h.
NOTE: For inflation of the lung with formalin, a standardized procedure should be applied to obtain comparable results. Example protocols for such procedures are published elsewhere10,11. - Trim the organ according to experimental needs. Transversally cut the lungs in half to capture all parts of the bronchial tree in a section.
- Transfer samples to an automatic tissue processor for dehydration in alcohol sequence, clearing in xylol and paraffin coating as follows: 70% ethanol, 70% ethanol, 80% ethanol, 96% ethanol, 100% ethanol (1 h each), 100% ethanol, 100% ethanol, xylol (2 h each), xylol (1 h), and paraffin 3x for 2 h.
- Melt paraffin and embed samples in the correct orientation in Polyoxymethylen embedding cassettes. Take care to embed the samples with the cut surface facing downwards. Use a rotary microtome to cut 5 µm sections consecutively. Let the slides dry for 24 h at 37 °C before further processing.
2. Deparaffinization and hydration of paraffin slides
- Remove paraffin by incubating the samples 2x in 100% xylol for 5 min each.
CAUTION: Xylol is flammable and hazardous to health. Only use under the fume hood. - Hydrate the samples in 100% ethanol, 100% ethanol, 95% ethanol, 70% ethanol, and 50% ethanol for 2 min each. Then, wash for 2 min in distilled water.
3. Staining with Picrosirius Red
- Stain slides for 8 min in Meyer's hemalum solution. Wash slides for 10 min under running tap water.
- Incubate slides for 1 h in Picrosirius Red solution. For Picrosirius Red solution, either use a premixed staining solution or use 0.1% of Direct Red 80 or Sirius Red F 3B (CI 35780) diluted in saturated picric acid.
CAUTION: Picric acid is flammable and highly explosive and should be treated carefully.
NOTE: There are many different suppliers for Picrosirius Red staining kits available, as listed in the Table of Materials. Be aware of differences in price and staining methods, and always adhere to suppliers' instructions. It is recommended to only use staining kits with the specific color index for Sirius Red F 3B (CI 35780). - Dip the slides 5x-10x in an acetic acid solution (0.5%). Gently shake the slides to physically remove the solution.
- Dehydrate the slides in 95% ethanol, 100% ethanol, 100% ethanol, 100% xylol, and 100% xylol for 5 min each.
- Embed by adding one drop of mounting medium on each sample and cover with a coverslip. Let slides dry under a fume hood.
4. Digitalization and image processing
- Focus the samples under a light microscope with appropriate imaging software until the picture becomes clear and sharp. Add a polarizing filter and adjust the degree of polarization until the background is completely dark or black. When the background is as dark as possible, the correct polarization is achieved.
NOTE: It is also possible to visualize Picrosirius Red staining with conventional transmitted light and without a polarizing filter. For this purpose, it is recommended to use Sirius Red with Fast Green, as it has been shown to be more sensitive than Sirius Red alone12,13. However, polarizing microscopy is necessary to distinguish between thick and thin collagen fibers. - Scan all samples completely under 20x magnification and export as .tiff. Transfer to Fiji software14. Make sure to clean slides properly in advance and use standardized settings regarding exposure time and light source intensity.
- Surround the sample manually and cut it out in the software using Edit > Clear Outside. Measure the total surface area by clicking Analyze > Measure.
NOTE: The selection of the compartment to measure is a critical step in creating reliable and comparable results. By digitalizing and selecting the entire sample, a good cross-section is displayed, and heterogeneous tissues are considered as a whole. However, it is also possible to confine oneself to specific areas within the samples, such as pleura or individual bronchi. Keep in mind to always select compartments to compare in a standardized fashion. Should the samples show a large variability in ventilation, it is advisable to exclude the airspace area from the analyzed compartment15. - Measure total collagen content per sample using color threshold by clicking Image > Adjust > Color Threshold and adjust the settings by clicking Analyze > Analyze Particles. Set Size = 1 - Infinity, Clear, Summarize. Brightness = 35 - 255. Hue = 2 - 130
NOTE: A reasonable setting of the color thresholds is critical to achieve reliable results. - Repeat for thick and thin collagen fibers by adjusting Hue as Red
 Thick Collagen Fibers (Hue 2-30) and Yellow-Green
Thick Collagen Fibers (Hue 2-30) and Yellow-Green  Thin Collagen Fibers (Hue 31-130). The result will be the area of stained pixels.
Thin Collagen Fibers (Hue 31-130). The result will be the area of stained pixels. - Configure Macro for automated processing by clicking Plugins > Macros > Record and repeat with all samples.
- To receive final values standardized to the cross-section's surface area, divide the area of stained pixels for all parameters by the total surface area as measured in steps 4.3 and 4.5 for each sample, respectively.
Results
The protocol described above allows for an objective semiquantitative evaluation of collagen deposition in murine lung tissue. Fibrotic remodeling is the pathophysiological hallmark of chronic rejection after lung transplantation. Therefore, Picrosirius Red staining was applied in chronic lung allograft rejection models using left-sided murine orthotopic lung transplantation. The major MHC-mismatched lung transplantation from a BALB/c donor to a C57BL/6 recipient under mild immunosuppression results in fibrotic changes comparable to human chronic lung allograft dysfunction5. In contrast, minor MHC-mismatched transplantation from a C57BL/10 donor to a C57BL/6 recipient primarily results in lymphocytic bronchiolitis6. Figure 1 depicts the experimental setup in detail.
Figure 2 provides an overview of representative images from transplanted left lungs of the two models as well as a C57BL/6J isograft. Masson's Trichrome16, Herovici's17, and Picrosirius Red18 staining illustrates the extensive peribronchial and perivascular collagen deposition in the major MHC-mismatched model (Model 1, Figure 2B,D). In contrast, the minor mismatched model (Model 2) primarily shows dense lymphocytic infiltrates as reflected by Hematoxylin and Eosin staining (Figure 2F) and less intense collagen deposition (Figure 2G-J). In Picrosirius Red staining, the addition of the polarization filter reveals a primarily green appearance of the deposited fibers across both models (Figure 2E,J). In isograft controls, the presence of collagen is restricted to the direct peribronchial and perivascular borders (Figure 2K-O), similar to the structure of naïve murine lungs (Figure 3).
Both naïve left-sided lungs from BALB/c animals and right-sided lungs from C57BL/6J recipients show only a thin lining of collagen along the peribronchial and perivascular borders (Figure 3).
Digital image analysis of whole lung sections stained with Picrosirius Red revealed an increased presence of total collagen in the lung grafts in the major MHC-mismatched model when compared to the grafts from the minor mismatched model (p=0.0038), right-sided naïve lung tissue (p=0.0006) or naïve BALB/c left lungs (p=0.0003,One-way ANOVA, Tukey's multiple comparisons test, Figure 4A). For thick collagen fibers, which appear red under polarized light, differences between the groups could not be demonstrated (p=0.5512, One-way ANOVA, Figure 4B). However, the analysis of thin collagen fibers with a green appearance under polarized light revealed an increased presence in the major mismatched model when compared to the minor mismatched model (p=0.0005), naïve right-sided lung tissue (p<0.0001) or naïve left-sided BALB/c lungs (p<0.0001, One-way ANOVA, Tukey's multiple comparisons test, Figure 4C).

Figure 1: Overview of the experimental design. Orthotopic left lung transplantation was performed using a major (Model 1) and a minor mismatched strain combination (Model 2). Recipients of an MHC major mismatched graft received daily immunosuppression with 10 mg/kg Cyclosporine and 1.6 mg/kg Methylprednisolone by subcutaneous injection. All recipient animals were euthanized at eight weeks (day 56) postoperatively. Lung tissue was processed by formalin fixation, paraffin embedding, and sectioning. Consecutive sections were stained with Hematoxylin and Eosin (H&E), Masson's Trichrome (MTC), Herovici's, and Picrosirius Red (PSR) staining. Please click here to view a larger version of this figure.

Figure 2: Representative images of the peribronchiovascular region in transplanted lungs. (A-E) The major MHC-mismatched Model 1 results in peribronchial and perivascular fibrosis, as evidenced by Masson's Trichrome (MTC), Herovici's, and Picrosirius Red staining (PSR). This is also reflected by adding the polarization filter (PSR (pol)). (F-J) The minor MHC-mismatched Model 2 presents with prominent peribronchiovascular lymphocytic infiltrates, as demonstrated by Hematoxylin and Eosin staining (H&E). MTC and PSR staining only show a limited extent of collagen deposition in this model. (K-O) In isograft controls, collagen deposition is restricted to the direct peribronchiovascular regions. Lymphocytic infiltrates are scarce. Please click here to view a larger version of this figure.
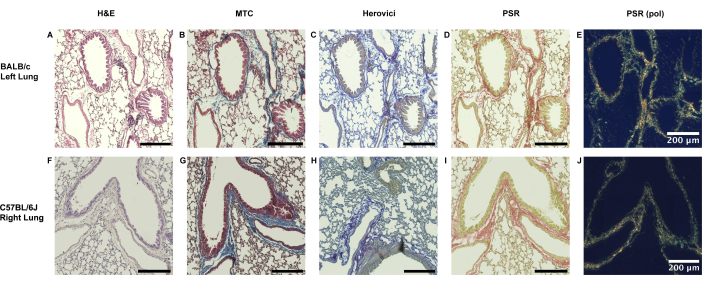
Figure 3: Physiological extent of peribronchiovascular collagen presence in murine lungs. (A-E) Collagen presence in murine lung tissue is physiological to a certain extent without major strain dependence. In left-sided naïve BALB/c lungs, as used for the major mismatched model (Model 1), a thin lining of collagen can be seen in the peribronchiovascular area as evidenced by Masson's Trichrome (MTC), Herovici's and Picrosirius Red staining (PSR). (F-J) Similarly, a collagen lining restricted to the direct peribronchiovascular border is seen in the contralateral right lungs of recipient C57BL/6J origin. Abbreviations: H&E = Hematoxylin and Eosin staining; PSR (pol) = Picrosirius Red staining after adding the polarization filter. Please click here to view a larger version of this figure.

Figure 4: Picrosirius Red staining under polarized light reveals increased deposition of thin collagen fibers in the major MHC-mismatched murine transplantation model. Image analysis of polarized Picrosirius Red staining over the whole area of transversal lung sections. (A) Analysis of total collagen deposition reveals increased collagen deposition in the major mismatched model compared to minor mismatched allografts, contralateral recipient lungs, or naïve BALB/c left lung grafts. (B) No significant differences were found between the groups regarding thick collagen fibers. (C) Analysis of thin collagen fiber presence reveals increased deposition in major mismatched allografts compared to the minor mismatched group, contralateral recipient lungs, or naïve BALB/c left lung grafts. Graphs show mean ± standard error of the mean, One-way ANOVA, Tukey's multiple comparisons used for significance, * p < 0.05, ** p < 0.005, *** p < 0.0005, **** p < 0.0001, ns not significant. Please click here to view a larger version of this figure.
Discussion
Standard histologic methods such as Hematoxylin and Eosin, and Masson's Trichrome staining are widely used to detect fibrotic changes in murine lungs in a spatially resolved manner16,19. However, additional methods are often necessary to quantify these changes and evaluate the tissue's collagen composition.
Picrosirius Red staining was first described in 1964 to identify collagen: under transmitted light microscopy, collagen appears red while muscle and cytoplasm emerge yellow18. Slotting a polarizing filter ahead and taking advantage of birefringence enabled the differentiation of collagen fiber thickness. The correlation between fibrillar hue and collagen fiber thickness has been demonstrated repeatedly since and is frequently used for assessing total collagen content and estimating collagen subtypes in fibrotic remodeling9,20,21,22,23,24,25. However, more recent studies question the direct association of collagen subtype and hue26,27. Therefore, a cautious reporting of collagen fiber thickness should be preferred over directly inferring collagen subtype discrimination.
Staining collagen with Picrosirius Red and using polarizing microscopy allows the localization of tissue components containing collagen molecules28. In comparison to other collagen staining methods like Van Gieson or Masson's trichrome, it also enables the visualization of very thin collagen fibers7, which mainly arise during fibrosis in allograft rejection29. Additionally, it enables the quantitative detection of these fiber types in only one staining. However, this method only allows semiquantitative evaluation and no absolute statements. Therefore, it is required to follow the protocol precisely and, whenever possible, stain all sections simultaneously. The same applies to settings for microscopy and image processing options.
When selecting the compartment of interest for evaluation, it is crucial to use an individually standardized approach. This protocol describes a whole sample selection of entire cross-sections. Furthermore, the use of a circular polarizing filter is recommended, as linear polarization may lead to underestimated values7.
A simple staining analysis can be achieved with the freely available Fiji software package, as described in the protocol presented here. However, more sophisticated methods for digital image analysis of the staining have been described in the literature and can be employed according to the individual researcher's needs13,15. Recently, artificial intelligence tools have gained interest in digital imaging analysis and could likewise be trained to evaluate Picrosirius Red staining30.
Fibrotic tissue remodeling is the histopathological hallmark of chronic graft failure in solid organ transplantation. Therefore, Picrosirius Red staining can be a valuable read-out in experimental transplantation. Indeed, the method was used in the clinic for the evaluation of tissue biopsies in heart transplant recipients31. The clinical use of Picrosirius Red staining in lung transplantation has not been reported, even though the early detection of thin collagen III deposition is discussed as a potential biomarker for CLAD diagnosis32. In preclinical murine models of the disease, it can be a valuable tool as it allows for a semiquantitative evaluation of collagen deposition with relatively little tissue consumption.
The representative results presented here demonstrate that digital image analysis of Picrosirius Red Staining can show the enhanced deposition of collagen fibers in a major-mismatched model of chronic rejection after orthotopic lung transplantation when compared to a minor-mismatched strain combination. Our group previously reported this observation in a more descriptive manner, relying on Masson's Trichrome Staining16. Picrosirius Red Staining, with the addition of a polarizing filter, allows for a semiquantitative analysis and statistical reporting of fibrotic change in lung allografts. The major benefits of the method are its technical simplicity and small tissue consumption, which are important aspects to consider during read-out method selection following technically complex animal models, such as murine orthotopic lung transplantation.
Researchers should be aware that even though staining has historically been used for collagen subtype estimation, recent publications have found that the method cannot reliably distinguish collagen types I and III27. The method can provide information on collagen organization by reflecting fiber thickness26, it may not be sufficient for a comprehensive evaluation of collagen deposition.
Disclosures
The authors have nothing to disclose.
Acknowledgements
Birte Ohm is supported by the Berta-Ottenstein-Program for Clinician Scientists, Faculty of Medicine, University of Freiburg. Steffen U Eisenhardt is a Heisenberg Professor of the German Research Foundation (DFG) and supported this work with personal grants. Furthermore, we would like to thank Sheena Kreuzaler for her technical assistance. Figure 1 was created with the help of Biorender.com. Imaging was performed at the Lighthouse Core Facility, which is funded in part by the Medical Faculty, University of Freiburg (Project Numbers 2023/A2-Fol; 2021/B3-Fol), the DKTK, and the DFG (Project Number 450392965).
Materials
| Name | Company | Catalog Number | Comments |
| Acetic acid | Honeywell, Charlotte, USA | 33209 | |
| Axio Observer | Zeiss, Oberkochen, Germany | 4633000956 (serial number) | |
| Coverslip 1.5 | Roth, Karlsruhe, Germany | KCY5.1 | |
| Formaldehyde 37% | Fisher Scientific, Leicestershire, UK | F/1501/PB15 | |
| Meyer's hemalum solution | Merck, Darmstadt, Germany | 109249 | |
| Picrosirius Red Solution | Morphisto, Offenbach am Main, Germany | 13422 | alternatives that can be used: ab150681, abcam, Cambridge, UK; SRS250 ScyTek Laboratories, Logan City US |
| Polarizing filter | Zeiss, Oberkochen, Germany | 000000-1121-813 | |
| Rotary microtome, HistoCore AUTOCUT | Leica, Wetzlar, Germany | 149AUTO00C1, 14051956472 | |
| ROTI Histokitt mounting medium | Roth, Karlsruhe, Germany | 6638.1 | |
| ROTI Plast Paraffin | Roth, Karlsruhe, Germany | 6642.5 | |
| Rotilabo-embedding cassettes, POM | Roth, Karlsruhe, Germany | K113.1 | |
| Superfrost Plus Adhesion Microscope slide | epredia, Portsmouth, UK | J1800AMNZ | |
| Tissue Processor | Leica, Wetzlar, Germany | TP 1020 | |
| Software | |||
| Fiji software version 2.14.0/1.54f | Open Source | ||
| Imaging Software ZEN 3.4.91 | Zeiss, Oberkochen, Germany |
References
- Weigt, S., DerHovanessian, A., Wallace, W., Lynch, J., Belperio, J. Bronchiolitis Obliterans Syndrome: The Achilles' heel of lung transplantation. Semin Respir Crit Care Med. 34 (3), 336-351 (2013).
- Verleden, G. M., et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. 38 (5), 493-503 (2019).
- Lama, V. N., et al. Models of lung transplant research: A consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight. 2 (9), e93121 (2017).
- Martinu, T., et al. Spectrum of chronic lung allograft pathology in a mouse minor-mismatched orthotopic lung transplant model. Am J Transplant. 19 (1), 247-258 (2019).
- de Vleeschauwer, S., et al. Chronic rejection pathology after orthotopic lung transplantation in mice: The development of a murine BOS model and its drawbacks. PLoS One. 7 (1), e29802 (2012).
- Yamada, Y., et al. Chronic airway fibrosis in orthotopic mouse lung transplantation models-An experimental reappraisal. Transplantation. 102 (2), e49-e58 (2018).
- Whittaker, P., Kloner, R. A., Boughner, D. R., Pickering, J. G. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 89 (5), 397-410 (1994).
- Puchtler, H., Waldrop, F. S., Valentine, L. S. Polarization microscopic studies of connective tissue stained with picro-sirius red FBA. Beitr Pathol. 150 (2), 174-187 (1973).
- Junqueira, L. C. U., Cossermelli, W., Brentani, R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch Histol Jpn. 41 (3), 267-274 (1978).
- Davenport, M. L., Sherrill, T. P., Blackwell, T. S., Edmonds, M. D. Perfusion and inflation of the mouse lung for tumor histology. J Vis Exp. (162), e60605 (2020).
- Limjunyawong, N., Mock, J., Mitzner, W. Instillation and fixation methods useful in mouse lung cancer research. J Vis Exp. (102), e52964 (2015).
- Segnani, C., et al. Histochemical detection of collagen fibers by Sirius Red/Fast Green is more sensitive than van Gieson or Sirius Red alone in normal and inflamed rat colon. PLoS One. 10 (12), e0144630 (2015).
- Courtoy, G. E., et al. Digital image analysis of Picrosirius Red staining: A robust method for multi-organ fibrosis quantification and characterization. Biomolecules. 10 (11), 1585 (2020).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Ségard, B. D., et al. Quantification of fibrosis extend and airspace availability in lung: A semi-automativ ImageJ/Fiji toolbox. PloS. 2 (2), e0298015 (2024).
- Zhou, X., Moore, B. B. Lung section staining and microscopy. Bio Protoc. 7 (10), e2286 (2017).
- Herovici, C. Picropolychrome: histological staining technic intended for the study of normal and pathological connective tissue. Rev Fr Etud Clin Biol. 8, 88-89 (1963).
- Sweat, F., Puchtler, H., Rosenthal, S. Sirius Red F3BA as a stain for connective tissue. Arch Pathol. 78, 69-72 (1964).
- Cardiff, R. D., Miller, C. H., Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014 (6), 655-658 (2014).
- Montes, G. S., Junqueira, L. C. The use of the Picrosirius-polarization method for the study of the biopathology of collagen. Mem Inst Oswaldo Cruz. 86, 1-11 (1991).
- Rittié, L. Method for Picrosirius Red-polarization detection of collagen fibers in tissue sections. Methods Mol Biol. 1627, 395-407 (2017).
- Diehm, Y. F., et al. Stem cell-enriched hybrid breast reconstruction reduces risk for capsular contracture in a hybrid breast model. Plast Reconstr Surg. 152 (3), 572-580 (2023).
- Rich, L., Whittaker, P. Collagen and Picrosirius Red staining: A polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Sci. 22 (2), 97-104 (2005).
- Datar, U. V., et al. Clinicopathologic study of a series of giant cell fibroma using picrosirius red polarizing microscopy technique. Arch Iran Med. 17 (11), 746-749 (2014).
- Nishat, R., Kumar, H. Collagen fibers in oral submucous fibrosis - A polarizing microscopy study using two special stains. Indian J Pathol Microbiol. 62 (4), 537-543 (2019).
- Lattouf, R., et al. Picrosirius red staining: a useful tool to appraise collagen networks in normal and pathological tissues. J Histochem Cytochem. 62 (10), 751-758 (2014).
- López De Padilla, C. M. Picrosirius Red staining: Revisiting its application to the qualitative and quantitative assessment of collagen type I and type III in tendon. J Histochem Cytochem. 69 (10), 633-643 (2021).
- Junqueira, L. C. U., Bignolas, G., Brentani, R. R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 11 (4), 447-455 (1979).
- Zheng, L., et al. Scar collagen deposition in the airways of allografts of lung transplant recipients. Am J Respir Crit Care Med. 155 (6), 2072-2077 (1997).
- Astbury, S., et al. Reliable computational quantification of liver fibrosis is compromised by inherent staining variation. J Pathol Clin Res. 7 (5), 471-481 (2021).
- Feingold, B., et al. Diffuse myocardial fibrosis among healthy pediatric heart transplant recipients: Correlation of histology, cardiovascular magnetic resonance, and clinical phenotype. Pediatr Transplant. 21 (5), 12986 (2017).
- vander Ploeg, E. A., Melgert, B. N., Burgess, J. K., Gan, C. T. The potential of biomarkers of fibrosis in chronic lung allograft dysfunction. Transplant Rev (Orlando). 35 (3), 100626 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved