Method Article
Standardized Hemorrhagic Shock Induction Guided by Cerebral Oximetry and Extended Hemodynamic Monitoring in Pigs
In This Article
Summary
Hemorrhagic shock is a severe complication in seriously injured patients, which leads to life-threatening oxygen undersupply. We present a standardized method to induce hemorrhagic shock via blood withdrawal in pigs that is guided by hemodynamics and microcirculatory cerebral oxygenation.
Abstract
Hemorrhagic shock ranks among the main reasons for severe injury-related death. The loss of circulatory volume and oxygen carriers can lead to an insufficient oxygen supply and irreversible organ failure. The brain exerts only limited compensation capacities and is particularly at high risk of severe hypoxic damage.This article demonstrates the reproducible induction of life-threatening hemorrhagic shock in a porcine model by means of calculated blood withdrawal. We titrate shock induction guided by near-infrared spectroscopy and extended hemodynamic monitoring to display systemic circulatory failure, as well as cerebral microcirculatory oxygen depletion. In comparison to similar models that primarily focus on predefined removal volumes for shock induction, this approach highlights a titration by means of the resulting failure of macro- and microcirculation.
Introduction
Massive blood loss is among the main causes of injury-related deaths1,2,3. The loss of circulatory fluid and oxygen carriers leads to hemodynamic failure and severe oxygen undersupply and can cause irreversible organ failure and death. The severity level of shock is influenced by additional factors like hypothermia, coagulopathy, and acidosis4. Particularly the brain, but also the kidneys lack compensation capacity due to high oxygen demand and the incapability of adequate anaerobic energy generation5,6. For therapeutic purposes, fast and immediate action is pivotal. In clinical practice, fluid resuscitation with a balanced electrolyte solution is the first option for treatment, followed by the administration of red blood cell concentrates and fresh frozen plasma. Thrombocyte concentrates, catecholamines, and the optimization of coagulation and the acid-base status support the therapy to regain normal physiological conditions after sustained trauma. This concept focuses on the restoration of hemodynamics and macrocirculation. Several studies, however, show that microcirculatory perfusion does not recover simultaneously with the macrocirculation. Especially, cerebral perfusion remains impaired and further oxygen undersupply may occur7,8.
The use of animal models allows scientists to establish novel or experimental strategies. The comparable anatomy, homology, and physiology of pigs and humans enable conclusions on specific pathological factors. Both species have a similar metabolic system and response to pharmacologic treatments. This is a great advantage in comparison to small animal models where differences in blood volume, hemodynamics, and overall physiology make it almost impossible to mimic a clinical scenario9. Furthermore, authorized medical equipment and consumables can be easily used in porcine models. Additionally, it is easily possible to obtain pigs from commercial suppliers, which allows a high diversity of genetics and phenotypes and is cost reducing10. The model of blood withdrawal via vessel cannulation is quite common11,12,13,14,15.
In this study, we extend the concept of hemorrhagic shock induction via arterial blood withdrawal with an exact titration of hemodynamic failure and cerebral oxygenation impairment. Hemorrhagic shock is achieved if the cardiac index and mean arterial pressure drops below 40% of the baseline value, which has been shown to cause considerable deterioration of the cerebral regional oxygenation saturation8. Pulse contour cardiac output (PiCCO) measurement is used for continuous hemodynamic monitoring. First, the system has to be calibrated by transpulmonary thermodilution, which enables the calculation of the cardiac index of the extravascular lung water content and the global end-diastolic volume. Subsequently, the continuous cardiac index is calculated by pulse contour analysis and also provides dynamic preload parameters like pulse pressure and stroke volume variation.
This technique is well established in clinical and experimental settings.Near-infrared spectroscopy (NIRS) is a clinically and experimentally established method to monitor changes in cerebral oxygen supply in real-time. Self-adherent sensors are attached to the left and right forehead and calculate the cerebral oxygenation non-invasively in the cerebral frontal cortex. Two wavelengths of infrared light (700 and 900 nm) are emitted and detected by the sensors after being reflected from the cortex tissue. To assess the cerebral oxygen content, contributions of arterial and venous blood are calculated in 1:3 relations and updated in 5 s intervals. The sensitivity in depth of 1-4 cm is exponential decreasing and influenced by the penetrated tissue (e.g., skin and bone), although the skull is translucent to infrared light. The technique facilitates quick therapeutic actions to prevent patients from adverse outcomes like delirium or hypoxic cerebral injury and serves as the target parameter in case of impaired cardiac output16,17. The combination of both techniques during experimental shock enables an exact titration of macrocirculation, as well as cerebral microcirculatory impairment, to study this life-threatening event.
Protocol
The experiments in this protocol were approved by the State and Institutional Animal Care Committee (Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany; Chairperson: Dr. Silvia Eisch-Wolf; reference number: 23 177-07/G 14-1-084; 02.02.2015). The experiments were conducted in accordance with the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines. The study was planned and conducted between November 2015 and March 2016. After extended literature research, the pig model was chosen as a well-established model for hemorrhagic shock. Seven anesthetized male pigs (Sus scrofa domestica) with a mean weight of 28 ± 2 kg and an age of 2-3 months were included in the protocol. The animals were cared for by a local breeder that was recommended by the State and Institutional Animal Care Committee. The animals were kept in their known environment as long as possible to minimize stress. Food, but not water was denied 6 h before the experiment was scheduled, to reduce the risk of aspiration. The representative time course is displayed in Figure 1.
1. Anesthesia, Intubation, and Mechanical Ventilation
- Sedate pigs with a combined injection of ketamine (4 mg·kg-1) and azaperone (8 mg·kg-1) in the neck or the gluteal muscle with a needle for intramuscular injection (1.2 mm). Ensure that the animals remain stable until the sedation sets in.
CAUTION: Gloves are absolutely necessary when handling animals. - Transport the sedated animals to the laboratory.
NOTE: The animals fall deeply asleep and do not awake during normal handling, like when they are lifted into the transport cage. In this setting, the transport time was about 20 min with a special van for animal transport. - Monitor the peripheral oxygen saturation (SpO2) with a sensor clipped to the pig's tail or ear directly after arrival.
- Disinfect the skin with colorless disinfection tincture and wait for 3 min before inserting a peripheral vein catheter (1.2 mm) into an ear vein. Then, induce anesthesia by an intravenous injection of fentanyl (4 µg·kg-1) and propofol (3 mg·kg-1).
- When all reflexes are absent and spontaneous breathing expires, place the pigs in supine position on a stretcher and fix them with bandages.
NOTE: Adequate levels of anesthesia have to be confirmed by an experienced researcher by the absence of an eyelid reflex and other reactions to external stimuli. - Immediately start noninvasive ventilation with a dog ventilation mask (size 2). Use the following ventilation parameters: inspiratory oxygen fraction (FiO2) = 1.0; respiratory rate = 14-16 min-1; peak inspiratory pressure <20 cm H2O, positive end-expiratory pressure (PEEP) = 5 cm H2O.
- Maintain anesthesia via a continuous infusion of fentanyl (0.1-0.2 µg·kg-1·h-1) and propofol (8-12 mg·kg-1·h-1) and start an infusion of balanced electrolyte solution (5 mL·kg-1·h-1).
- Facilitate endotracheal intubation by the application of a muscle relaxant (atracurium 0.5 mg·kg-1).
- Secure the airway via intubation with a common endotracheal tube (ID 6-7) and an introducer. Use a common laryngoscope with a Macintosh blade (size 4). Two persons facilitate the procedure.
- Person 1: Fix the tongue outside with a piece of tissue and open the snout with the other hand.
- Person 2: Perform a laryngoscopy.
- Person 2: When the epiglottis comes into view, move the laryngoscope ventrally. Lift up the epiglottis and make sure the vocal cords are visible.
NOTE: If the epiglottis does not move dorsally, it sticks to the soft palatine and can be mobilized by the tip of the tube. Alternatively, a blade with another size (3 or 5) or type (Miller blade) can be used.
- Move the tube carefully through the vocal cords.
NOTE: The narrowest point of the trachea is not on the level of the vocal cords but subglottic. If tube insertion is not possible, try to rotate the tube or use a smaller tube. - Pull the introducer out of the tube, use a 10 mL syringe to block the cuff with 10 mL of air, and control the cuff pressure with a cuff manager (30 cm H2O).
- Start mechanical ventilation after the tube is connected to a ventilator (PEEP = 5 cm H2O; tidal volume = 8 mL·kg-1; FiO2 = 0.4; inspiration-to-expiration ratio = 1:2; respiratory rate = variable to achieve an end-tidal CO2 of <6 kPa).
NOTE: Avoid fluctuation of the CO2 to minimize any respiratory effects on the cerebral perfusion. - Make sure that tube position is correct by regular and periodic exhalation of CO2 via capnography, and check the double-sided ventilation through auscultation.
NOTE: If the tube is placed incorrectly, air inflation into the stomach rapidly forms a visible bulge in the abdominal wall, even before the capnography is installed. In this case, replacement of the tube and the insertion of a gastric tube are absolutely necessary. - With two persons, place a gastric tube into the stomach to avoid reflux and vomiting.
- Person 1: Fix the tongue outside with a piece of tissue and open the snout with the other hand.
- Person 2: Perform a laryngoscopy of the porcine larynx.
- Person 2: Visualize the esophagus.
- Person 2: Push the gastric tube inside the esophagus with a pair of Magill forceps until gastric fluid is drained.
NOTE: Sometimes, visualization is not easy. In this case, move the laryngoscope dorsally to the tube and push it ventrally to open the esophagus. During the procedure, the animal body is covered with blankets to avoid hypothermia. If the animal's body temperature decreases, use a heating system to stabilize the temperature on a physiological level (see the Table of Materials). The body temperature is displayed on the screen of the PiCCO.
2. Instrumentation
- Use bandages to pull back the hind legs to smoothen the folds in the femoral area for vessel catheterization.
- Prepare the following materials: one 5 mL syringe, one 10 mL syringe, one 50 mL syringe, one Seldinger needle, introducer sheaths (2 mm, 2.7 mm, 2.7 mm), guidewires for the sheaths, a central venous catheter with three ports (2.3 mm, 30 cm) with guidewire, and a PiCCO catheter (1.67 mm, 20 cm).
- Disinfect the inguinal area with colored disinfection, wait for 2 min, and wipe the disinfection off with a sterile tissue. Repeat this procedure 3x. After the third time, do not remove the disinfection.
- Fill all catheters with saline solutions.
- Apply ultrasound gel to the ultrasound probe. Cover the inguinal area with a sterile fenestrated drape and scan the right femoral vessels with ultrasound. Use the Doppler technique to distinguish between the artery and the vein18.
- Bright red pulsating blood confirms the aspired needle position. Disconnect the syringe and insert the guidewire into the right femoral artery.
- Visualize the longitudinal axis of the right femoral vein and insert the Seldinger needle under permanent aspiration with the 5 mL syringe.
- Aspirate dark red nonpulsating venous blood.
- Visualize the right femoral artery axially and switch to a longitudinal view of the artery by rotating the probe 90°.
- Puncture the right femoral artery under ultrasound visualization with the Seldinger needle under permanent aspiration with the 5 mL syringe.
NOTE: The ultrasound-guided Seldinger technique is associated with significantly lower blood loss, tissue trauma, and time consumption than other methods of vascular access19,20.- If the correct position of the needle in the different vessels cannot be established for certain, take the blood probes and analyze the blood gas content with a blood gas analyzer (see the Table of Materials). A high oxygen level is a good sign of arterial blood, and a low oxygen level is a sign of venous blood.
- Insert the guidewire for the central venous catheter into the right femoral vein after disconnecting the syringe and retracting the Seldinger needle.
- Visualize both right vessels with ultrasound to control the correct wire position.
- Push the arterial introducer sheath (2 mm) over the guidewire into the right artery and secure the position with blood aspiration.
- Use the Seldinger technique to position the central venous line into the right femoral vein. Aspirate all ports and flush them with saline solution.
- Perform the same procedure on the left inguinal side to insert the other introducer sheaths in the Seldinger technique into the left femoral artery (2.7 mm) and femoral vein (2.7 mm).
- Connect the right arterial introducer sheath and the central venous catheter with two transducer systems for the measurement of invasive hemodynamics, and position both transducers on the heart level to get appropriate values.
- Switch the three-way-stopcocks of both transducers open to the atmosphere to calibrate the systems to 0 as is prescribed in the operation instructions.
NOTE: It is absolutely necessary to avoid any air bubbles and bloodstains in the systems to generate plausible values. - Switch all infusions for maintaining anesthesia from the peripheral vein to the central venous line.
- Take baseline values (hemodynamics, spirometry, NIRS (see section 4) and PiCCO (see section 3) after 15 min of recovery.
- Initiate hemorrhagic shock (see section 5).
3. PiCCO Measurement
NOTE: For the PiCCO equipment, see the Table of Materials.
- Insert the PiCCO catheter into the right arterial introducer sheath.
NOTE: In clinical medicine, PiCCO catheters are directly placed by the Seldinger technique. However, placement via an introducer sheath is feasible as well. - Connect the catheter with the arterial wire of the PiCCO system and the arterial transducer directly with the PiCCO port. Then, recalibrate as described in step 2.17.
- Connect the venous measuring unit of the PiCCO system with the left venous introducer sheath.
NOTE: It is necessary to connect the venous and arterial probes at some distance from each other. Otherwise, the measurement will be disturbed, because the application of cold saline solution into the venous system will influence the arterial measurement. For more details on PiCCO, see Mayer and Suttner21. - Turn on the PiCCO system and confirm that a new patient is measured.
- Enter the animal's size and weight and switch the category to adults.
- Enter the protocol name and ID and enter Exit.
- Set the injection volume to 10 mL.
NOTE: The volume of the chosen injection solution can be varied. A higher volume makes the measured values more valid. Chose a small volume to avoid any hemodilution effects through repetitive application. - Enter the central venous pressure.
- Open the three-way stopcock to the atmosphere, click on Zero for system calibration, and click on Exit.
- Calibrate the continuous cardiac output measurement as described next and click on TD (Thermodilution). Prepare physiological saline solution with a temperature of 4 °C in a 10 mL syringe and click on Start.
- Inject 10 mL of the cold saline solution quickly and steadily into the venous measuring unit and wait until the measurement is completed and the system requests a repetition.
- Repeat this procedure until three measurements are completed.
- Let the system calculate the mean of all parameters and click on Exit.
- After complete calibration, immediately start the measurement. To monitor shock induction, focus on the PiCCO-derived parameter cardiac index.
4. Cerebral Regional Oxygenation Saturation
NOTE: For the equipment to monitor cerebral regional oxygenation, see the Table of Materials.
- Shave the forehead of the pig with a disposable razor and water and stick two self-adherent sensors (see the Table of Materials) for NIRS to the forehead of the pig.
- Connect the preamplifier to the monitor and connect the sensor cable connectors color-coded to the preamplifier.
- Close the preamp locking mechanism and attach the sensors to the sensor cables.
NOTE: In order to record real-time data, a USB flash drive has to be connected to the NIRS monitor. - Switch on the monitor, click on New Patient, enter the study name, and click on Done.
- Check the incoming signal. When the signal is stable, click on Baseline Menu and click on Set Baselines. If the baseline has already been entered, confirm the new baseline by clicking Yes and click on Event Mark.
- Chose the event with the arrow buttons on the keyboard and with Next Event; select the event 3 Induction and press Select Event.
NOTE: If further information is necessary, consult the operation manual of the NIRS system22.
5. Hemorrhagic Shock Induction
- Connect left introducer sheath with a tree-way stopcock. Connect one port of the three-way stopcock with a 50 mL syringe and one with an empty infusion bottle.
NOTE: Alternatively, the withdrawn blood may be collected in citrated bags for later autotransfusion. This is a major advantage of controlled blood withdrawal. - Measure and document the exact hemodynamic parameters and calculate 40% of the cardiac index and mean arterial pressure as hemodynamic targets. Set the event 93 Blood Loss in the NIRS system as described in step 4.6.
NOTE: Hemorrhagic shock is achieved if the cardiac index and mean arterial pressure drops below 40% of the baseline value. A considerable cerebral regional oxygenation saturation (crSO2) decline of 20% is preferable to depict microcirculatory impairment. The average blood loss to achieve this lies within a range of 25-35 mL·kg-1. - Aspirate 50 mL of blood into the syringe and switch the three-way stopcock. Push the blood into the empty bottle.
- Note the removed blood volume.
- Monitor the arterial blood pressure, the cardiac index, and the crSO2 closely. Repeat the blood withdrawal until the target blood pressure and cardiac index are achieved (after 20-30 min).
- Set the event 97 Hypotension in the NIRS device as described in step 4.6.
NOTE: Do not withdraw the blood too quickly, because this bears the risk of immediate cardio-circulatory failure. After finishing the shock induction procedure, the animals can be used for various therapeutic interventions.
6. End of the Experiment and Euthanasia
- Inject 0.5 mg of fentanyl into the central venous line and wait for 5 min.
- Inject 200 mg of propofol into the central venous line and euthanize the animal with 40 mmol potassium chloride.
Results
After starting the shock induction, a short time of compensation can be registered. With ongoing blood removal, the aforementioned cardio-circulatory decompensation, as monitored by a significant decrease of crSO2, the cardiac index, the intrathoracic blood volume index, and the global end-diastolic volume index (Figure 2, Figure 3, and Figure 4), occurs. Furthermore, significant tachycardia and a decrease of arterial blood pressure are observed as common manifestations of hemorrhagic shock (Figure 2). Stroke volume variation increases significantly (Figure 3). Extravascular lung water content and systemic vascular resistance are usually unaffected (Figure 3). After ending the blood withdrawal (28 ± 2 mL·kg-1), the hemodynamic values remain on a critically low level. Parallelly, crSO2 also drops down significantly. These sensors do not regularly start on the same level, but the percental dropdown is comparable. Figure 4 shows a representative recording from one animal. Hemoglobin content and hematocrit do not directly decrease in the process, but lactate levels rise and the central venous oxygen saturation decreases (Figure 5).

Figure 1: Experimental flow chart. The baseline is set after preparation and a 30 min stabilization. Shock is induced for 30 min. Pulse contour cardiac output parameters and cerebral regional oxygenation are measured during the whole experiment. The times of measurement are termed as Preparation, Baseline, and Shock.
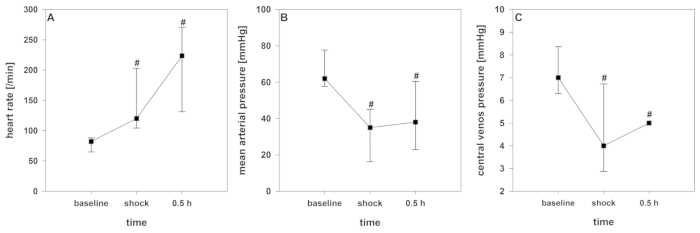
Figure 2: Development of hemodynamics during hemorrhagic shock. Effects over time are analyzed by ANOVA and post hoc Student-Newman-Keuls method. #p < 0.05 to baseline. Data are presented as mean and standard deviation. (A) Heart rate (B) mean arterial pressure, and (C) central venous pressure are considerably influenced in this model. Please click here to view a larger version of this figure.
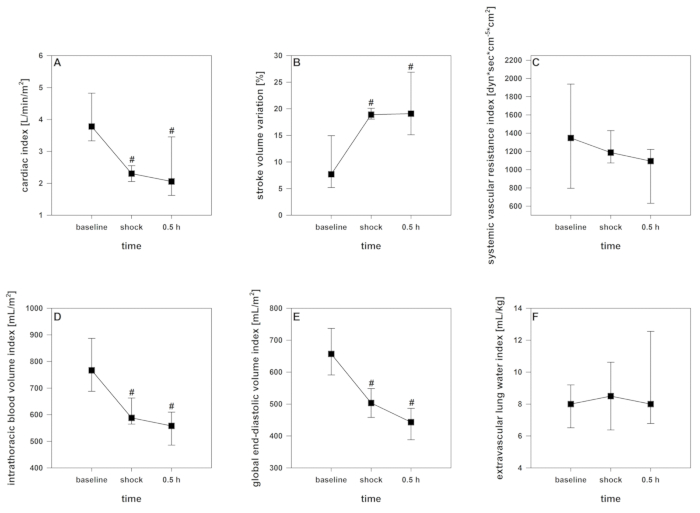
Figure 3: Development of the pulse contour cardiac output and thermodilution-derived parameters during hemorrhagic shock. Effects over time are analyzed by ANOVA and post hoc Student-Newman-Keuls method. #p < 0.05 to baseline. Data are presented as mean and standard deviation. (A) Cardiac index decreases, (B) Stroke volume variation increases, (D) intrathoracic blood volume index and (E) global end-diastolic volume index decrease, (C) systemic vascular resistance index and (F) extravascular lung water index remain unaffected. Please click here to view a larger version of this figure.
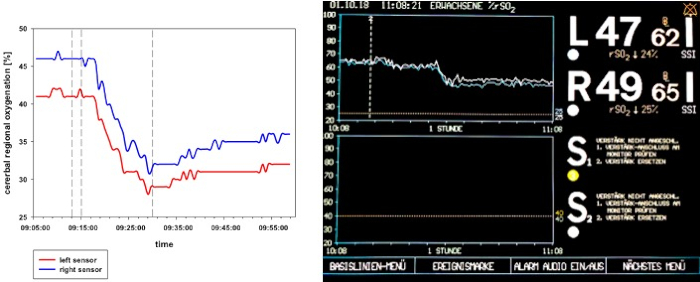
Figure 4: crSO2 flow chart during hemorrhagic shock in one representative animal. The left panel shows a schematic presentation of the crSO2 during hemorrhagic shock. The right panel shows the display of the NIRS system. crSO2 significantly breaks down through shock induction and remains at a low level after the blood withdrawal is ended.

Figure 5: Development of hematologic parameters during hemorrhagic shock. Effects over time are analyzed by ANOVA and post hoc Student-Newman-Keuls method. #p < 0.05 to baseline. Data are presented as mean and standard deviation. (A) Hemoglobin and (D) base excess remain unaffected, (C) lactate level rises significantly, (B) central venous oxygen saturation decreases. Please click here to view a larger version of this figure.
Discussion
The protocol describes one method of inducing hemorrhagic shock via controlled arterial bleeding in pigs that is guided by systemic hemodynamics, as well as by cerebral microcirculatory impairment. Shock conditions were achieved by a calculated blood withdrawal of 25-35 mL kg-1 and confirmed by the mentioned composite of surrogate parameters indicating considerable cardio-circulatory failure. If untreated, this procedure was lethal within 2 h in 66% of the animals, which underlines the severity and reproducibility of the model. Adequate fluid resuscitation, on the other hand, restabilized the circulation and approved the patency to mimic a clinical scenario8. However, less blood loss may not lead to the hemodynamic instability that also affected crSO2 leading to experimental failure. The amount of removed blood needs to be adapted to the animal's body weight, which corresponded with the total blood volume8.
This method allows scientists to examine different aspects of this life-threatening condition and opens up the opportunity to study a wide array of therapeutic interventions in a pseudoclinical scenario. In this context, it is important to note that during manifest hemorrhagic shock the macrocirculation alone hardly indicates an intact or impaired microcirculation and organ oxygen supply7. The advantage of the procedure lies in its simple design and usability. The transfer to other medium-sized mammals appears uncomplicated, although different species may exhibit specific challenges. The design provides high flexibility as different levels of cardio-circulatory impairment can be easily chosen by titrating the effect variables. The combination with NIRS provides information about the otherwise unrecognized microcirculatory oxygen supply during hemorrhagic shock.
Some of the model's critical steps have to be highlighted and require attention. Adequate sedation prior to transport is essential to avoid stress that could complicate the animal handling and falsify results by endogenic catecholamine release. The porcine snout, with its long oropharyngeal cavity, complicates intubation and makes the assistance of a second person reasonable. Regularly, the epiglottis sticks to the palate and has to be mobilized with the tip of the tube. The narrowest part of the airway is not at the level of the vocal cords but subglottic, like in pediatric patients23. These aspects make adequate muscle relaxation essential because intubation is facilitated. Ultrasound-guided vessel catheterization is preferable, although surgical access can also be used in reproducible fashion. The minimally invasive technique needs special training and experience but can minimize uncontrolled bleeding, tissue damage, complication rates, access time, and pain24. The induction of the hemorrhagic shock itself appears to be very simple, but the user should be aware of several pitfalls. It is important to reduce the blood removal speed to recognize hemodynamic instability. Arterial removal is efficient, but when it is performed too fast, it can lead to unplanned cardio-circulatory and experimental failure. The calculation of the approximate extraction volume helps to manage the removal and avoids critically low cardio-circulatory levels25,26,27. Other published protocols vary in terms of targeted hemodynamic failure, amount of removed blood volume, and period of blood withdrawal. The punctuated vessel can differ as well27,28.
NIRS enables real-time measurements of the crSO2. In several clinical settings, this method has been used to recognize an impaired cerebral oxygen supply: particularly during cardiac and major vascular surgery, NIRS represents a valuable tool. NIRS-derived parameters can predict a worse neurological outcome and patient survival caused by insufficient tissue oxygenation29. Interestingly, the intracerebral lactate level decreases in correlation with the NIRS values. Studies have shown that during oxidative stress lactate can be utilized as a source of pyruvate, and the intracranial lactate level decreases10. These findings and measurements are not considered in this basic model description. Changes of mean arterial pressure that influence the cerebral perfusion, PaO2, PaCO2, or the hemoglobin directly affect NIRS-derived crSO230,31. NIRS has a prognostic value in patients suffering from hemorrhagic shock and hemodynamic instability as well32,33,34,35,36,37,38,39. However, several limitations and disadvantages have to be noted. Extracranial tissue below the sensors, like skin, muscles, and fat, may influence the measurements and can lead to false negative results. The spatial resolution is low, and the penetration depth is limited32,33,34,40,41,42,43. The method neither differentiates between arterial and venous blood nor between oxygen delivery and demand41,44,45. The device is primarily approved for human application. The used sensors are designed for human adults. Smaller sensors for children and newborns exist, but these were not available for this protocol. In pigs, the technique is widely accepted, and crSO2 correlates with a partial pressure of oxygen, quantitative electroencephalography, and cerebral venous oxygen saturation46,47. Several devices directly measure the oxygen partial pressure in the cerebral tissue. For this purpose, the probes have to be inserted surgically into the brain. This enables unaffected measurements in the respective region of interest and avoids disturbances by surrounding noncerebral tissue. This approach is highly invasive and rather suitable for special scenarios like neurosurgical procedures48,49,50,51. The use of porcine models to simulate human pathomechanisms is a very common approach11,12,13,15. The advantage lies in the physiologic comparability between both species. Experiments that simulate life-threatening clinical conditions require fundamental expertise in intensive care medicine and anesthesia but also in specific species-related features. This allows mimicking clinical scenarios in realistic fashion for the translational testing of novel devices or therapeutic regimes on the threshold to clinical application8,52. However, we have to be aware that direct or immediate conclusions concerning clinical application can hardly be drawn from experimental models. Some relevant differences and limitations have to be noted: regarding shock or hemorrhage, the porcine coagulation system appears to be more effective and the hemoglobin content is significantly lower. Also, lactate and succinate plasma levels differ53. The porcine blood consists of an "A0" blood group system, compared to the human "AB0" system54. Some studies discuss if splenectomy should be performed to exclude the occurrence of intrinsic autotransfusion in porcine shock models. On the other hand, during splenectomy, oxidative stress, pain, and sympathetic stimulation occur, and the procedure is associated with autotransfusion reactions by itself. For these reasons, splenectomy is not recommended55,56. The use of clinically approved devices has some systemic sources of error. The PiCCO system requires calculation of the body surface area, which differs between pigs and humans. This can cause a systemic error, but the trending ability of the device will be unaffected. Other methods of cardiac output measurement, like echocardiography or a pulmonary arterial catheter, can be discussed in this setting.
In conclusion, this protocol presents a standardized hemorrhagic shock model initiated by arterial blood withdrawal and controlled by extended hemodynamic monitoring, as well as crSO2. In comparison to similar models that primarily focus on predefined removal volumes for shock induction, this approach highlights a titration by means of the resulting failure of macro- and microcirculation.
Disclosures
The NIRS device was provided unconditionally by Medtronic PLC, USA, for experimental research purposes. Alexander Ziebart, Andreas Garcia-Bardon, and Erik K. Hartmann received instructor honoraria for physician training courses from Medtronic PLC. None of the authors report financial or other conflicts of interest.
Acknowledgements
The authors want to thank Dagmar Dirvonskis for her excellent technical support.
Materials
| Name | Company | Catalog Number | Comments |
| 3-way-stopcock blue | Becton Dickinson Infusion Therapy AB Helsingborg, Sweden | 394602 | Drug administration |
| 3-way-stopcock red | Becton Dickinson Infusion Therapy AB Helsingborg, Sweden | 394605 | Drug administration/Shock induction |
| Atracurium | Hikma Pharma GmbH , Martinsried | AM03AC04* | Anesthesia |
| Canula 20 G | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | 301300 | Vascular access |
| Datex Ohmeda S5 | GE Healthcare Finland Oy, Helsinki, Finland | - | Hemodynamic monitor |
| Desinfection | Schülke & Mayr GmbH, Germany | 104802 | Desinfection |
| Heidelberger Verlängerung 75CM | Fresenius Kabi Deutschland GmbH | 2873112 | Drug administration/Shock induction |
| INVOS 5100C Cerebral | Medtronic PLC, USA | - | Monitore for cerebral regional oxygenation |
| INVOS Cerebral/Somatic Oximetry Adult Sensors | Medtronic PLC, USA | 20884521211152 | Monitoring of the cerebral regional oxygenation |
| Endotracheal tube | Teleflex Medical Sdn. Bhd, Malaysia | 112482 | Intubation |
| Endotracheal tube introducer | Wirutec GmbH, Sulzbach, Germany | 5033062 | Intubation |
| Engström Carestation | GE Heathcare, Madison USA | - | Ventilator |
| Fentanyl | Janssen-Cilag GmbH, Neuss | AA0014* | Anesthesia |
| Gloves | Paul Hartmann, Heidenheim, Germany | 9422131 | Self-protection |
| Incetomat-line 150 cm | Fresenius, Kabi GmbH, Bad Homburg, Germany | 9004112 | Drug administration |
| Ketamine | Hameln Pharmaceuticals GmbH, Zofingen, Schweiz | AN01AX03* | Sedation |
| Laryngoscope | Teleflex Medical Sdn. Bhd, Malaysia | 671067-000020 | Intubation |
| Logical pressure monitoring system | Smith- Medical GmbH, Minneapolis, USA | MX9606 | Hemodynamic monitor |
| Logicath 7 Fr 3-lumen 30cm | Smith- Medical GmbH, Minneapolis, USA | MXA233x30x70-E | Vascular access/Drug administration |
| Masimo Radical 7 | Masimo Corporation, Irvine, USA | - | Hemodynamic monitor |
| Mask for ventilating dogs | Henry Schein, Melville, USA | 730-246 | Ventilation |
| Original Perfusor syringe 50ml Luer Lock | B.Braun Melsungen AG, Melsungen, Germany | 8728810F | Drug administration |
| PICCO Thermodilution. F5/20CM EW | MAQUET Cardiovascular GmbH, Rastatt, Germany | PV2015L20-A | Hemodynamic monitor |
| Percutaneous sheath introducer set 8,5 und 9 Fr, 10 cm with integral haemostasis valve/sideport | Arrow international inc., Reading, USA | AK-07903 | Vascular access/Shock induction |
| Perfusor FM Braun | B.Braun Melsungen AG, Melsungen, Germany | 8713820 | Drug administration |
| Potassium chloride | Fresenius, Kabi GmbH, Bad Homburg, Germany | 6178549 | Euthanasia |
| Propofol 2% | Fresenius, Kabi GmbH, Bad Homburg, Germany | AN01AX10* | Anesthesia |
| Pulse Contour Cardiac Output (PiCCO2) | Pulsion Medical Systems, Feldkirchen, Germany | - | Hemodynamic monitor |
| Sonosite Micromaxx Ultrasoundsystem | Fujifilm, Sonosite Bothell, Bothell, USA | - | Vascular access |
| Stainless Macintosh Size 4 | Teleflex Medical Sdn. Bhd, Perak, Malaysia | 670000 | Intubation |
| Sterofundin | B.Braun Melsungen AG, Melsungen, Germany | AB05BB01* | balanced electrolyte infusion |
| Stresnil 40mg/ml | Lilly Germany GmbH, Wiesbaden, Germany | QN05AD90 | Sedation |
| Syringe 10 mL | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | 309110 | Drug administration |
| Syringe 2 mL | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | 300928 | Drug administration |
| Syringe 20 mL | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | 300296 | Drug administration |
| Syringe 5 mL | Becton Dickinson S.A. Carretera Mequinenza Fraga, Spain | 309050 | Drug administration |
| venous catheter 22G | B.Braun Melsungen AG, Melsungen, Germany | 4269110S-01 | Vascular access |
| *ATC: Anatomical Therapeutic Chemical / Defined Daily Dose Classification | |||
References
- Kutcher, M. E., et al. A paradigm shift in trauma resuscitation: evaluation of evolving massive transfusion practices. JAMA Surgery. 148 (9), 834-840 (2013).
- Allen, B. S., Ko, Y., Buckberg, G. D., Sakhai, S., Tan, Z. Studies of isolated global brain ischaemia: I. A new large animal model of global brain ischaemia and its baseline perfusion studies. European Journal of Cardio-Thoracic Surgery. 41 (5), 1138-1146 (2012).
- Noll, E., et al. Comparative analysis of resuscitation using human serum albumin and crystalloids or 130/0.4 hydroxyethyl starch and crystalloids on skeletal muscle metabolic profile during experimental haemorrhagic shock in swine: A randomised experimental study. European Journal of Anaesthesiology. 34 (2), 89-97 (2017).
- Tisherman, S. A., Stein, D. M. ICU Management of Trauma Patients. Critical Care Medicine. , (2018).
- Nielsen, T. K., Hvas, C. L., Dobson, G. P., Tonnesen, E., Granfeldt, A. Pulmonary function after hemorrhagic shock and resuscitation in a porcine model. Acta Anaesthesiologica Scandinavica. 58 (8), 1015-1024 (2014).
- Bogert, J. N., Harvin, J. A., Cotton, B. A. Damage Control Resuscitation. Journal of Intensive Care Medicine. 31 (3), 177-186 (2016).
- Gruartmoner, G., Mesquida, J., Ince, C. Fluid therapy and the hypovolemic microcirculation. Current Opinion in Critical Care. 21 (4), 276-284 (2015).
- Ziebart, A., et al. Effect of gelatin-polysuccinat on cerebral oxygenation and microcirculation in a porcine haemorrhagic shock model. Scandinavian Journal Trauma Resuscitation Emergency Medicin. 26 (1), 15 (2018).
- Bassols, A., et al. The pig as an animal model for human pathologies: A proteomics perspective. Proteomics Clinical Applications. 8 (9-10), 715-731 (2014).
- Alosh, H., Ramirez, A., Mink, R. The correlation between brain near-infrared spectroscopy and cerebral blood flow in piglets with intracranial hypertension. Journal of Applied Physiology. 121 (1985), 255-260 (2016).
- Hartmann, E. K., et al. Ventilation/perfusion ratios measured by multiple inert gas elimination during experimental cardiopulmonary resuscitation. Acta Anaesthesiologica Scandinavica. 58 (8), 1032-1039 (2014).
- Hartmann, E. K., Duenges, B., Baumgardner, J. E., Markstaller, K., David, M. Correlation of thermodilution-derived extravascular lung water and ventilation/perfusion-compartments in a porcine model. Intensive Care Medicine. 39 (7), 1313-1317 (2013).
- Hartmann, E. K., et al. An inhaled tumor necrosis factor-alpha-derived TIP peptide improves the pulmonary function in experimental lung injury. Acta Anaesthesiologica Scandinavica. 57 (3), 334-341 (2013).
- Ortiz, A. L., et al. The influence of Ringer's lactate or HES 130/0.4 administration on the integrity of the small intestinal mucosa in a pig hemorrhagic shock model under general anesthesia. Journal of the Veterinary Emergency and Critical. 27 (1), 96-107 (2017).
- Ziebart, A., et al. Low tidal volume pressure support versus controlled ventilation in early experimental sepsis in pigs. Respiratory Research. 15, 101 (2014).
- Hoffman, G. M., et al. Postoperative Cerebral and Somatic Near-Infrared Spectroscopy Saturations and Outcome in Hypoplastic Left Heart Syndrome. The Annals of Thoracic Surgery. 103 (5), 1527-1535 (2017).
- Hickok, R. L., Spaeder, M. C., Berger, J. T., Schuette, J. J., Klugman, D. Postoperative Abdominal NIRS Values Predict Low Cardiac Output Syndrome in Neonates. World Journal for Pediatric and Congenital Heart Surgery. 7 (2), 180-184 (2016).
- Weiner, M. M., Geldard, P., Mittnacht, A. J. Ultrasound-guided vascular access: a comprehensive review. Journal of Cardiothoracic and Vascular Anesthesia. 27 (2), 345-360 (2013).
- Kumar, A., Chuan, A. Ultrasound guided vascular access: efficacy and safety. Best Practice & Research: Clinical Anaesthesiology. 23 (3), 299-311 (2009).
- Lamperti, M., et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Medicine. 38 (7), 1105-1117 (2012).
- Mayer, J., Suttner, S. Cardiac output derived from arterial pressure waveform. Current Opinion in Anesthesiology. 22 (6), 804-808 (2009).
- Medtronic. . Operations Manual INVOS ® System, Model 5100C. , (2013).
- Wani, T. M., Rafiq, M., Akhter, N., AlGhamdi, F. S., Tobias, J. D. Upper airway in infants-a computed tomography-based analysis. Paediatric Anaesthesia. 27 (5), 501-505 (2017).
- Tuna Katircibasi, M., Gunes, H., Cagri Aykan, A., Aksu, E., Ozgul, S. Comparison of Ultrasound Guidance and Conventional Method for Common Femoral Artery Cannulation: A Prospective Study of 939 Patients. Acta Cardiologica Sinica. 34 (5), 394-398 (2018).
- Teeter, W. A., et al. Feasibility of basic transesophageal echocardiography in hemorrhagic shock: potential applications during resuscitative endovascular balloon occlusion of the aorta (REBOA). Cardiovascular Ultrasound. 16 (1), 12 (2018).
- Kontouli, Z., et al. Resuscitation with centhaquin and 6% hydroxyethyl starch 130/0.4 improves survival in a swine model of hemorrhagic shock: a randomized experimental study. European Journal of Trauma and Emergency Surgery. , (2018).
- Nikolian, V. C., et al. Improvement of Blood-Brain Barrier Integrity in Traumatic Brain Injury and Hemorrhagic Shock Following Treatment With Valproic Acid and Fresh Frozen Plasma. Critical Care Medicine. 46 (1), e59-e66 (2018).
- Williams, T. K., et al. Endovascular variable aortic control (EVAC) versus resuscitative endovascular balloon occlusion of the aorta (REBOA) in a swine model of hemorrhage and ischemia reperfusion injury. The Journal of Trauma and Acute Care Surgery. 85 (3), 519-526 (2018).
- Aly, S. A., et al. Cerebral tissue oxygenation index and lactate at 24 hours postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenital Heart Disease. 12 (2), 188-195 (2017).
- Sorensen, H. Near infrared spectroscopy evaluated cerebral oxygenation during anesthesia. The Danish Medical Journal. 63 (12), (2016).
- Cem, A., et al. Efficacy of near-infrared spectrometry for monitoring the cerebral effects of severe dilutional anemia. Heart Surgery Forum. 17 (3), E154-E159 (2014).
- Edmonds, H. L., Ganzel, B. L., Austin, E. H. Cerebral oximetry for cardiac and vascular surgery. Seminars in Cardiothoracic and Vascular Anesthesia. 8 (2), 147-166 (2004).
- Murkin, J. M., et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesthesia & Analgesia. 104 (1), 51-58 (2007).
- Hong, S. W., et al. Prediction of cognitive dysfunction and patients' outcome following valvular heart surgery and the role of cerebral oximetry. European Journal of Cardio-Thoracic Surgery. 33 (4), 560-565 (2008).
- Al Tayar, A., Abouelela, A., Mohiuddeen, K. Can the cerebral regional oxygen saturation be a perfusion parameter in shock?. Journal of Critical Care. 38, 164-167 (2017).
- Torella, F., Cowley, R. D., Thorniley, M. S., McCollum, C. N. Regional tissue oxygenation during hemorrhage: can near infrared spectroscopy be used to monitor blood loss?. Shock. 18 (5), 440-444 (2002).
- Yao, F. S., Tseng, C. C., Ho, C. Y., Levin, S. K., Illner, P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia. 18 (5), 552-558 (2004).
- Slater, J. P., et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. The Annals of Thoracic Surgery. 87 (1), 36-44 (2009).
- Brodt, J., Vladinov, G., Castillo-Pedraza, C., Cooper, L., Maratea, E. Changes in cerebral oxygen saturation during transcatheter aortic valve replacement. Journal of Clinical Monitoring and Computing. 30 (5), 649-653 (2016).
- Yoshimura, A., et al. Altered cortical brain activity in end stage liver disease assessed by multi-channel near-infrared spectroscopy: Associations with delirium. Scintific Reports. 7 (1), 9258 (2017).
- Douds, M. T., Straub, E. J., Kent, A. C., Bistrick, C. H., Sistino, J. J. A systematic review of cerebral oxygenation-monitoring devices in cardiac surgery. Perfusion. 29 (6), 545-552 (2014).
- Forman, E., et al. Noninvasive continuous cardiac output and cerebral perfusion monitoring in term infants with neonatal encephalopathy: assessment of feasibility and reliability. Pediatric Research. 82 (5), 789-795 (2017).
- Tweddell, J. S., Ghanayem, N. S., Hoffman, G. M. Pro: NIRS is " standard of care " for postoperative management. Seminars in Thoracic and Cardiovascular Surgery: Pediatric Cardiac Surgery Annual. 13 (1), 44-50 (2010).
- Lewis, C., Parulkar, S. D., Bebawy, J., Sherwani, S., Hogue, C. W. Cerebral Neuromonitoring During Cardiac Surgery: A Critical Appraisal With an Emphasis on Near-Infrared Spectroscopy. Journal of Cardiothoracic and Vascular Anesthesia. 32 (5), 2313-2322 (2018).
- Thudium, M., Heinze, I., Ellerkmann, R. K., Hilbert, T. Cerebral Function and Perfusion during Cardiopulmonary Bypass: A Plea for a Multimodal Monitoring Approach. Heart Surgery Forum. 2 (1), E028-E035 (2018).
- Putzer, G., et al. Monitoring of brain oxygenation during hypothermic CPR - A prospective porcine study. Resuscitation. 104, 1-5 (2016).
- Weenink, R. P., et al. Detection of cerebral arterial gas embolism using regional cerebral oxygen saturation, quantitative electroencephalography, and brain oxygen tension in the swine. Journal of Neuroscience Methods. 228, 79-85 (2014).
- Mader, M. M., et al. Evaluation of a New Multiparameter Brain Probe for Simultaneous Measurement of Brain Tissue Oxygenation, Cerebral Blood Flow, Intracranial Pressure, and Brain Temperature in a Porcine Model. Neurocritical Care. , (2018).
- Mikkelsen, M. L. G., et al. The influence of norepinephrine and phenylephrine on cerebral perfusion and oxygenation during propofol-remifentanil and propofol-remifentanil-dexmedetomidine anaesthesia in piglets. Acta Veterinaria Scandinavica. 60 (1), 8 (2018).
- Nelskyla, A., et al. The effect of 50% compared to 100% inspired oxygen fraction on brain oxygenation and post cardiac arrest mitochondrial function in experimental cardiac arrest. Resuscitation. 116, 1-7 (2017).
- Klein, K. U., et al. Intraoperative monitoring of cerebral microcirculation and oxygenation--a feasibility study using a novel photo-spectrometric laser-Doppler flowmetry. European Journal of Trauma and Emergency Surgery. 22 (1), 38-45 (2010).
- Ziebart, A., et al. Pulmonary effects of expiratory-assisted small-lumen ventilation during upper airway obstruction in pigs. Anaesthesia. 70 (10), 1171-1179 (2015).
- Reisz, J. A., et al. All animals are equal but some animals are more equal than others: Plasma lactate and succinate in hemorrhagic shock-A comparison in rodents, swine, nonhuman primates, and injured patients. The Journal of Trauma and Acute. 84 (3), 537-541 (2018).
- Smith, D. M., Newhouse, M., Naziruddin, B., Kresie, L. Blood groups and transfusions in pigs. Xenotransplantation. 13 (3), 186-194 (2006).
- Boysen, S. R., Caulkett, N. A., Brookfield, C. E., Warren, A., Pang, J. M. Splenectomy Versus Sham Splenectomy in a Swine Model of Controlled Hemorrhagic. Shock. 46 (4), 439-446 (2016).
- Wade, C. E., Hannon, J. P. Confounding factors in the hemorrhage of conscious swine: a retrospective study of physical restraint, splenectomy, and hyperthermia. Circulatory Shock. 24 (3), 175-182 (1988).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved