Method Article
In Vivo Immunofluorescence Localization for Assessment of Therapeutic and Diagnostic Antibody Biodistribution in Cancer Research
In This Article
Summary
The in vivo immunofluorescence localization (IVIL) method can be used to examine in vivo biodistribution of antibodies and antibody conjugates for oncological purposes in living organisms using a combination of in vivo tumor targeting and ex vivo immunostaining methods.
Abstract
Monoclonal antibodies (mAbs) are important tools in cancer detection, diagnosis, and treatment. They are used to unravel the role of proteins in tumorigenesis, can be directed to cancer biomarkers enabling tumor detection and characterization, and can be used for cancer therapy as mAbs or antibody-drug conjugates to activate immune effector cells, to inhibit signaling pathways, or directly kill cells carrying the specific antigen. Despite clinical advancements in the development and production of novel and highly specific mAbs, diagnostic and therapeutic applications can be impaired by the complexity and heterogeneity of the tumor microenvironment. Thus, for the development of efficient antibody-based therapies and diagnostics, it is crucial to assess the biodistribution and interaction of the antibody-based conjugate with the living tumor microenvironment. Here, we describe In Vivo Immunofluorescence Localization (IVIL) as a new approach to study interactions of antibody-based therapeutics and diagnostics in the in vivo physiological and pathological conditions. In this technique, a therapeutic or diagnostic antigen-specific antibody is intravenously injected in vivo and localized ex vivo with a secondary antibody in isolated tumors. IVIL, therefore, reflects the in vivo biodistribution of antibody-based drugs and targeting agents. Two IVIL applications are described assessing the biodistribution and accessibility of antibody-based contrast agents for molecular imaging of breast cancer. This protocol will allow future users to adapt the IVIL method for their own antibody-based research applications.
Introduction
Monoclonal antibodies (mAb) are large glycoproteins (approximately 150 kDa) of the immunoglobulin superfamily that are secreted by B cells and have a primary function in the immune system to identify and either inhibit the biological function of, or mark for destruction, bacterial or viral pathogens, and can recognize abnormal protein expression on cancer cells1. Antibodies can have an extremely high affinity to their specific epitopes down to femtomolar concentrations making them highly promising tools in biomedicine2. With the development of hybridoma technology by Milstein and Köhler (awarded the Nobel Prize in 1984), the production of mAbs became possible3. Later, human mAbs were generated using the phage display technology or transgenic mouse strains and revolutionized their use as novel research tools and therapeutics4,5.
Cancer is a worldwide health issue and a major cause of death creating the need for novel approaches for prevention, detection, and therapy6. To date, mAbs have allowed extrication of the role of genes and their proteins in tumorigenesis and when directed against cancer biomarkers, can enable tumor detection and characterization for patient stratification. For cancer therapy, bispecific mAbs, antibody-drug conjugates, and smaller antibody fragments are being developed as therapeutics, and for the targeted drug delivery to enhance therapeutic efficacy7. Additionally, antibodies serve for the biomarker targeting of contrast agents for molecular imaging modalities such as fluorescence-guided surgery, photoacoustic (PA) imaging, ultrasound (US) molecular imaging, and clinically used positron emission tomography (PET) or single photon emission computed tomography (SPECT)8. Finally, antibodies can also be used as theranostic agents enabling stratification of patients and response monitoring for targeted therapies9. Therefore, novel mAbs are beginning to play a critical role in cancer detection, diagnosis, and treatment.
Despite critical advancements in the development and production of novel and highly specific mAbs, diagnostic and therapeutic applications can be rendered ineffective due to the complexity of the tumor environment. Antibody interactions are dependent on the type of epitope, i.e., whether it is linear or conformational10. In addition to the recognition of antigens, antibodies need to overcome natural barriers such as vessel walls, basal membranes, and the tumor stroma to reach target cells expressing the antigen. Antibodies interact with the tissue not only through the variable fragment antigen binding (Fab) domain but also through the constant crystalline fragment (Fc) which further leads to off-site interactions11. Targeting is also complicated by the heterogeneous expression of tumor markers throughout the tumor bulk and heterogeneity in tumor vascularization and the lymphatics system12,13. In addition, the tumor microenvironment is composed of cancer-associated fibroblasts which support tumor cells, tumor immune cells that suppress anti-tumor immune reactions, and the tumor endothelium which supports the transport of oxygen and nutrients, all of which interfere with the penetration, distribution, and availability of antibody-based therapeutics or diagnostics. Overall, these considerations can limit therapeutic or diagnostic efficacy, reduce treatment response, and may result in tumor resistance.
Therefore, for the development of efficient antibody-based therapies and diagnostics, it is crucial to assess the biodistribution and interaction of the antibody-based conjugate within the tumor microenvironment. Currently, in preclinical studies, marker expression in tumor research models is analyzed ex vivo by immunofluorescence (IF) staining of tumor sections14. Standard IF staining is performed with primary marker-specific antibodies which are then highlighted by secondary fluorescently labeled antibodies on ex vivo tumor tissue slices that have been isolated from the animal. This technique highlights the static location of the marker at the time of tissue fixation and does not provide insight into how the antibody-based therapeutics or diagnostics might distribute or interact in physiological conditions. Molecular imaging by PET, SPECT, US, and PA can provide information about the antibody-conjugated contrast agent distribution in living preclinical models8,15. As these imaging modalities are non-invasive, longitudinal studies can be performed and time-sensitive data can be collected with a minimal number of animals per group. However, these non-invasive molecular imaging approaches are not sensitive enough and do not have enough resolution for the localization of antibody distribution at the cellular level. Additionally, the physical and biological characteristics of the primary antibody may be drastically changed by the conjugation of a contrast agent16.
In order to take the in vivo physiological and pathological conditions into consideration of how antibody-based therapeutics and diagnostics interact within the tumor environment and to obtain high-resolution cellular and even sub-cellular distribution profiles of non-conjugated antibodies, we propose an IF approach, deemed In Vivo Immunofluorescence Localization (IVIL), in which the antigen-specific antibody is intravenously injected in vivo. The antibody-based therapeutic or diagnostic, acting as a primary antibody, circulates in functional blood vessels and binds to its target protein in the highly accurate, living tumor environment. After isolation of in vivo-labeled tumors with the primary antibody, a secondary antibody is used to localize accumulated and retained antibody conjugates. This approach is similar to a previously described IF histology approach injecting fluorescently labeled antibodies17. Though here, the use of non-conjugated antibodies avoids a potential change in biodistribution characteristics induced by antibody modification. Furthermore, ex vivo application of fluorescent secondary antibody avoids a possible loss of fluorescence signal during tissue collection and processing and provides amplification of fluorescence signal intensity. Our labeling approach reflects in vivo biodistribution of antibody-based drugs and targeted agents and can provide important insights for the development of novel diagnostic and therapeutic agents.
Here, we describe two applications of the IVIL method as applied in previous studies investigating the biodistribution and accessibility of antibody-based contrast agents for molecular imaging approaches for breast cancer detection. First, the biodistribution of an antibody-near infrared dye conjugate (anti-B7-H3 antibody bound to the near infrared fluorescence dye, indocyanine green, B7-H3-ICG) and the isotype control agent (Iso-ICG) for fluorescence and photoacoustic molecular imaging is explored18. This application's method is described in the protocol. Next, the biodistribution results of a conformationally sensitive antibody to netrin-1, typically not detectable with traditional IF imaging, used with ultrasound molecular imaging, is quantified and presented in the representative results19. At the conclusion of this protocol paper, readers should feel comfortable adopting the IVIL method for their own antibody-based research applications.
Protocol
All methods described here have been approved by the Institutional Administrative Panel on Laboratory Animal Care (APLAC) of Stanford University.
1. Transgenic mouse model of breast cancer development
- Observe mice from the desired cancer model for the appropriate tumor growth via palpation or caliper measurement before proceeding.
NOTE: Here, the transgenic murine model of breast cancer development (FVB/N-Tg(MMTV-PyMT)634Mul/J) (MMTV-PyMT) was used. These animals spontaneously develop invasive breast carcinomas between 6 and 12 weeks of age in each mammary gland20. Normal mammary glands were used as controls from the transgene-negative, age-matched littermates.
2. Intravenous injection of specific and nonspecific antibody agents
- Purify rabbit anti-mouse B7-H3 and rabbit IgG isotype control antibodies on a desalting column (e.g., PD-10) to remove preservatives and storage buffers following manufacturer instructions.
NOTE: Some antibodies may need further purification with Protein-A agarose beads based protocol21. - Aliquot dosages of 33 µg of each antibody conjugate in individual microcentrifuge tubes.
NOTE: Dosage of the administered agent may vary depending on the application and matches the dosages at which the antibody conjugate is routinely used. The concentration of the antibody solutions is required if the volume is more than 100 µL for animal safety. - At the desired time point before the tissue collection, here 96 h, anesthetize the tumor bearing animal with 2% isoflurane flowing in oxygen at 2 L/min, and place on a 37 °C heated stage. Do a toe pinch to make sure the proper level of anesthsia was reached prior to the procedure.
- To prepare for the tail vein inoculation of the antibody solutions, disinfect the tail of the animal by wiping three times with an alcohol wipe. Dilate the tail veins by warming with a heat pad for approximately 30 s. Avoid heating the entire animal. Wipe the tail once more with an alcohol wipe after removing the heat pad.
- Using a 27G tail vein catheter, insert the butterfly needle into one of the two lateral tail veins and carefully fix the tail with the inserted needle to the stage with a piece of surgical tape.
NOTE: Visible blood backflow into the catheter indicates the proper location of the needle within the tail vein. - Flush the catheter with 25 µL of sterile phosphate buffered saline (PBS), then inject the antibody solution into the catheter using insulin syringes. Flush the catheter once again with 25 µL of sterile PBS.
- Remove the needle from the tail and apply pressure to stop any bleeding.
- Turn off anesthesia and observe the animal until fully awake for any signs of distress.
3. Collection and preparation of target tumor tissues
- At the desired time point, humanely euthanize the animal according to the acceptable institutional procedure, here, by gradual inhalation of 100% CO2 from a compressed gas tank with a chamber displacement flow rate of 10-30% volume/min.
NOTE: Some applications of the IVIL technique require euthanasia by cardiac perfusion with PBS to remove freely circulating antibody19,22. - After confirmation of humane euthanasia via cessation of respiratory and cardiac movement, lack of toe pinch response, and greying of mucous membranes, excise tumor tissues using surgical scissors and forceps as follows:
- Lay the mouse in the supine position and grasping only the outer layer of skin between the set of mammary glands closest to the tail (5th) with forceps, make a small incision with a pair of surgical scissors.
- Introduce the closed scissors into the cut and slowly open the tip to carefully separate the skin from the underlying abdominal wall membrane keeping it intact.
- Make a vertical incision up the abdomen, continuing to separate the skin from the inner membrane. Between the 3rd and 4th mammary glands, make a horizontal cut across the abdomen to allow retraction of the skin and visualization of the mammary glands.
NOTE: Mammary tumors and glands are located superficially under the skin. - Grasping each tumor or normal gland with forceps, carefully trim away the attached skin using surgical scissors.
- Place excised tissues into tissue disposable base molds, prelabeled and filled with optimal cutting temperature (OCT) embedding medium and freeze the molds quickly by placement on dry ice. In order to study off-target delivery, excise other tissues or organs of interest (e.g., the liver or lungs).
NOTE: To pause the protocol at this point, store frozen tissue blocks at -80 °C until ready to proceed. - Using a cryostat, section frozen tissue blocks at 10 µm thickness and place adjacent sections onto prelabeled adhesion glass slides.
NOTE: To pause the protocol at this point, store slides at -80 °C until ready to proceed.
4. Ex vivo staining protocol
NOTE: For the quantitative comparison between fluorescence microscopy images, all slides are stained at the same time with the same prepared solutions.
- Rinse frozen tissue slides with room temperature PBS for 5 min to remove OCT.
- Demarcate tissue sections with a hydrophobic barrier pen to reduce the volume of solutions needed during staining.
NOTE: Be careful not to allow the pen to run over the tissue samples as it may either remove the tissue from the slide or prevent proper staining on the affected tissue portion. Do not allow the tissue sections to dehydrate at any point. - Fix the tissue sections with 4% paraformaldehyde solution for 5 min.
- Rinse slides in PBS for 5 min.
- Permeabilize tissue sections with 0.5% Triton-X 100 in PBS for 15 min.
- Rinse slides in PBS for 5 min.
- Block the tissues with 3% w/v bovine serum albumin (BSA) and 5% v/v goat serum, both in PBS (blocking solution) for 1 h at room temperature.
NOTE: Match the blocking solution serum to the secondary antibody host animal. - Rinse slides in PBS for 5 min.
- Incubate sections with record-keeping primary antibodies as desired, possibly a common nuclear (e.g., DAPI), vascular (e.g., CD31), or cytoplasmic marker (e.g., actin). Here, rat anti-mouse CD31 (vascular marker) at a 1:100 dilution was used according to manufacturer's instructions in the blocking solution overnight at 4 °C protected from dehydration on a slide tray.
NOTE: Do not add additional primary antibody or antibody conjugate. The conjugate that was injected and allowed to accumulate in the tissues in vivo act as the primary antibody. - Rinse slides in PBS for 5 min three times, changing the PBS each time.
- Incubate slides with secondary antibodies to label primary antibodies. For this application, visualize anti-B7-H3 antibody using AlexaFluor-546 conjugated goat anti-rabbit antibody (1:200 dilution, optimized according to manufacturer's instructions) and CD31 with AlexaFluor-488 goat anti-rat secondary antibody (1:200 dilution, optimized according to manufacturer's instructions) in blocking solution, protected from light and dehydration on a slide tray, for 1 h at room temperature.
NOTE: Secondary antibodies are from the same host animal but match the affinity of the secondary antibodies to the host species of the respective primary antibody. Slides are protected from light from this point onwards. - Rinse slides in PBS for 5 min three times, changing the PBS each time.
- Apply one drop of the mounting medium into the center of the tissue slice and carefully place a coverslip avoiding entrapment of air bubbles.
- Seal the edges of the coverslip with clear nail polish and allow to dry.
NOTE: To pause the protocol for up to a week at this point, store slides at -20 °C until ready to proceed.
5. Confocal microscopy imaging and quantitative image analysis
NOTE: Preparing the confocal microscope and imaging parameters will depend on the confocal system used. The microscope used here was purchased commercially (e.g., Zeiss LSM 510 Meta system) and the associated acquisition software was used (e.g., Zen 2009). However, many of these steps will apply to any confocal microscope and assume basic confocal microscopy knowledge.
- After the system has been turned on and warmed up, select the desired objective; here a 20x (numerical aperture = 0.8) objective was used.
- Load a positive control slide, coverslip down, to allow for setting the optimization for the brightest signal. Imaging in the red channel, focus the system on the sample in Live imaging mode.
- For each laser channel used, optimize the laser intensity, master gain, and pinhole size as follows:
- Switch to Continuous mode for imaging.
- Optimize the Laser Intensity (which controls the laser power) and Gain (Master) (which controls the voltage for photomultiplier tube) slide bars while monitoring the Look Up Table (LUT) histogram. Adjust these two settings until the dynamic range of the histogram is filled without saturating pixels.
NOTE: If the laser intensity is too high photobleaching will occur. If the Gain (Master) is too high, the image will become noisy. Ideally, the Gain (Master) will be in the middle of its range. - Set the pinhole to 1 airy unit (AU), which gives the highest resolution and the thinnest z-slice.
- Slide the Digital Offset bar to minimize the noise floor on the LUT histogram for a true black background.
NOTE: Once microscopy settings are optimized for each laser channel and objective, keep them constant throughout the imaging session and for imaging of all slides, to allow for the quantitative comparison between slides.
- Under the Acquisition Mode tab, under Averaging, select the desired Number and Bit Depth. Click the Optimal button to set the optimal pixel size.
- Use the Snap acquisition button to collect a high-quality image. Here, random fields of view were selected from within the tumor, but other areas of interest may apply for different applications (vessels, tumor margins, penetration depth, etc.)
- Save image files in the format used by the confocal software (here, ".lsm") for offline processing and quantification.
NOTE: If not all slides are imaged during the same session, save the settings and reload them on subsequent visits, though reimaging the same slide is not recommended due to photobleaching. - Perform the quantitative fluorescence intensity measurements. Open Fiji (Fiji Is Just ImageJ software)19,23 and load an .lsm image file by dragging onto the status bar.
- Split the color channel data. Go to Image > Stacks > Split Channels.
- Preprocess the fluorescence images as needed, i.e., subtract the background signal (Process > Subtract Background) or reduce the noise via a filtering method.
- Segment the color channel corresponding to the reference protein (vascular, nuclear, cellular stain) by setting a threshold on the signal intensity (Image > Adjust > Threshold).
NOTE: Manual thresholding introduces subjectivity into the image analysis, therefore, using an automated thresholding algorithm or referencing image histograms makes image analysis results less biased. - Use this threshold to create a binary mask (Process > Binary > Convert to Mask).
- Measure and label ROIs within the mask (Analyze > Analyze Particles > Check Add to Manager > OK).
- Apply ROIs to the color channel corresponding to the antibody of interest (Click channel image, Analyze > Tools > ROI Manager > Measure). This will apply the mask ROIs to the antibody image and provide image measurements for label sections in the Results window. Save results window (File > Save).
- Calculate the desired statistic of interest, such as the mean fluorescence intensity as described here24.
- Perform all processing steps identically to each image within a set of slides. Create a macro to automatically do this for large image batches23.
- For image display only after quantitative measurements, apply qualitative image adjustments to optimize visualization of biodistribution patterns by adjusting minimum, maximum, brightness, and contrast to the same levels on all slides (Image > Adjust > Brightness/Contrast).
- Convert the image type (Image > Type > RGB Color) and save files in a lossless image type such as .tiff (File > Save As > Tiff…) for use in presentations and publications.
Results
The IVIL method was used here to examine the in vivo biodistribution and tissue interaction of B7-H3-ICG and Iso-ICG, by allowing the agents, after intravenous injection into a living animal, to interact with the target tissue for 96 h, and then once the tissues are harvested, to act as the primary antibodies during ex vivo immunostaining. The IVIL method was also compared to the standard ex vivo IF staining of the tissues for the B7-H3 marker. Normal murine mammary glands do not express the B7-H3 marker, confirmed in the ex vivo, standard IF staining, and do not accumulate antibodies through other passive mechanisms, and, therefore, no accumulation of B7-H3-ICG or Iso-ICG was detected, representative of a negative result (Figure 1, top row). However, murine mammary tumors do express B7-H3 both in the vasculature and epithelium (as confirmed by standard IF staining), and IVIL was able to highlight the B7-H3-ICG agent that had accumulated strongly on the vasculature and was able to partially extravasate from the vasculature to bind to the cancer cells themselves though in a heterogeneous manner compared to the uniform distribution of the B7-H3 marker throughout the tumors, also shown in Figure 1, bottom row. Furthermore, even though the Iso-ICG agent has no molecular specificity, it was still shown to accumulate in a nonspecific fashion, due to Fc interactions, poor interstitial drainage, and the enhanced permeability and retention effect within murine mammary tumors (Figure 1). This difference is highlighted in the biodistribution patterns shown between the two agents, and further enhances the specific binding results of the specific antibody agent, and represents two possible outcomes of a positive result.
The B7-H3-ICG representative results demonstrate how the IVIL method can be used as a qualitative method to describe general antibody/antibody conjugate biodistribution. However, at times, a more quantitative interpretation may be needed, or an antibody may have highly sensitive, conformation-specific binding capacity. In these situations, IVIL can also provide the desired information. In a second example, in which the expression of netrin-1 protein in tumor tissues and its co-localization with CD31-positive tumor endothelium was to be studied, the IVIL technique was also successfully applied19.
Netrin-1 is a secreted 65 kDa protein which is over-expressed in certain types of cancer such as metastatic breast cancer25,26. In order to develop a molecular imaging approach for netrin-1, the in vivo availability of the protein had to be determined19. Also, as the anti-netrin-1 antibody does not have sufficient affinity for its antigen after tissue fixation, an alternative staining protocol to classical immunohistochemistry or IF staining was required and IVIL was implemented. Humanized anti-netrin-1 antibody and a human IgG isotype control antibody were intravenously injected 24 h prior to cardiac perfusion and tumor collection. Cardiac perfusion was applied to reduce unspecific background staining in the vasculature and to enhance the detection of significant anti-netrin-1 antibody accumulation in comparison to isotype control antibody signal when the target expression is limited. Tumor sections were labeled ex vivo with vasculature highlighting anti-CD31 antibody (rat anti-mouse CD31 antibody (Table of Materials) and fluorescent secondary antibodies, Alexa 488-coupled goat anti-rat IgG, 1:500 dilution and Alexa Fluor 594-coupled goat anti-human IgG, 1:500 dilution respectively) were used to reveal anti-netrin-1/isotype and anti-CD31 mAbs. The fluorescence signal intensities of the anti-netrin-1 and isotype control antibody were quantified to determine differences in staining co-localized with CD31. Figure 2 shows that the anti-netrin-1 antibody specifically accumulated in the epithelial tumor compartment of MMTV-PyMT mammary tumors (a positive result), while there was no signal in normal mammary glands (a negative result). The antibody further co-localized with the endothelial marker CD31. Comparison with isotype antibody-injected tumors and normal glands showed that epithelial accumulation was specific for the tumor tissue. There was signal accumulation in the endothelial cells of both tumors and normal glands due to netrin-1 binding (tumors) and non-specific Fc interactions (tumors and normal mammary glands)11. Thus, the fluorescence signal quantification using a CD31-based binary mask was required and demonstrated that the anti-netrin-1 antibody signal was significantly higher than the isotype control antibody signal in the tumor tissue, which suggests netrin-1 specifically accumulated in the tumor endothelium.

Figure 1: Representative confocal micrographs of the comparison of specific B7-H3 antibody-ICG conjugate and nonspecific isotype control antibody-ICG conjugate localization in murine mammary glands containing normal or carcinoma tissues.(Top) Normal murine mammary glands from an animal intravenously injected with 33 µg of Iso-ICG or B7-H3-ICG (red) and counter-stained with CD31 (green) showing no staining of the antibody conjugates. Normal tissues were shown to have no expression of B7-H3 by standard ex vivo IF staining. (Bottom) Invasive mammary tumors from an animal intravenously injected with 33 µg of B7-H3-ICG or Iso-ICG (red) and vascular counter-stained CD31 (green) showing extensive, heterogeneous staining, though with different distribution patterns, for both the B7-H3-ICG and Iso-ICG agents. B7-H3-ICG strongly binds to the vasculature, the first point of contact in vivo, and then is able to extravasate from the vasculature to heterogeneously stain the tumor epithelium. Iso-ICG shows non-specific accumulation within the tumor tissues. Standard ex vivo IF staining shows uniform expression of the B7-H3 marker on epithelial and endothelial cells. Yellow color indicates co-localized red and green channel signal. Scale bar is 100 µm and consistent between panels. Please click here to view a larger version of this figure.
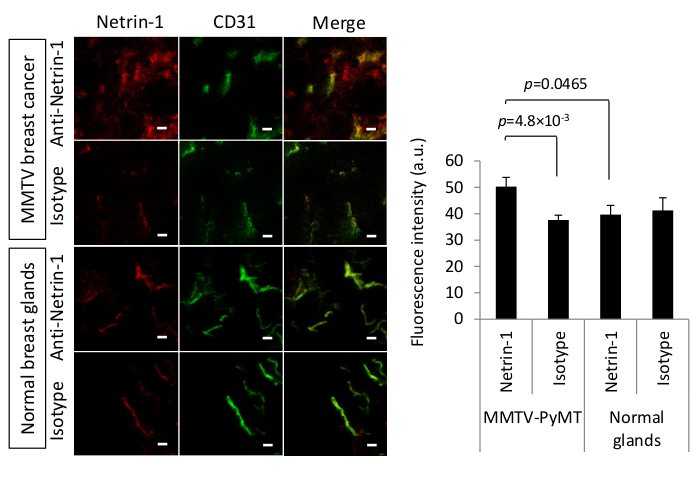
Figure 2: In vivo immuno-localization of netrin-1 in MMTV-PyMT mammary tumors. (Left) Representative confocal micrographs of the IVIL method to detect netrin-1 expression (red) or isotype control expression (red) in murine carcinoma and normal mammary glands. IVIL confirms epithelial signal for netrin-1 in MMTV-PyMT tumors but not in normal mammary glands, and strong netrin-1 signal on endothelial cells (CD31 staining, green) in breast tumors and significantly weaker signal of netrin-1 in normal mammary glands. Yellow color indicates co-localized red and green channel signal. Scale bars indicate 20 µm. For the detection of netrin-1 protein in the tumor endothelium, 100 µg of primary humanized NET1-H-mAb or 100 µg of human IgG isotype control antibody were intravenously injected 24 h prior to tumor collection. Freely circulating antibody was removed by cardiac perfusion with PBS and tumor tissue was isolated, flash frozen, and sectioned at 15 µm thickness on a cryostat. Endothelial cells were labeled with primary rat anti-mouse CD31 antibody followed by secondary Alexa 488-coupled goat anti-rat IgG. To reveal the primary antibody targeting netrin-1, secondary Alexa Fluor 594-coupled goat anti-human IgG was used. To avoid unspecific interaction of the goat secondary antibodies, tissue samples were blocked with goat serum. (Right) Bar graph highlighting the quantification of anti-netrin-1 or isotype control antibody signal that colocalizes with anti-CD31 antibody signal. N=13 tumors (of two mice) per group of MMTV-PyMT and N=7 mammary glands (of one mouse) per group of normal glands; Error bars present SEM; Two-group comparisons were performed with Student's t-test. Figure reprinted with permission from 19 under the creative commons license CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Please click here to view a larger version of this figure.
Discussion
This method has several critical steps and requires potential modifications to ensure successful implementation. First, the dosage and timing of the antibody/antibody conjugate intravenous injection must be tailored to the specific application. Generally, dosages should be used that are consistent with how the antibody conjugate will typically be used, i.e., matching dosages of the therapeutic antibody or antibody-based contrast agent. Also, the timing of the collection of target tissues should be carefully considered. Antibodies and antibody conjugates have longer circulation times than standard drugs and contrast agents, however, these are highly variable and should be optimized for the desired application, but it is recommended to allow at least 24 h circulation time27. Second, during the target tissue collection, it may be required to implement an intracardiac perfusion technique to remove freely circulating antibody from the blood pool19. While looking at tumor distribution of B7-H3-ICG, it was found unnecessary due to sufficiently long circulation times (96 h) and the extravascular localization of the agent. However, for the netrin-1 application of the IVIL method, cardiac perfusion was considered pertinent due to the endothelial expression of netrin-1 and its relatively low expression level. Third, during ex vivo tissue staining it is imperative to stain any slides that will be compared quantitatively during the same session and with the same solutions to prevent normal inter-batch variations. Ensure slides are properly rinsed between steps and the hydrophobic pen solution does not come in contact with the tissue to avoid suboptimal staining. Finally, during confocal microscopy, maintain the same imaging settings between slides to allow for relative quantification and work quickly to prevent photobleaching of the slides. These key points will increase the likelihood of a successful IVIL implementation.
There are several advantages to the IVIL method. First, while the method is similar to the standard IF techniques, the two provide significantly different information. IF staining indicates the anatomical location of molecular markers within tissue at a given time point (when the tissue was collected and fixed). However, IVIL provides information on how an antibody or antibody conjugate, as demonstrated by localization of B7-H3-ICG, is distributing and interacting, whether that be specific or non-specific accumulation, internalization, or retention in the target tissue. This is critical to pharmacokinetic/dynamic and biodistribution studies. Second, traditional IF methods are, at times, limited by fixation of the tissues which can cause antigen distortion or masking preventing binding by the primary antibody28. Therefore, IVIL may be useful when standard IF staining or immunohistochemistry fail. The IVIL technique can be considered a complementary method to verify in vivo antibody activity that would otherwise be undetectable. Finally, those familiar with traditional IF staining techniques will find the method straightforward in execution and will have most of the required materials on hand.
The IVIL method has a few limitations. First, due to the necessity to euthanize the animal to harvest the target tissues, IVIL only provides biological interaction information at a single time point. Injection of the primary antibody or antibody conjugate at varying time points into different animals will allow for a broader time window of information on the biodistribution and in vivo interaction. Second, if users are unfamiliar with in vivo animal techniques, such as intravenous tail vein injection or potential intracardiac perfusion, this can be a limitation to the method. These methods require skilled personnel to perform reliably. However, use of a catheter during tail vein injection ensures administration of the entire dose as proper needle placement can be confirmed before injection. Additionally, one alternative to tail vein injection that could be considered is retro-orbital injection, which typically has a higher success rate with less experienced personnel. The need to stain and image all tissue slides in the same batch to allow for quantitative assessment limits the number of samples and markers that can be included in a single study. The study might need to be organized into serial staining on different days and on adjacent tissue slices to collect the desired information. Finally, the use of species-matched antibodies, i.e., murine anti-mouse antibodies in murine preclinical models, raises an issue of non-specific background staining with secondary antibodies. Thus, the use of species-mismatched primary antibodies is required as presented in this study, or fluorescently labeled species-matched primary antibodies would have to be used.
The IVIL technique is useful for biodistribution analysis of diagnostic and therapeutic antibodies for many applications. Different applications for the intravenous antibody or ligand injection and ex vivo tissue analysis have been reported previously and confirm the interest to widely introduce this staining procedure. Robertson and colleagues labeled vascular elements such as endothelial capillaries and larger vessels with intravenous injections of Lycopersicon esculentum agglutinin (tomato lectin)29.The technique was compatible with histochemistry and immunocytochemistry and revealed vascular patterns and functional parenchymal characteristics. Though, the use of fluorescent ligands was limited by the rapid degradation of fluorescence upon injection30. As demonstrated in the IVIL protocol, the injection of a primary antibody in vivo followed by ex vivo labeling with a secondary fluorescent antibody might be a more robust approach. Another interesting application of this type of in vivo IF staining is the labeling of intravascular lymphocytes as demonstrated by Anderson and colleagues31. Intravenously injected antibody was used to label lymphocytes localized in blood vessels, which were subsequently collected and further processed for flow cytometry analysis of immunological markers. Finally, to assess the delivery and histological localization of antibody therapeutics that were delivered to patients with head and neck squamous cell carcinoma (HNSCC), a first in-human study of antibody distribution was performed using systemically administered near-infrared fluorescently labeled therapeutic antibody cetuximab-IRDye800CW32. The data were compared with histological analyses and showed that the fluorescent signal decreased with distance from the tumor confirming specificity. Though, the therapeutic antibody did not reach all regions of antigen expression, especially the well-differentiated tumor regions with high levels of the epidermal growth factor receptor (EGFR) antigen. In the current context of cancer research focusing on the development of targeted therapies and immunotherapies which encounter strong resistance and lack of efficacy, the IVIL method would be an excellent tool to study the distribution of therapeutic antibodies such as PD-1/PD-L-1 or HER233,34. These results are extremely valuable to understand why targeted therapies are effective in certain cases promoting the development of novel approaches. Taken together, these examples highlight the potential importance of the IVIL approach.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Andrew Olson (Stanford Neuroscience Microscopy Service) for discussions and equipment use. We thank Dr. Juergen K. Willmann for his mentorship. This study was supported by NIH R21EB022214 grant (KEW), NIH R25CA118681 training grant (KEW), and NIH K99EB023279 (KEW). The Stanford Neuroscience Microscopy Service was supported by NIH NS069375.
Materials
| Name | Company | Catalog Number | Comments |
| Animal Model | |||
| FVB/N-Tg(MMTV-PyMT)634Mul/J | The Jackson Laboratory | 002374 | Females, 4-6 weeks of age |
| Animal Handling Supplies | |||
| 27G Catheter | VisualSonics | Please call to order | Vevo MicroMarker Tail Vein Access Cannulation Kit |
| Alcohol Wipes | Fisher Scientific | 22-246073 | |
| Gauze Sponges (4" x 4" 16 Ply) | Cardinal Health | 2913 | |
| Heat Lamp | Morganville Scientific | HL0100 | |
| Isoflurane | Henry Schein Animal Health | 29404 | |
| Ophthalmic Ointment | Fisher Scientific | NC0490117 | |
| Surgical Tape | 3M | 1530-1 | |
| Tissue Collection | |||
| Disposable Base Molds | Fisher Scientific | 22-363-556 | |
| Optimal Cutting Temperature (OCT) Medium | Fisher Scientific | 23-730-571 | |
| Surgical London Forceps | Fine Science Tools | 11080-02 | |
| Surgical Scissors | Fine Science Tools | 14084-08 | |
| Antibodies | |||
| AlexaFluor-488 goat anti-rat IgG | Life Technologies | A-11006 | |
| AlexaFluor-546 goat anti-rabbit IgG | Life Technologies | A-11010 | |
| AlexaFluor-594 goat anti-human IgG | Life Technologies | A11014 | |
| Human IgG Isotype Control | Novus Biologicals | NBP1-97043 | |
| Humanized anti-netrin-1 antibody | Netris Pharma | contact@netrispharma.com | |
| Rabbit anti-Mouse CD276 (B7-H3) | Abcam | ab134161 | EPNCIR122 Clone |
| Rat anti-Mouse CD31 | BD Biosciences | 550274 | MEC 13.3 Clone |
| Reagents | |||
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A2153-50G | |
| Clear Nail Polish | Any local drug store | ||
| Indocyanine Green - NHS | Intrace Medical | ICG-NHS ester | |
| Mounting Medium | ThermoFisher Scientific | TA-006-FM | |
| Normal Goat Serum | Fisher Scientific | ICN19135680 | |
| Paraformaldehyde (PFA) | Fisher Scientific | AAJ19943K2 | |
| Sterile Phosphate Buffered Saline (PBS) | ThermoFisher Scientific | 14190250 | |
| Triton-X 100 | Sigma-Aldrich | T8787 | |
| Supplies | |||
| Adhesion Glass Slides | VWR | 48311-703 | |
| Desalting Columns | Fisher Scientific | 45-000-148 | |
| Glass Cover Slips | Fisher Scientific | 12-544G | |
| Hydrophobic Barrier Pen | Ted Pella | 22311 | |
| Microcentrifuge Tubes | Fisher Scientific | 05-402-25 | |
| Slide Staining Tray | VWR | 87000-136 | |
| Software | |||
| FIJI | LOCI, UW-Madison. | Version 4.0 | https://fiji.sc/ |
References
- Forthal, D. N. Functions of Antibodies. Microbiology Spectrum. 2 (4), 1-17 (2014).
- Boder, E. T., Midelfort, K. S., Wittrup, K. D. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proceedings of the National Academy of Sciences of the United States of America. 97 (20), 10701-10705 (2000).
- Köhler, G., Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 256 (5517), 495-497 (1975).
- Lonberg, N., et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 368 (6474), 856-859 (1994).
- McCafferty, J., Griffiths, A. D., Winter, G., Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 348 (6301), 552-554 (1990).
- Ferlay, J., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 136 (5), E359-E386 (2015).
- Reichert, J. M., Valge-Archer, V. E. Development trends for monoclonal antibody cancer therapeutics. Nature Reviews Drug Discovery. 6 (5), 349-356 (2007).
- Kircher, M. F., Willmann, J. K. Molecular Body Imaging: MR Imaging, CT, and US. Part I. Principles. Radiology. 263 (3), 633-643 (2012).
- Fleuren, E. D. G., et al. Theranostic applications of antibodies in oncology. Molecular Oncology. 8 (4), 799-812 (2014).
- Forsström, B., Bisławska Axnäs, B., Rockberg, J., Danielsson, H., Bohlin, A., Uhlen, M. Dissecting Antibodies with Regards to Linear and Conformational Epitopes. PLoS ONE. 10 (3), (2015).
- Woof, J. M., Burton, D. R. Human antibody-Fc receptor interactions illuminated by crystal structures. Nature Reviews Immunology. 4 (2), 89-99 (2004).
- Brooks, J. D. Translational genomics: The challenge of developing cancer biomarkers. Genome Research. 22 (2), 183-187 (2012).
- Tabrizi, M., Bornstein, G. G., Suria, H. Biodistribution Mechanisms of Therapeutic Monoclonal Antibodies in Health and Disease. The AAPS Journal. 12 (1), 33-43 (2009).
- Duraiyan, J., Govindarajan, R., Kaliyappan, K., Palanisamy, M. Applications of immunohistochemistry. Journal of Pharmacy & Bioallied Sciences. 4 (Suppl 2), S307-S309 (2012).
- Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nature Reviews. Cancer. 2 (9), 683-693 (2002).
- Freise, A. C., Wu, A. M. In vivo Imaging with Antibodies and Engineered Fragments. Molecular Immunology. 67 (200), 142-152 (2015).
- Cilliers, C., Menezes, B., Nessler, I., Linderman, J., Thurber, G. M. Improved Tumor Penetration and Single-Cell Targeting of Antibody-Drug Conjugates Increases Anticancer Efficacy and Host Survival. Cancer Research. 78 (3), 758-768 (2018).
- Wilson, K. E., et al. Spectroscopic Photoacoustic Molecular Imaging of Breast Cancer using a B7-H3-targeted ICG Contrast Agent. Theranostics. 7 (6), 1463-1476 (2017).
- Wischhusen, J., et al. Ultrasound molecular imaging as a non-invasive companion diagnostic for netrin-1 interference therapy in breast cancer. Theranostics. 8 (18), 5126-5142 (2018).
- Guy, C. T., Cardiff, R. D., Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Molecular and Cellular Biology. 12 (3), 954-961 (1992).
- Hober, S., Nord, K., Linhult, M. Protein A chromatography for antibody purification. Journal of Chromatography B. 848 (1), 40-47 (2007).
- Gage, G. J., Kipke, D. R., Shain, W. Whole Animal Perfusion Fixation for Rodents. Journal of Visualized Experiments JoVE. (65), (2012).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Bachawal, S. V., et al. Earlier detection of breast cancer with ultrasound molecular imaging in a transgenic mouse model. Cancer Research. 73, 1689-1698 (2013).
- Fitamant, J., et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proceedings of the National Academy of Sciences. 105 (12), 4850-4855 (2008).
- Kennedy, T. E., Serafini, T., de la Torre, J. R., Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 78 (3), 425-435 (1994).
- Ryman, J. T., Meibohm, B. Pharmacokinetics of Monoclonal Antibodies. CPT: Pharmacometrics & Systems Pharmacology. 6 (9), 576-588 (2017).
- Scalia, C. R., et al. Antigen Masking During Fixation and Embedding, Dissected. Journal of Histochemistry and Cytochemistry. 65 (1), 5-20 (2017).
- Robertson, R. T., et al. Use of labeled tomato lectin for imaging vasculature structures. Histochemistry and Cell Biology. 143 (2), 225-234 (2015).
- Chen, C. Y., et al. Blood flow reprograms lymphatic vessels to blood vessels. The Journal of Clinical Investigation. 122 (6), 2006-2017 (2012).
- Anderson, K. G., et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature Protocols. 9 (1), 209-222 (2014).
- de Boer, E., et al. In Vivo Fluorescence Immunohistochemistry: Localization of Fluorescently Labeled Cetuximab in Squamous Cell Carcinomas. Scientific Reports. 5, (2015).
- Jenkins, R. W., Barbie, D. A., Flaherty, K. T. Mechanisms of resistance to immune checkpoint inhibitors. British Journal of Cancer. 118 (1), 9-16 (2018).
- Rexer, B. N., Arteaga, C. L. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Critical Reviews in Oncogenesis. 17 (1), 1-16 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved