Method Article
Investigating Flagella-Driven Motility in Escherichia coli by Applying Three Established Techniques in a Series
In This Article
Summary
Many bacteria use flagella-driven motility to navigate their environment and colonize favorable surroundings both individually and as a collective. Demonstrated here is the use of three established methods that exploit motility as a selection tool to identify components/pathways contributing to swimming and swarming motility.
Abstract
Motility is crucial to the survival and success of many bacterial species. Many methodologies exist to exploit motility to understand signaling pathways, to elucidate the function and assembly of flagellar parts, and to examine and understand patterns of movement. Here we demonstrate a combination of three of these methodologies. Motility in soft agar is the oldest, offering a strong selection for isolating gain-of-function suppressor mutations in motility-impaired strains, where motility is restored through a second mutation. The cell-tethering technique, first employed to demonstrate the rotary nature of the flagellar motor, can be used to assess the impact of signaling effectors on the motor speed and its ability to switch rotational direction. The “border-crossing” assay is more recent, where swimming bacteria can be primed to transition into moving collectively as a swarm. In combination, these protocols represent a systematic and powerful approach to identifying components of the motility machinery, and to characterizing their role in different facets of swimming and swarming. They can be easily adapted to study motility in other bacterial species.
Introduction
Bacteria employ many appendages for movement and dispersal in their ecological niches1. Flagella-driven motility is the fastest of these, promoting the colonization of favorable locales in response to environmental signals, and contributing significantly to the pathogenic ability of some species2,3. Flagellated bacteria can swim individually in bulk liquid, or swarm as a collective over a semi-solid surface4. Extracellular flagella attach to and are driven by rotary motors embedded in the membrane, which harness the power of ion gradients to generate torque that causes rotation1,2,4,5,6,7,8. In E. coli, whose motors run at a constant torque9, the motor output can be categorized in terms of rotational speed and switching of the rotor between counter-clockwise (CCW) and clockwise (CW) directions. CCW rotation promotes formation of a coherent flagellar bundle that propels the cell forward (run), while a transient switch in rotational direction (CW) causes the bundle to disassemble either partially or fully10, and the cell to reorient its swimming direction (tumble). E. coli typically run for a second and tumble for a tenth of a second. Switching frequency of the rotor or ‘tumble bias’ is controlled by the chemotaxis signaling system, wherein transmembrane chemoreceptors detect external chemical signals and transmit them via phosphorelay to the flagellar motor to extend runs in response to attractants, or suppress them in response to toxic chemicals11,12. Swimming motility is assayed in 0.3% soft agar.
During swarming, bacteria navigate on a semi-solid surface as a dense collective, where packs of bacteria stream in a continuous swirling motion2,13,14,15. E. coli swarms exhibit altered chemosensory physiology (lower tumble bias), higher speeds, and higher tolerance to antimicrobials over cells swimming in bulk liquid16,17. Swarmers vary in their deployment of a plethora of strategies that aid movement, including surfactant production, hyperflagellation, and cell elongation2. Swarming offers bacteria a competitive advantage in both ecological and clinical settings18,19,20. There are two categories of swarming bacteria: temperate swarmers, which can swarm only on media solidified with 0.5-0.8% agar, and robust swarmers, which can navigate across higher agar concentrations21.
A variety of assays exist to interrogate swimming motility and its regulation. When impaired by mutations or environmental conditions, motility itself offers a strong selection for identifying gain-of-function suppressor mutations. These suppressors can be genuine revertants of the original mutation, or pseudo-revertants, where a second mutation restores functionality. Such mutants can be identified by whole genome sequencing (WGS). An alternative to unbiased suppressor selection is a biased targeted mutagenesis strategy (e.g., PCR mutagenesis). These methodologies often shed light on the function or environmental regulation of the motility apparatus. If the goal is to study motor function, then the restoration of wild-type motility as measured in soft agar may not necessarily indicate restoration of wild-type motor output. The cell-tethering assay, in which cells are attached to a glass surface by a single flagellum and rotation of the cell body is subsequently monitored, can be the initial assay of choice for assessing motor behavior. Although more sophisticated methodologies are now available to monitor motor properties, the required high-speed camera set-up and application of software packages for motion analysis limit their widespread use22,23,24,25. The cell-tethering assay requires only that the flagella be sheared, allowing attachment of the short filaments to a glass slide, followed by videotaping the rotation of the cell body. Although the recorded motor speeds are low in this assay because of the high load the cell body exerts on the flagellum, this assay has nonetheless contributed to valuable insights into chemotactic responses26,27,28,29, and remains a valid investigative tool as discussed below.
Swarming motility poses a different set of challenges to researchers. Selection of gain-of-function suppressors only works in swarmers that produce copious surfactants and swarm readily13. Surfactant non-producers such as E. coli are fastidious with respect to the choice of agar, media composition and humidity of the environment2,13,14,21. Once swarming conditions are established, the border-crossing assay17 is a useful methodology to interrogate the ability of a swarm to navigate new/harsh conditions. Though the protocols presented below relate to E. coli, they can be readily adapted for application in other species.
Protocol
1. Isolation of suppressor mutants in motility-deficient strains
NOTE: Use this method as a broad ‘catch-all’ to identify the general nature of the motility defect.
- Soft-agar plate preparation
NOTE: Soft-agar, also referred to as motility- or swim-agar, is a low percentage agar (~0.2-0.35% w/v), long used to assay chemotaxis31,32.- Add 3 g of bacto-agar (0.3% w/v) and 20 g of LB to a 2 L round bottom flask. Add 1 L of ddH2O (double-distilled water) to the flask and evenly mix the suspension using a stir rod and magnetic stirring plate.
- Autoclave for 20 min at 121 °C.
- Allow to cool with gentle agitation using the rod/plate as above. When the temperature reaches approximately 50 °C, pour 25 mL into sterile Petri dishes (100 mm x 15 mm), and allow the molten agar to set with lid in place for at least 1 h, for use within 16 h.
- Culture preparation, inoculation, and isolation of suppressor mutants
NOTE: E. coli inoculated in the center of nutrient rich media solidified with soft agar consume nutrients locally, creating a nutrient gradient that they follow. As they move outward, defined ‘rings’ appear (Figure 1A), which are related to specific chemoattractants the bacteria respond to. Defects in either the chemotaxis system or structural components of the flagella motor can compromise performance in this assay. Often, mutants with a motility advantage arise during screening, and can be seen emerging from single or from multiple points along the periphery of the ring, from where they ‘flare’ out (Figure 1C). One will notice that the outermost edge of the swimming front contrasts readily against the uncolonized virgin soft agar.- Grow overnight cultures of the desired motility-deficient strain in 5 mL of Lennox Broth (LB; 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl, Table of Materials) at 30 °C with horizontal shaking (220 r.p.m.). The following day, sub-culture (1:100 dilution) in fresh LB, growing under the same conditions to exponential phase (OD600 of 0.6).
- Inoculate 6 µL of the culture into the center of a soft-agar plate (1.1) using a pipette, pushing the loaded sterile tip into the agar to gently expel the contents. Transfer to 30 °C and incubate (Figure 1B), until motility ‘flares’ are evident, emanating from the inoculation point or the periphery of the motility rings, typically in 24-36 h (Figure 1C).
NOTE: In motility assays, inoculate a wild-type strain alongside the mutant isolates for comparison. This wild-type strain will show the characteristic concentric chemotactic rings (Figure 1A) and fill the plate within 8-10 h. - Use a sterile wire-loop to lift the cells from the ‘flare’ region and streak to purify single colonies onto an LB hard agar plate (LB prepared as above, solidified with 15 g/L bacto-agar).
- Pick single colonies from the streak plate using a sterile wire loop and re-purify by streaking for single colonies to ensure isolation of a ‘pure’ colony isolate.
- Confirmation, and characterization of suppressor mutants
- Confirm that the isolated suppressor mutant(s) have restored motility. Prepare soft-agar plates (1.1), and cultures for strains of interest (as in 1.2.1), including wild-type and the starting ‘motility-deficient’ strain for comparison.
- Inoculate the plates (as in 1.2.2) and incubate at 30 °C for 8-10 h.
- Record the diameter of the outermost ring (edge of the circle) and compare to establish which of the isolates have substantially restored motility.
NOTE: It is recommended that plates be photographed throughout the time-course of the experiment. For best results, use a “bucket of light” device30, where a digital camera is mounted above a light source for better illumination to measure the diameter of the swim colony and distinguish it from uncolonized agar. - Subject verified isolates to WGS as required, allowing for sufficient ‘sequence coverage’ to positively identify the mutations that restored wild-type function.
2. Quantifying flagella motor behavior via cell tethering
NOTE: Use this method when normal run-tumble behavior (chemotaxis) appears to be compromised.
- Culture preparation and flagella shearing
- Prepare an exponential phase culture of the strain of interest as described in step 1.2.1.
- Pellet 10 mL of cells by centrifugation at 2,000 x g for 3 min before resuspending in 10 mL of filter-sterilized Motility Buffer (MB; 10 mM potassium phosphate buffer [0.0935 M K2HPO4, 0.0065 M KH2PO4, pH 7.0], 0.1 mM EDTA [pH 7.0], 10 mM NaCl, 75 mM KCl).
NOTE: MB supports motility, but does not support bacterial growth - Repeat step 2.1.2 two more times before resuspending the final pellet in 1 mL of MB.
- Transfer the cell suspension into a 1 mL syringe and attach a 23G needle to the end. Assemble an identical syringe/needle apparatus and attach the two together via 6 inches of polyethylene tubing (inner diameter of 0.58 mm) tightly sheathed over each needle tip.
- Shear the flagella (they are fragile and break easily) by gently passing the cell suspension back and forth from one syringe to the other 50x, with 1 min pauses between every 10 passes.
- Centrifuge the sheared cells at 2,000 x g for 3 min and resuspend in a final volume of 500 µL of MB.
- Slide preparation and cell tethering
- Prepare a cell fixation chamber by stacking an 18 mm x 18 mm coverslip over a 3 inch x 1 inch x 1 mm glass microscope slide, separated by double-sided tape (Figure S1).
- Flush the chamber with 0.01% (w/v) poly-lysine solution by applying to the top of the chamber. Tilt the bottom edge (the coverslip flush with the microscope slide) onto a task wiper (tissue paper) to help draw solution through the chamber (Figure S1). Then incubate at room temperature for 10 min.
- Wash the chamber three times with 40 µL of MB using as described in step 2.2.2
- Add 40 µL of the sheared cell suspension (prepared above) to the top of the chamber and incubate at room temperature for 10 min to allow cells to attach to the coverslip.
- Gently flush the chamber with 40 µL of MB as in 2.2.3 to remove unattached cells.
- Cell rotation recording and quantification
- Transfer the microscope slide loaded with tethered cells to the microscope stage.
- Using phase-contrast microscopy and a 100x objective, scan the population for cells that are fixed in place, and rotating on a single axis, i.e., smooth rotations on a fixed point rather than presenting at an angle wherein the cell moves in and out of focus (Video 1).
- Use a commercial microscope and associated camera. Open the associated software, ensure cells of interest are in focus and click video acquisition to record the cell rotation for one minute (at 10 frames per second or higher).
- From video playback, quantify the number of complete rotations per minute and the number of times the cell changes direction (switching frequency).
NOTE: Rotational speeds and switching frequency may be too fast to gauge by eye so it is recommended to use video software that offers slow/fine playback, or adopts an automated software system to quantify rotational patterns33. An alternative would be to increase the viscosity of the MB using methyl cellulose (or similar agent) to help slow and resolve the rotation of faster bacteria or compensate when cameras with low framerates are in use. - Repeat step 2.3.2 with biological replicates to compile a representation of the population of interest.
3. Preparation of swarms in a border-crossing assay
NOTE: Use this method for assessing the impact of a mutation or condition on group motility. Swarm-agar refers to agar where the percentage is typically higher than that of soft-agar. In soft agar (0.3 %), cells swim individually inside the agar. In swarm agar (0.5% and above), cells move as a group on the surface. While swarm plates must be used as detailed here, swim plates have a longer shelf life, and may be used for several days. Our personal preference is to use in 1-2 days.
- Preparation of swarm agar
- Add 5 g of Eiken agar (0.5% w/v) and 20 g of LB to a 2 L round bottom flask. Add 1 L of ddH2O to the flask and evenly mix the suspension using a stir rod and magnetic stirring plate.
- Autoclave for 20 min at 121 °C.
- Allow to cool with gentle agitation to avoid any air bubbles using the rod/plate as above. When approximately 50 °C, add filter-sterilized glucose for a final concentration of 0.5 %.
- Pour 25 mL into sterile Petri dishes (100 mm x 15 mm) and allow to set at room temperature for at least 14 h and no more than 20 h. Do not store for future use.
- Inoculation and incubation of swarm plates
- Inoculate 6 µL of a mid-exponential culture (prepared as in 1.2) by spotting on top of the agar.
- Leave the lid off for 5-10 min and replace when the inoculum has dried into the agar surface.
- Incubate at 30 °C for 8 h. Avoid the temptation to inspect swarm progress by removing the lid, as this will contribute to drying of the agar and impair swarming.
NOTE: Incubation time may vary depending on the strain phenotype. Some isolated mutations may hamper swarming ability and will require a reduced percentage of agar or a more prolonged period of incubation.
- Preparation of border-crossing assay plates
NOTE: This assay utilizes a modified Petri dish, where a plastic divide (border) creates two chambers, rather than one (Figure 2A). Each chamber can be prepared independently of the other, offering differing conditions for swarming, prior to ‘connecting’ the two. Depending on experimental design, the first chamber (designated left) can be prepared with either swim agar (0.3% w/v) or swarm agar (0.5% w/v) from where the bacteria can migrate across the border into the right chamber containing swarm agar +/- any required supplement or challenge (e.g., antibiotics). Migration on either agar is typically measured/compared by recording the widest diameter of bacterial colonization (edge to edge) from the original point of inoculation.- Prepare swim agar as described in 1.1 if required.
- Prepare swarm agar as described in 3.1.
- Pour ~30 mL of swarm agar (with desired supplementation if required) into the right chamber of a dual-compartment Petri dish (100 mm x 15 mm), to the point where it is level with the plastic divider between chambers, but not overflowing into the left (Figure 2B).
- After the agar has hardened, fill the left chamber with ~30 mL of swim or swarm agar, again to the point of contact with the plastic divide (Figure 2C). Before it sets, use a sterile pipette tip to gently drag the agar over the border to connect the two sides with a ~1 mm tall agar bridge that spans the entire length of the divide (Figure 2D).
- Allow the plate to dry at room temperature (3.1).
NOTE: An alternative method of creating bridge is allow the left chamber agar to dry and then slowly pipette ~100 μL of molten swarm agar along the plastic divider to bridge the two chambers (3.4). Inoculate the plates on the left chamber as detailed above for swim (1.2.2) or swarm (3.2) agar, before incubating at 30 °C for 12-16 h, or until swarms have made sufficient progress over the right chamber to allow comparisons between strains of interest.
Results
The isolation of pseudo-revertants in an E. coli strain whose motility is impaired by high levels of the signaling molecule c-di-GMP, was detailed in recent work from our lab34. This strain (JP1442) harbored two mutations: ΔyhjH and ΔycgR. YhjH is the most active phosphodiesterase that degrades c-di-GMP in E. coli. Absence of YhjH leads to elevated c-di-GMP levels and inhibition of motility. YcgR is a c-di-GMP effector. In complex with c-di-GMP, YcgR binds to the flagellar rotor to first induce CCW motor rotation and subsequently decrease motor speed. Cell tethering and bead assays showed that motor behavior returned to normal in the double mutant, yet motility in soft agar did not34. So, we deployed step 1 of the protocol to isolate pseudo-revertant flares in the double mutant (Figure 1C). The majority of the mutations mapped by WGS (HiSeq 4000 platform, PE 2 x 150 setup34) to rssB, which codes for a response regulator/adaptor protein that normally directs ClpXP protease to target σS for degradation34. One of these revertants, which displayed motility close to wild-type (AW405, compare Figure 1A,D), was used to generate representative results for step 2 and 3 of the protocol section, using as controls both its double mutant parent (Figure 1B) and isogenic wild-type strain (Figure 1A).
For step 2 of the protocol section, video captures were analyzed to calculate rotations per minute (each 360° complete rotation), and CWBias (the fraction of time motors rotate in a CW direction, or tumble bias). The ΔyhjH showed fewer rotations per minute and a lower CW bias compared to the wild-type, as expected (Figure 3). Both the ΔyhjH ΔycgR double mutant and its suppressor showed motor behavior similar to wild-type, observations supported by a previous analysis using the higher-resolution ‘bead’ assay detailed in the introduction above in previous work34.
For step 3 of the protocol section, the border-crossing assay (Figure 2) was used to compare the abilities of the wild-type and the suppressor isolate, first to swarm, and then to move across the border and swarm on agar supplemented with kanamycin. Results show that both strains reached the border at a similar time (data not shown) indicating similar rates of swarming from an identical inoculation point. However, cross-over of the swarm to the right (antibiotic) chamber was marginally, but consistently greater for the wild-type than the suppressor at 20 µg/mL kanamycin (Figure 4). The difference between the two strains was more pronounced at 40 µg/mL kanamycin. Together, these data suggest that the mutations in rssB that restored motility on soft-agar plates (Figure 1D), negatively impact the antibiotic resistance of the suppressor strain during swarming (Figure 4).
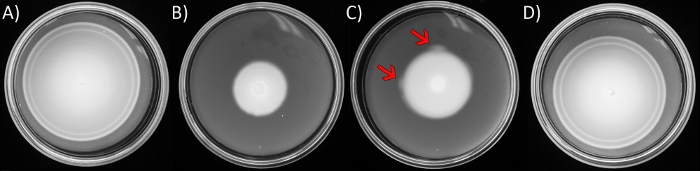
Figure 1: Soft-agar motility assays and emergence of suppressor flares.
The plates contain LB solidified with 0.3 % w/v agar. E. coli strains were inoculated in the center of each plate and incubated at 30°C for 8 h, except for C, which was incubated for 16 h. (A) Wild-type E. coli (AW405). (B) Motility-deficient variant ∆yhjH ∆ycgR (JP1442). (C) As in B, except longer incubation times. Arrows point to faster moving ‘flares’ emerging at the peripheral ring of the expanding swim colony. (D) A suppressor isolated from a flare in C. Please click here to view a larger version of this figure.
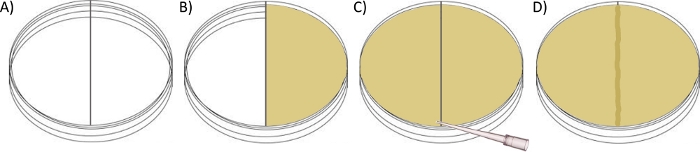
Figure 2: Schematic for setting up a Border-crossing plate assay.
(A) Pour ~30 mL of swarm agar (with desired antibiotic) into the right chamber of a divided petri dish until level with the plastic divider and allow to set with lid closed. (B) Fill the left chamber with ~30 mL of swim or swarm agar to the point of contact with the top of the plastic divider. (C) Use a sterile pipette tip to gently drag the molten swarm agar over the border, thereby connecting the two sides with a ~1 mm tall agar bridge and allow to set with lid closed. (D) Allow the plate to dry further at room temperature overnight before inoculating the left chamber with the desired strain, and incubating at 30 °C. Please click here to view a larger version of this figure.
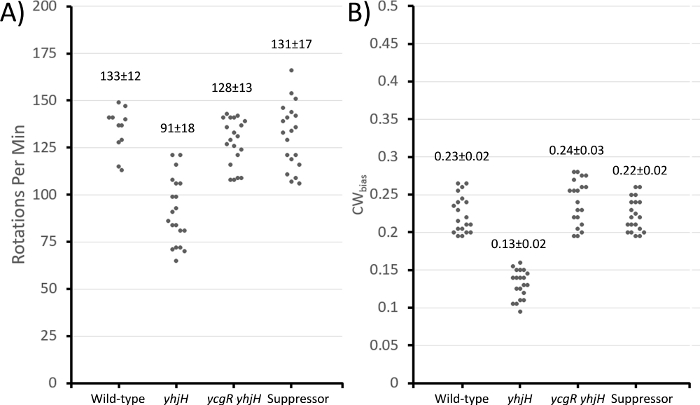
Figure 3: Motor properties of various strains as measured by the cell-tethering technique.
Wild-type (AW405), ∆yhjH (VN133), ∆ycgR ∆yhjH (JP1442), and its suppressor (JP1836) were grown in LB at 30 °C to mid-exponential phase prior to tethering. (A) Rotations per minute (completed 360° turns), and (B) CWbias (fraction of time motors rotate in a CW direction). Standard deviation of the mean (±). 20 tethered cells were observed for 60 sec in each strain. Please click here to view a larger version of this figure.
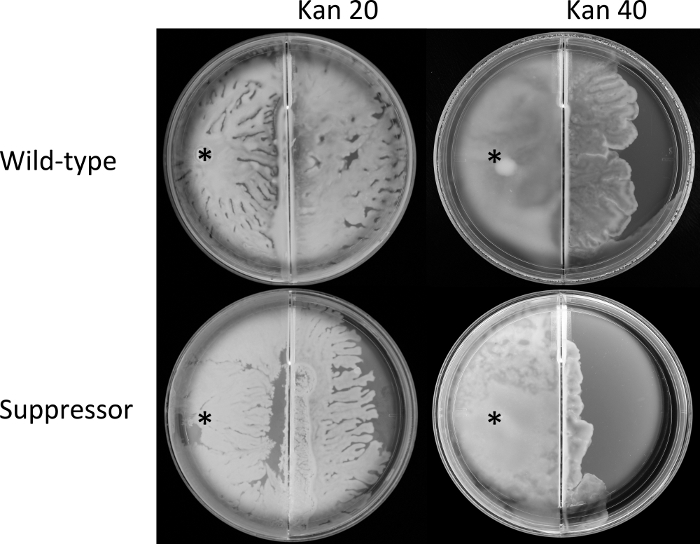
Figure 4: Border-crossing assays.
Mid-exponential phase cultures of wild-type E. coli (AW405) and the suppressor mutant (JP1836) were inoculated at the indicated position (*) in the left compartment of the divided plate containing swarm media, and incubated at 30 °C. They reached the border at comparable times. The plates were incubated for a further 6 h, during which the swarm crossed over to the right chamber, in which the media was supplemented with kanamycin (Kan; numbers indicate µg/mL). Plates are representative of three biological replicates each carried out in triplicate. Please click here to view a larger version of this figure.
Supplementary Figure 1: Preparation of a chamber slide for cell tethering. (A) Lay down two pieces of double-sided tape before (B) using a razor blade to trim away the excess. (C) Peel away the top layer to expose the adhesive before (D) affixing a coverslip and gently pressing it into position (indicated by [ ), ensuring all air is pushed out of the interface between the coverslip and tape below. (E) Load sample (shown here with DNA loading dye [30% v/v glycerol, 0.25% w/v bromophenol blue, and 0.25% w/v xylene cyanol] added to aid visualization) into the top of the created channel (arrow) while (F) angling onto clean, tissue task wipe to help draw the solution through the chamber as the tissue absorbs the liquid (arrow) in the channel and draws it through. Please click here to download this figure.
Video 1: Rotation of tethered E. coli cells. Please click here to download this video.
Video 2: An active E. coli swarm filmed under 60x magnification, demonstrating its characteristic swirling motion behind the edge of the moving front. Please click here to download this video.
Discussion
The isolation and characterization of suppressor mutations have successfully contributed to identifying key components of the chemotaxis system35,36,37, as well as the motor machinery itself38,39,40. While using Protocol 1, it is important to include multiple independent replicates to ensure the isolation of a large spectrum of possible mutations that could compensate for the loss of motility. Increasing the number of bacteria by streaking the culture in a line rather than a spot, can improve the odds of generating suppressors41. Isolation of the same mutation (as determined by DNA sequencing) multiple times increases confidence in its authenticity. WGS will invariably reveal the presence of other mutations in the genome. It is therefore important to verify the results by transducing (where possible) the identified mutation back into the original motility-deficient background. The suppressor mutant approach is rooted in restoring the function through a secondary mutation, so a limitation of this method is that if a critical structural gene is deleted, i.e., one that underpins the entire pathway or structure, there may be no scope for compensation. Despite being an old method, our recent work34 demonstrates its continuing utility in elucidation of new pathways that contribute to bacterial motility.
For the quantification of motor output, the cell-tethering approach remains a universally accessible tool requiring only a microscope with a camera attachment. Cell tethering has already been used in a diverse number of bacterial species including Salmonella42, Pseudomonas43, Streptococcus44, and Rhodobacter33. The success of the protocol is largely contingent on proper shearing and attachment of cells. Shearing too aggressively or omitting the pause between shears (2.1.5) tends to promote inconsistent or incomplete shearing of filaments, resulting in non-motile cells or cells tethered on a skewed axis. The enduring relevance of this protocol remains, despite the adoption of the higher-resolution bead assay by many research groups (including ours). The primary limitation of the bead assay comes from the need for the bead to adhere to the filament of the bacteria of interest. This technique has greatly benefitted from studies in E. coli that identified a ‘sticky’ flagellin allele, which facilitates adherence45. The sticky variant is also superior in the cell-tethering assay. Such a variant is not yet available for the majority of flagellated bacteria. The situation is complicated further with some organisms possessing multiple flagellin proteins46, and in the case of Vibrio sp., also possessing a membranous sheath47. Cell-tethering can also be performed using a species-specific anti-flagellin antibody or an antibody to an engineered epitope tag.
While bacteria can swim immediately upon introduction into a liquid medium, this is not true of swarming, where cells must be first primed into a swarming state. Surface contact triggers a physiological change required for cells to initiate swarming48,49,50,51, resulting in a lag phase and a buildup of high cell density. Physiological changes include remodeling of the chemotaxis system in E. coli16, and adaptations such as cell elongation and/or hyperflagellation for other bacteria13,14,21. Given the physiological changes required to initiate swarming, we strike a cautionary note about studies that have attempted to mimic select facets of swarming - such as high density or increased cell length – simply by concentrating planktonic cell cultures to increase density, and/or inducing cell elongation by use of antibiotics that inhibit cell division52,53. If using cell elongation as a marker, we caution also that compared to planktonic cells, there is only a marginal increase in the average length of swarmer cells in Salmonella or E. coli54. Swarm assays are harder to establish than swim assays. Variables include the commercial source of the agar used to solidify the media (the special Eiken agar is essential for swarming in E. coli, whereas the more standard Difco agar supports swarming for all other bacteria), use of rich versus minimal media (E. coli and Salmonella require glucose supplementation), and most critically ambient humidity2,13,14,21. Of these, maintaining optimal humidity can be the most frustrating. [An excellent, methodical, optimization for swarming in an organism of choice (Pseudomonas aeruginosa) has been demonstrated by Morales-Soto and co-workers55.] Swarm media must be sufficiently moist to promote swarming but not so moist as to allow passive spreading/sliding, which can be readily mistaken for active swarming56. It is therefore critical that swarms be checked under a microscope to confirm the distinct patterns of movement associated with this collective motility (Video 2). Temperature is also an important consideration for optimizing the swarming assay. Higher temperatures, for example 37 °C, will dry out the plates sooner than at 30 °C. Using an incubator with humidity control (~70-80%) can help mitigate these issues, including seasonal changes that could affect internal building temperatures and humidity. Once successfully established, protocol 3 provides a powerful way to investigate one of the most interesting aspects of swarming bacteria, elevated resistance to antibiotics17. All protocols described here can be applied to new organisms to identify pathways that specify and control flagella mediated motility.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institutes of Health grant GM118085 and in part by the Robert Welch Foundation (grant F-1811 to R.M.H.).
Materials
| Name | Company | Catalog Number | Comments |
| Reagents | |||
| Bacto Dehydrated Agar | Fisher Scientific | DF0140-15-4 | |
| EDTA Disodium Salt, Dihydrate | Fisher Scientific | 02-002-786 | |
| Eiken agar | Eiken Chemical Co. Japan | E-MJ00 | Essential for E. coli swarming |
| Glucose D (+) | Fisher Scientific | 410955000 | |
| LB (Lennox) Broth | Fisher Scientific | BP1427-500 | |
| Poly-L-lysine Solution (0.1%) | Sigma-Aldrich | P8920 | |
| Potassium chloride (KCl) | Fisher Scientific | 18-605-496 | |
| Potassium Phosphate monobasic (KH2PO4) | Fisher Scientific | BP362-500 | |
| Potassium Phosphate dibasic (K2HPO4) | Fisher Scientific | BP363-500 | |
| Sodium chloride (NaCl) | Fisher Scientific | S271-500 | |
| Materials and Equipment | |||
| CellSense microscope imaging software (V. 1.6) | Olympus | Or equivalent software for microscope used | |
| Electron Microscopy Sciences Scotch 666 Doube Sided Tape | Fisher | 50-285-28 | |
| Frosted microscope slides 3x1x1mm | Fisher | 12-550-343 | |
| Olympus BX53 microscope | Olympus | BX53 | Any upright or inverted phase microscope can be used |
| Petri dishes (100 mm diameter) | Fisher Scientific | FB0875712 | For soft-agar assays |
| Polyethylene Nebulizer Capillary Tubing (0.58mm x 99mm 3.0m) | Perkin Elmer | 9908265 | |
| Round Petri Dish with 2 Compartments | VWR | 89200-944 | For border-crossing assays |
| Safety Hypodermic Needles (23G) | Fisher Scientific | 14-826A | |
| Sterile Syringe - 1 mL | Fisher scientific | 14-955-450 | |
| Task/Tissue wipes | Fisher scientific | 06-666 | Or equivalent single use tissue wipes |
| VWR micro cover-glass 18x18mm | VWR | 48366205 | |
| XM10 camera | Olympus | XM10 | Or equivalent microscope camera |
References
- Jarrell, K. F., McBride, M. J. The surprisingly diverse ways that prokaryotes move. Nature Reviews in Microbiology. 6 (6), 466-476 (2008).
- Harshey, R. M. Bacterial motility on a surface: many ways to a common goal. Annual Reviews Microbiology. 57, 249-273 (2003).
- Duan, Q., Zhou, M., Zhu, L., Zhu, G. Flagella and bacterial pathogenicity. Journal of Basic Microbiology. 53 (1), 1-8 (2013).
- Nakamura, S., Minamino, T. Flagella-Driven Motility of Bacteria. Biomolecules. 9 (7), (2019).
- Haiko, J., Westerlund-Wikstrom, B. The role of the bacterial flagellum in adhesion and virulence. Biology (Basel). 2 (4), 1242-1267 (2013).
- Berg, H. C. . E. coli in Motion. 1 edn. , (2004).
- Berg, H. C. The rotary motor of bacterial flagella. Annual Review of Biochemistry. 72, 19-54 (2003).
- Xing, J., Bai, F., Berry, R., Oster, G. Torque-speed relationship of the bacterial flagellar motor. Proceedings of the National Academy of Sciences U. S. A. 103 (5), 1260-1265 (2006).
- Chen, X., Berg, H. C. Torque-speed relationship of the flagellar rotary motor of Escherichia coli. Biophysics Journal. 78 (2), 1036-1041 (2000).
- Turner, L., Ryu, W. S., Berg, H. C. Real-time imaging of fluorescent flagellar filaments. Journal of Bacteriology. 182 (10), 2793-2801 (2000).
- Brown, M. T., Delalez, N. J., Armitage, J. P. Protein dynamics and mechanisms controlling the rotational behaviour of the bacterial flagellar motor. Current Opinion in Microbiology. 14 (6), 734-740 (2011).
- Parkinson, J. S., Hazelbauer, G. L., Falke, J. J. Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends in Microbiology. 23 (5), 257-266 (2015).
- Kearns, D. B. A field guide to bacterial swarming motility. Nature Reviews Microbiology. 8 (9), 634-644 (2010).
- Harshey, R. M., Partridge, J. D. Shelter in a Swarm. Journal of Molecular Biology. 427 (23), 3683-3694 (2015).
- Ariel, G., et al. Swarming bacteria migrate by Levy Walk. Nature Communications. 6, 8396 (2015).
- Partridge, J. D., Nhu, N. T. Q., Dufour, Y. S., Harshey, R. M. Escherichia coli Remodels the Chemotaxis Pathway for Swarming. mBio. 10 (2), (2019).
- Butler, M. T., Wang, Q., Harshey, R. M. Cell density and mobility protect swarming bacteria against antibiotics. Proceedings of the National Academy of Science U. S. A. 107 (8), 3776-3781 (2010).
- Mobley, H. L., Belas, R. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends in Microbiology. 3 (7), 280-284 (1995).
- Burall, L. S., et al. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infections and Immunity. 72 (5), 2922-2938 (2004).
- Mazzantini, D., et al. FlhF Is Required for Swarming Motility and Full Pathogenicity of Bacillus cereus. Frontiers in Microbiology. 7, 1644 (2016).
- Partridge, J. D., Harshey, R. M. Swarming: flexible roaming plans. Journal of Bacteriology. 195 (5), 909-918 (2013).
- Yuan, J., Berg, H. C. Resurrection of the flagellar rotary motor near zero load. Proceedings of the National Academy of Science U. S. A. 105 (4), 1182-1185 (2008).
- Yuan, J., Fahrner, K. A., Berg, H. C. Switching of the bacterial flagellar motor near zero load. Journal of Molecular Biology. 390 (3), 394-400 (2009).
- Terasawa, S., et al. Coordinated reversal of flagellar motors on a single Escherichia coli cell. Biophysics Journal. 100 (9), 2193-2200 (2011).
- Nord, A. L., Sowa, Y., Steel, B. C., Lo, C. J., Berry, R. M. Speed of the bacterial flagellar motor near zero load depends on the number of stator units. Proceedings of the National Academy of Science. 114 (44), 11603-11608 (2017).
- Block, S. M., Segall, J. E., Berg, H. C. Adaptation kinetics in bacterial chemotaxis. J Bacteriol. 154 (1), 312-323 (1983).
- Segall, J. E., Block, S. M., Berg, H. C. Temporal comparisons in bacterial chemotaxis. Proceedings of the National Academy of Science U. S. A. 83 (23), 8987-8991 (1986).
- Wolfe, A. J., Conley, M. P., Kramer, T. J., Berg, H. C. Reconstitution of signaling in bacterial chemotaxis. Journal of Bacteriology. 169 (5), 1878-1885 (1987).
- Blair, D. F., Berg, H. C. Restoration of torque in defective flagellar motors. Science. 242 (4886), 1678-1681 (1988).
- Parkinson, J. S. A "bucket of light" for viewing bacterial colonies in soft agar. Methods Enzymol. 423, 432-435 (2007).
- Adler, J. Chemotaxis in bacteria. Science. 153 (3737), 708-716 (1966).
- Wolfe, A. J., Berg, H. C. Migration of bacteria in semisolid agar. Proceedings of the National Academy of Science U. S. A. 86 (18), 6973-6977 (1989).
- Kojadinovic, M., Sirinelli, A., Wadhams, G. H., Armitage, J. P. New motion analysis system for characterization of the chemosensory response kinetics of Rhodobacter sphaeroides under different growth conditions. Applied and Environmental Microbiology. 77 (12), 4082-4088 (2011).
- Nieto, V., et al. Under Elevated c-di-GMP in Escherichia coli, YcgR Alters Flagellar Motor Bias and Speed Sequentially, with Additional Negative Control of the Flagellar Regulon via the Adaptor Protein RssB. Journal of Bacteriology. 202 (1), (2019).
- Parkinson, J. S., Parker, S. R., Talbert, P. B., Houts, S. E. Interactions between chemotaxis genes and flagellar genes in Escherichia coli. Journal of Bacteriology. 155 (1), 265-274 (1983).
- Roman, S. J., Meyers, M., Volz, K., Matsumura, P. A chemotactic signaling surface on CheY defined by suppressors of flagellar switch mutations. Journal of Bacteriology. 174 (19), 6247-6255 (1992).
- Sanna, M. G., Simon, M. I. Isolation and in vitro characterization of CheZ suppressors for the Escherichia coli chemotactic response regulator mutant CheYN23D. Journal of Biological Chemistry. 271 (13), 7357-7361 (1996).
- Sockett, H., Yamaguchi, S., Kihara, M., Irikura, V. M., Macnab, R. M. Molecular analysis of the flagellar switch protein FliM of Salmonella typhimurium. Journal of Bacteriology. 174 (3), 793-806 (1992).
- Irikura, V. M., Kihara, M., Yamaguchi, S., Sockett, H., Macnab, R. M. Salmonella typhimurium fliG and fliN mutations causing defects in assembly, rotation, and switching of the flagellar motor. Journal of Bacteriology. 175 (3), 802-810 (1993).
- Ishida, T., et al. Sodium-powered stators of the bacterial flagellar motor can generate torque in the presence of phenamil with mutations near the peptidoglycan-binding region. Molecular Microbiology. 111 (6), 1689-1699 (2019).
- Barker, C. S., Meshcheryakova, I. V., Kostyukova, A. S., Samatey, F. A. FliO regulation of FliP in the formation of the Salmonella enterica flagellum. PLoS Genetics. 6 (9), 1001143 (2010).
- Paul, K., Nieto, V., Carlquist, W. C., Blair, D. F., Harshey, R. M. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a "backstop brake" mechanism. Molecular Cell. 38 (1), 128-139 (2010).
- Qian, C., Wong, C. C., Swarup, S., Chiam, K. H. Bacterial tethering analysis reveals a "run-reverse-turn" mechanism for Pseudomonas species motility. Applied and Environmental Microbiology. 79 (15), 4734-4743 (2013).
- Manson, M. D., Tedesco, P. M., Berg, H. C. Energetics of flagellar rotation in bacteria. Journal of Molecular Biology. 138 (3), 541-561 (1980).
- Kuwajima, G. Construction of a minimum-size functional flagellin of Escherichia coli. Journal of Bacteriology. 170 (7), 3305-3309 (1988).
- Kuhn, M. J., et al. Spatial arrangement of several flagellins within bacterial flagella improves motility in different environments. Nature Communication. 9 (1), 5369 (2018).
- Hranitzky, K. W., Mulholland, A., Larson, A. D., Eubanks, E. R., Hart, L. T. Characterization of a flagellar sheath protein of Vibrio cholerae. Infections and Immun. 27 (2), 597-603 (1980).
- Wang, Q., Frye, J. G., McClelland, M., Harshey, R. M. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Molecular Microbiology. 52 (1), 169-187 (2004).
- Gode-Potratz, C. J., Kustusch, R. J., Breheny, P. J., Weiss, D. S., McCarter, L. L. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Molecular Microbiology. 79 (1), 240-263 (2011).
- McCarter, L., Silverman, M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Molecular Microbiology. 4 (7), 1057-1062 (1990).
- Pearson, M. M., Rasko, D. A., Smith, S. N., Mobley, H. L. Transcriptome of swarming Proteus mirabilis. Infections and Immunity. 78 (6), 2834-2845 (2010).
- Swiecicki, J. M., Sliusarenko, O., Weibel, D. B. From swimming to swarming: Escherichia coli cell motility in two-dimensions. Integrative Biology (Cambridge). 5 (12), 1490-1494 (2013).
- Colin, R., Drescher, K., Sourjik, V. Chemotactic behaviour of Escherichia coli at high cell density. Nature Communication. 10 (1), 5329 (2019).
- Partridge, J. D., Harshey, R. M. More than motility: Salmonella flagella contribute to overriding friction and facilitating colony hydration during swarming. Journal Bacteriology. 195 (5), 919-929 (2013).
- Morales-Soto, N., et al. Preparation, imaging, and quantification of bacterial surface motility assays. Journal Visualized Experiment. (98), e52338 (2015).
- Chawla, R., Ford, K. M., Lele, P. P. Torque, but not FliL, regulates mechanosensitive flagellar motor-function. Science Reports. 7 (1), 5565 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved