Method Article
Precise, High-throughput Analysis of Bacterial Growth
In This Article
Summary
Quantitative evaluation of bacterial growth is essential to understanding microbial physiology as a systems-level phenomenon. A protocol for experimental manipulation and an analytical approach are introduced, allowing for precise, high-throughput analysis of bacterial growth, which is a key subject of interest in systems biology.
Abstract
Bacterial growth is a central concept in the development of modern microbial physiology, as well as in the investigation of cellular dynamics at the systems level. Recent studies have reported correlations between bacterial growth and genome-wide events, such as genome reduction and transcriptome reorganization. Correctly analyzing bacterial growth is crucial for understanding the growth-dependent coordination of gene functions and cellular components. Accordingly, the precise quantitative evaluation of bacterial growth in a high-throughput manner is required. Emerging technological developments offer new experimental tools that allow updates of the methods used for studying bacterial growth. The protocol introduced here employs a microplate reader with a highly optimized experimental procedure for the reproducible and precise evaluation of bacterial growth. This protocol was used to evaluate the growth of several previously described Escherichia coli strains. The main steps of the protocol are as follows: the preparation of a large number of cell stocks in small vials for repeated tests with reproducible results, the use of 96-well plates for high-throughput growth evaluation, and the manual calculation of two major parameters (i.e., maximal growth rate and population density) representing the growth dynamics. In comparison to the traditional colony-forming unit (CFU) assay, which counts the cells that are cultured in glass tubes over time on agar plates, the present method is more efficient and provides more detailed temporal records of growth changes, but has a stricter detection limit at low population densities. In summary, the described method is advantageous for the precise and reproducible high-throughput analysis of bacterial growth, which can be used to draw conceptual conclusions or to make theoretical observations.
Introduction
Microbiological studies often start with the culture of bacterial cells and the assessment of the bacterial growth curves, which represent a fundamental phenomenon of bacterial physiology1,2,3. Basic culture principles are widely available in the published research literature and textbooks because bacterial culture is a fundamental methodology. At the bench level, substantial attention has traditionally been focused on optimizing growth media and culturing conditions, but controlling the growth rate, which would likely provide even greater understanding of microbial physiology, has not been extensively studied4. For exponentially growing bacteria, a key parameter of the cellular state is the growth rate, which has been reported to be coordinated with the genome, transcriptome, and proteome5,6,7,8. Thus, quantitative evaluation of bacterial growth is crucial for understanding microbial physiology.
To evaluate bacterial growth, the experimental methods used to estimate biomass are well established9,10 and are based on the detection of biochemical, physical, or biological parameters, such as optical turbidity. In addition, the analytical methods used to capture the dynamic properties of growth changes are commonly based on established nonlinear models11,12,13, for example, logistic equations. Growth dynamics are generally acquired by timed sampling of cell growth in culture by either measuring optical turbidity or performing colony-forming unit (CFU) assays. The limitation of these culturing and detection methods is that the data points are not a true reflection of population dynamics because the measurement intervals are often in hours and because the culture condition (e.g., changes in temperature and aeration) is disturbed at the time of sampling. Culture and analysis techniques must be updated using recent developments in technology and understanding. Recent advances in microplate readers allow the real-time observation of bacterial growth and significantly decrease labor costs. Using these advanced devices, the latest studies on bacterial growth have reported analytical methods for high-throughput measurements14,15.
The purpose of this protocol is to evaluate the precise growth dynamics in a high-throughput manner, which will be valuable for quantitative studies that ultimately address the questions of how the growth rate is determined and what factors affect the growth rate. The protocol addresses all factors that should be taken into account for the repeatable and precise quantitation of bacterial growth. The experimental method and analysis are described in detail in the main text. This method permits the precise and reproducible analysis of bacterial growth in a high-throughput manner. Microbiologists can use this protocol to derive additional quantitative results from their experimental evidence. This protocol can also be used for studies in systems biology that attempt to draw conceptual conclusions or to achieve a theoretical overview of growth.
Protocol
1. Preparing the Growth Medium
NOTE: The chemical composition of minimal medium M63 is as follows: 62 mM K2HPO4, 39 mM KH2PO4, 15 mM (NH4)2SO4, 1.8 µM FeSO4, 15 µM thiamine-HCl, 0.2 mM MgSO4, and 22 mM glucose. M63 is made by mixing three stock solutions: Five X solution, 20% glucose and MgSO4 thiamine solution. Store all solutions at 4 °C.
- Preparing the Five X solution
- To prepare FeSO4 solution, use an electrical pipette and a disposable serological pipette to add ddH2O to a 50-mL centrifuge tube. Use a P-200 to add 0.06 mL HCl to obtain 0.01 M HCl. Add 36 mg FeSO4-7H2O and mix well.
- Measure 160 mL ddH2O in a measuring cylinder and add it to a 500-mL beaker. Add the following in this order: 10.72 g K2HPO4, 5.24 g KH2PO4, 2.0 g (NH4)2SO4, and 0.5 mL FeSO4 solution (prepared in step 1.1.1).
- Using a precision pH meter, adjust the pH to 7.0 by adding 2 M KOH. Pour the solution in a measuring cylinder and add ddH2O to a total volume of 200 mL. Sterilize the solution using a 250-mL filtration unit (PVDF, 0.22 µM).
- Preparing the 20% glucose solution
- Measure 200 mL ddH2O in a measuring cylinder and pour it to a 500-mL beaker. While mixing using a magnetic stirrer, add 50 g of glucose. Dilute the solution in a measuring cylinder to a total volume of 250 mL. Sterilize the solution as described in step 1.1.3.
- Preparing the MgSO4 thiamine solution
- Add 150 mL ddH2O to a 500-mL beaker. While mixing with a magnetic stirrer, add 1.0 g of thiamine-HCl and 10 g of MgSO4-7H2O. Dilute the solution in a measuring cylinder to a total volume of 200 mL. Sterilize the solution as described in step 1.1.3.
- Preparing minimal medium M63
- Place the Five X solution, the 20% glucose solution, the MgSO4 thiamine solution, an electronic pipette, a P-200 pipette, and 200-µL pipette tips on a clean bench.
- Measure 155.8 mL ddH2O in a measuring cylinder and pour it into a 500-mL beaker.
- Using the electronic pipette and disposable serological pipette, add 40 mL of Five X solution and 4 mL 20% glucose solution to the measured ddH2O. Then, add 0.2 mL MgSO4 thiamine solution with the P-200. Sterilize the solution as described in step 1.1.3.
2. Preparing the Glycerol Stock
- Cell culture
NOTE: The bacterial strains (e.g., W3110 and its reduced genomes) are available from strain bank organizations. The strains are usually obtained in the form of colonies on agar plates.- Place five sterilized glass tubes with silicone rubber stoppers, an electronic pipette, P-1,000, 1,000-µL pipette tips, P-200, 200-µL pipette tips, and the target strains (colonies on plates) on a clean bench.
- Expose the mouth of the glass tube to a Bunsen burner before opening the silicone rubber stopper. Expose the silicone rubber stopper to the flame after the tube is opened, and then lightly place the cap back on the glass tube.
- Use the electronic pipette and disposable serological pipette to add 5 mL M63 to one of the glass tubes and 4.5 mL M63 to the other four tubes.
- Use the P-200 tip to pick a colony and inoculate it in the glass tube containing 5 mL M63.
- Vortex the tube to make a suspension. Then, dilute the solution 10-fold by transferring 0.5 mL of this solution to one of the four tubes containing 4.5 mL M63.
- Repeat the process described in step 2.1.5 for the remaining tubes. A dilution series with five different concentrations (dilutions of 1, 10, 100, 1000, and 10,000) is now ready.
- Sterilize the mouths of the glass tubes and the silicone rubber stoppers as described in step 2.1.2. Cap the tubes with the stoppers. Avoid contamination by not wrinkling the silicone rubber stoppers.
- Place the five tubes in a pre-warmed shaking incubator at 37 °C and shake at 200 rpm. Incubate the culture overnight or for 10 to 30 h.
- Selection of the culture for the glycerol stock
- Place the pre-warmed room temperature M63 medium, P-1,000, 1,000-µL pipette tips, and a disposable cuvette on a clean bench.
- Add 1000 µL M63 to a disposable cuvette with a P-1,000. Place the disposable cuvette in a spectrophotometer, start the program at a fixed wavelength of 600 nm, and measure the blank.
- Move the five glass tubes from the shaking incubator to the clean bench.
- Discard M63 from the disposable cuvette and add 1,000 µL culture to the same disposable cuvette with a P-1,000. Measure the optical turbidity of the cell culture (OD600) as described in step 2.2.2.
NOTE: To avoid any contamination and to achieve a precise measurement, expose the glass tubes and the stoppers to the flame as described and vortex the culture before sampling. To ensure reliable results, repeated measurements are recommended, particularly when the cell density is low. - Of the five cell cultures, choose one that is in the early exponential growth phase (OD600= 0.01 - 0.05) for the glycerol stock.
NOTE: If multiple cultures have densities within the optimal range, the one closest to 0.05 is commonly selected.
- Make the glycerol stocks for repeated tests.
NOTE: This is described for preparing ten stocks. Larger or smaller amounts can be made according to the experimental requirements.- Place the sterilized 60% glycerol solution, ten sterilized 1.5-mL microtubes, P-1,000 and P-200, 1,000- and 200-µL pipette tips, and a microtube stand on a clean bench.
- Add 250 µL sterilized 60% glycerol solution and 750 µL of the selected cell culture to the 1.5-mL microtube and mix by pipetting.
NOTE: The stock volume is variable, but always maintain a 1:3 ratio of 60% glycerol to cell culture; this results in a final concentration of 15% glycerol. - Place the remaining nine microtubes in the microtube stand and dispense 100 µL of the mixture prepared in step 2.3.2 to each tube. There are now ten identical glycerol stocks for future use.
- Store the stocks in a deep freezer at -80 °C.
3. Acquiring the Growth Curves
- Setting up the microplate reader.
NOTE: The terms shown in quotation marks show the specific phrasing used on the plate reader used here (see the Table of Materials).- Open the software. Open "Protocols" in "Task Manager" and choose "Create New". Choose "Standard Protocol".
- Open "Procedure", and adjust the settings. Open "Set Temperature", and select "Incubator On". Set "Temperature" to 37 °C and "Gradient" to 0 °C. Check "Preheat" before continuing with the next step. Open "Start Kinetic", set "Run time" for 24:00:00 or 48:00:00, and "Interval" for 00:30:00 or 01:00:00.
NOTE: It takes approximately 1 min to read an entire 96 well plate. - Open "Shake" and set "Shake Mode" as "Linear". Check "Constitution Shake" and set "Frequency" at 567 cpm. Open "Read", check "Absorbance", "Endpoint/Kinetic", and "Monochromators." Set "Wavelength" to 600.
- Click "Validate" to confirm that the procedure is correct. Click "Save" to save it as a new program for future use.
- Real-time recording of growth
- Draw a 96-well plate pictogram (8 × 12 table) to indicate the positions of the inoculated culture samples on the 96-well plate. Print out the table and use it as a reference for the experiment.
NOTE: The wells located on the edges of the microplate should only contain blank medium because of evaporation. - Place a sterilized 96-well flat bottom microplate with lid, P-1000, 1000-µL pipette tips, P-200, 200-µL pipette tips, several 1.5-mL microtubes, an 8-multichannel pipette, a sterilized reagent reservoir, room temperature M63, and the glycerol stocks (prepared in step 2.3) on a clean bench.
- Add approximately 25 mL M63 to the reagent reservoir. Use this reservoir stock for all the following steps.
- Add 900 µL M63 to the microtubes in preparation for making serial dilutions.
- Thaw the glycerol stock at room temperature. Add 900 µL M63 to the thawed glycerol stock and vortex. This results in a 10-fold dilution of the original glycerol stock.
- Transfer 100 µL of the 10-fold dilution to another microtube containing 900 µL of M63 and vortex. This results in a 100-fold dilution.
- Repeat step 3.2.6 until the desired number of dilutions are achieved.
- Fill the wells at the edge of the microplate with 200 µL M63 using an 8-channel pipette (P-200).
NOTE: These wells can be used as the blank. - Load 200 µL of each diluted sample prepared in steps 3.2.4 - 3.2.7 to the microplate wells according to the reference table (step 3.2.1). Vortex the diluted samples prior to loading and load the same sample in multiple wells at varied locations on the plate.
- Place the 96-well microplate on the plate reader.
- Open "Read Now" in "Task Manager" and choose the program (section 3.1). Click "OK" to start measuring. Save the recording as a new experimental file for data analysis (section 4).
- Draw a 96-well plate pictogram (8 × 12 table) to indicate the positions of the inoculated culture samples on the 96-well plate. Print out the table and use it as a reference for the experiment.
4. Data Analysis
- Export the results of the real-time growth-rate data (section 3.2) to a USB memory stick as a text file.
NOTE: A 96-well formatted results (curves) will display on the plate reader in real-time. The hourly records (OD values) can be exported as a table; the rows and columns represent the well numbers (e.g., A1, B1) and the measuring time (e.g., 00:00:59), respectively. - Open the text file with a spreadsheet software.
- Copy the hourly OD600 reads of the 96-well microplate to a new worksheet for further analysis.
- Subtract the background reads at time zero from the hourly reads of each sample well.
NOTE: For convenience, the mean value of the blank wells containing M63 (step 3.2.8) can be used as the background value. - Calculate the mean of five consecutive OD600 reads to estimate the maximal population density.
NOTE: The largest mean value of the five consecutive OD600 reads is defined as the maximal OD600 of the corresponding growth curve. - Calculate the growth rate, µ (h-1), by applying the following equation for all pairs of consecutive values of OD600:
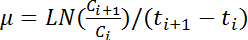
NOTE: In this equation, Ci and Ci+1 represent the OD600 values of any two consecutive time points (ti and ti+1, respectively). LN indicates natural logarithm. - Calculate the changes in the growth rate over time based on the hourly OD600 reads according to step 4.6. Calculate the mean and the standard deviation of five consecutive growth rates to estimate the maximal growth rate.
NOTE: The largest mean with the smallest standard deviation is defined as the maximal growth rate of the corresponding growth curve.
5. Confirming the Global Bias of the 96-well Reads (Optional)
NOTE: Both the plate reader and the consumable 96-well plate can cause biased measurements. To achieve highly precise and reproducible quantitative results, confirming the global bias of the 96-well plate is highly recommended.
- Prepare the 96-well plate and the plate reader for the bias test as described in section 3.2.2.
- Add 20 mL M63 to a sterilized 50-mL centrifuge tube. Add the glycerol stock to the same centrifuge tube and vortex. Transfer the suspended solution to a sterilized reagent reservoir.
- Use an 8-channel pipette to transfer 200 µL of the suspended solution to the 96-well plate.
- Place the 96-well plate on the plate reader and start the measurement.
- Record the hourly OD600 reads and analyze as described in section 4. Compare the calculated maximal growth rate and population density of each well to determine locational bias of the 96-well reads.
Results
The described method provides a means to capture dynamic bacterial growth in a continuous, high-throughput manner by utilizing a 96-well format reader that takes multiple optical density measurements at various time intervals (from minutes to hours to days). The growth curves of an assortment of E. coli strains expressing various genomes can be precisely acquired in a single experiment (Figure 1A). In comparison to the described method, the traditional method (the CFU assay) generally requires longer sampling time intervals (Figure 1B) and is labor intensive if multiple culture is required. In addition, each culture sampling time for the CFU assay requires the use of a small volume of cell culture that cannot be used for repeated measurements. Additionally, limited numbers of time points for detection may miss the sampling points that are needed for the correct calculation of the growth rate and the maximal OD600. However, the CFU assay has a lower detection limit, 103 - 104 cells/mL, making it at least two orders of magnitude more sensitive than the optical turbidity assay (OD600= 0.001, which has a detection limit of approximately 105 - 106 cells/mL). The described method provides a practical and efficient experimental tool for systems-level studies on growth dynamics.
A key advantage of this protocol is that it takes repeated measurements from a common glycerol stock. Having multiple cell cultures at various dilution rates in early exponential growth phase is highly useful because it allows researchers to determine the culture time at any timescale and avoids having saturated cultures due to unpredictable events. Repeated culturing and measurements of the common stocks, which were prepared from one of the five cultures initiated using different dilution rates as described in Protocol section 2.2 (Figure 2A), presented highly similar outcomes of the growth dynamics analysis. Multiple growth curves of repeated and independent measurements (N= 6) overlapped well (Figure 2B, top panel), with little variance (Figure 2B, bottom panel), particularly during the exponential phase (Figure 2B, shadowed area). These results suggest that the described method delivers highly reproducible results.
Estimating the global bias of the 96-well reads is highly recommended. In the present method, the global bias is estimated by monitoring the growth of the wild-type E. coli strain W3110 as described in Protocol section 5. For example, the growth rates calculated from the growth curves showed significant variation among the 96 wells (Figure 3A), representing the global fluctuation derived from both the data manipulation and the device errors. These errors can be masked by loading the same sample into multiple wells at varied locations on the plate. By contrast, the maximal OD600 showed a clear location-dependent bias for the results, as gradual changes were observed along the vertical direction, that is, the maximal OD600 gradually increased from the wells of column 2 to those of column 11 on the 96-well plate (Figure 3B). To avoid the biased results, the same sample is recommended to be loaded into the wells located on the both sides of the 96-well plate for repeated measurements. The locational bias in maximal OD600 values is assumed to result from the variation in evaporation rates, which are determined by both heating and sealing efficiencies. Compared with the wells in the middle of the 96-well microplate, the wells located at the edges tended to show relatively higher values, reflecting the varied evaporation rates resulting from the sealing effect of the microplate. In addition, since other 96-well microplates subjected to the same bias test showed similar directional changes based on where the well was located on the plate, the extent of the bias might depend on the plate reader. Thus, it is important to evaluate the global bias of the 96-well reads in studies that determine maximal population density. Commercially available plate readers all have their specific global bias caused by the heating and shaking efficiencies, and the 96-well microplates are largely differentiated by sealing efficiency.
This method is designed for the systematic analysis of bacterial growth, and it can be used to analyze two major parameters, i.e., growth rate and cell density, that are commonly applied in a wide variety of fields, such as systems biology, evolution, and ecology16,17. The manual calculation using spreadsheet is introduced to show the processing of the raw reads to produce the evaluated parameters of growth rate and cell density. The hourly growth rates are first calculated from the raw reads of the growth curve (Figure 4A) according to Protocol section 4.6 as a data set of sequential changes in growth rates (Figure 4B). The means and standard deviations of every five consecutive growth rates are acquired sequentially (Figure 4C-D), and the largest mean growth rate with the smallest standard deviation is defined as the maximal growth rate of the growth curve (Figure 4C-D, highlighted). The maximal cell density (OD600) is analyzed similarly (Figure 4E-F). The calculated growth rate and cell density are subsequently subjected to further analysis. Note that the described calculations can be automatically performed using computational platforms (programming languages), e.g., R and Python.

Figure 1 Growth Curves Acquired with Diverse Methods. (A) Multiple acquisitions of growth curves. Growth curves of four different E. coli strains expressing various genomes were obtained in a high-throughput manner. The E. coli cells were grown in minimal medium M63, and the changes in OD600 reads were recorded by a microplate reader at 1-h intervals. The strains from left to right are the wild type E. coli W3110 (Number 0) and its reduced genomes numbers 1, 10 and 18 (described previously5), respectively. (B) Growth curve obtained by using the CFU assay. The wild-type E. coli cells (Number 0) were cultured and were sampled at the indicated time points over several hours. The growth changes were estimated using the CFU assay. Open circles indicate the recording or sampling points. Please click here to view a larger version of this figure.
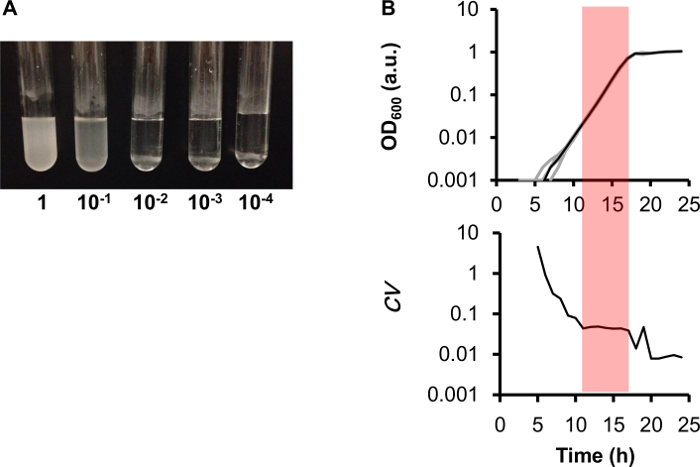
Figure 2. Reproducible Repeated Measurements. (A) Overnight cultures of varied dilution rates. The E. coli cells were inoculated in tubes at multiple dilution rates, which varied from 1- to 10,000-fold, as indicated. The cells were cultured at 37 °C with shaking at 200 rpm for approximately 12 h. The final OD600 values of the five cell cultures (from left to right) were 1.51, 0.74, 0.05, 0.008 and 0.001, respectively. The culture with OD600 of 0.05 was used for the common glycerol stock. (B) Highly reproducible measurements. Six independent growth curves (top, gray lines) of the same E. coli strain were acquired and calculated from six positions on the microplate and two vials of the common glycerol stock. The black line represents the average of the six growth curves. The coefficients of variation (CV) of the six repeated measurements were calculated (bottom), and the steady low points are shadowed in pink. Please click here to view a larger version of this figure.
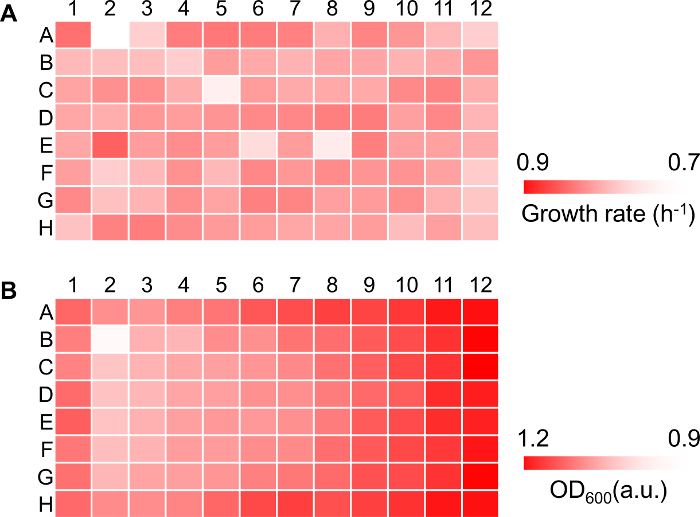
Figure 3 Global Bias of the 96-well Format. (A) Heat map of growth rates. The E. coli cell stock was suspended in minimal medium M63 and loaded equally in the 96 wells of the microplate (represented as a 12 × 8 table). The changes in OD600 were obtained using the microplate reader, as described in section 5 of the protocol. The gradation from white to red indicates the growth rates from 0.7 to 0.9 h-1. (B) Heat map of the maximal OD600. The gradation from white to red indicates the maximal OD600 values from 0.9 to 1.2. The calculations for the growth rate and maximal OD600 are as described in Figure 4. Please click here to view a larger version of this figure.

Figure 4 Analysis of the Growth Curves. (A) Raw data of growth curves. The hourly OD600 reads are plotted against the culture time. Open circles indicate the sampling times. (B) Temporal changes in growth rates. Hourly growth rates between two consecutive OD600 reads are calculated according to the equation described in 4.6. (C) Mean growth rates. The mean of five consecutive growth rates are calculated to give smooth growth rates. (D) Standard deviations of mean growth rates. The standard deviation of the same five consecutive growth rates is calculated. The maximal growth rate with the smallest standard deviation is highlighted in red. (E) Mean OD600. The mean of five consecutive OD600 values is calculated to determine the smooth OD600. (F) Standard deviations of mean OD600 values. Standard deviation of the same five consecutive OD600 values is calculated. The maximal OD600 with the smallest standard deviation is highlighted in red. Please click here to view a larger version of this figure.
Discussion
Critical steps in the protocol include the preparation of a common stock of exponentially growing cells and the replication of the same samples in multiple wells at various positions on the microplate. Previously, microbiologists started the culture from an overnight pre-culture. While this method may reduce the lag time of bacterial growth, it is difficult to achieve reproducible growth curves. As shown in Figure 2, the independent measurements using the common glycerol stocks resulted in nearly identical growth curves, which provide reproducible and precise results. As shown in Figure 3, wells at various locations give slightly different reads, which is considered the global background of the measurements. For the quantitative evaluation of bacterial growth, this background should be considered. Inoculation of samples to multiple wells at varied plate locations for experimental replication is highly recommended to account for global bias.
In addition to handling errors, the modification and troubleshooting derived from the experimental devices and supplies should be considered. Several advanced microplate readers are commercially available. They show a wide variation in characteristics, such as the method of adjusting the light wavelength, the mode of heating or cooling, and the manner and frequency of shaking. Based on our experience, evaporation should be addressed in high-throughput studies because changes in liquid volume greatly influence OD600 reads. Evaporation is caused by the method of heating used by the microplate readers and the architecture of the microplates. The two modes of heating commonly implemented in commercial microplate readers are heating by warm air and heating with a hot plate. The microplate reader used in this protocol used the hot plate as a heating method to decrease evaporation because the medium in the microplate would quickly dry up in the blowing warm air. The microplates should also be carefully selected. Different 96-well microplates (with lids) cause different levels of evaporation. The described method utilizes the plate format with a deep covered lid, which reduces evaporation.
Moreover, if the study requires stationary phase analysis of the growth curve, then the manner and frequency of shaking in the microplate reader should be taken into account. Shaking during culture ensures that the growing cells are suspended uniformly in the media. Improper shaking or low frequency may cause the cells to stack around the well edge or in the center at a high density, resulting in false reads that are either lower or higher than actual reads. The described method uses linear, bidirectional shaking. While the 8-shaped shaking mode is probably sufficient, we do not recommend the circular mode of shaking.
The major limitation of this method is the evaporation of the cell culture medium. Since the maximal culture volume is small, i.e., 200 µL, incubation at 37 °C causes significant evaporation. Thus, this method is unsuitable for culturing longer than 48 h. Additionally, cell cultures of low density should be avoided because optical detection (OD600) is limited at these densities. When considered together, very slow growing cells/strains or very low initial concentration cultures are not preferred, as they require longer time and are below the detection limit of OD600.
This method yields precise, high-throughput measurements, which are highly advantageous for systematic surveys. As a representative result, this method allows to evaluate an assortment of E. coli strains with various genomic sequences and/or medium easily and reproducibly5. When compared to traditional methods of culturing in tubes and manually measuring samples at different time points, the described method is less labor and time intensive. Future applications and development of the method are assumed to involve the automation of experimental manipulation and computational analysis. Automatic measurement and robotics application will lead to a highly efficient and reliable method of evaluating cell growth and performing survival tests using bacterial and mammalian cells.
Disclosures
We thank Kohei Tsuchiya for providing the CFU assay example. This work was partially financially supported by a Grant-in-Aid for Scientific Research (C) no. 26506003 (to BWY) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Acknowledgements
The authors have nothing to disclose.
Materials
| Name | Company | Catalog Number | Comments |
| K2HPO4 | Wako | 164-04295 | |
| KH2PO4 | Wako | 166-04255 | |
| (NH4)2SO4 | Wako | 019-03435 | |
| MgSO4-7H2O | Wako | 138-00415 | |
| Thiamine-HCl | Wako | 201-00852 | |
| glucose | Wako | 049-31165 | |
| HCl | Wako | 080-01066 | |
| Iron (II) sulfate heptahydrate (FeSO4-7H2O) | Wako | 094-01082 | |
| KOH | Wako | 168-21815 | |
| Glycerol | Wako | 075-00611 | |
| Centrifuge tube (50 mL, sterilized) | WATSON | 1342-050S | |
| Pipette Tips, 200 µL | WATSON | 110-705Y | |
| Pipette Tips, 1,000 µL | WATSON | 110-8040 | |
| Microtube (1.5 mL) | WATSON | 131-715C | |
| 8 multichannel-pipette | WATSON | NT-8200 | |
| PASORINA STIRRER | AS ONE | 2-4990-02 | |
| Glass cylinder (200 mL) | AS ONE | 1-8562-07 | |
| Precision pH mater | AS ONE | AS800 / 1-054-01 | |
| Pipetman P-200 | GILSON | 1-6855-05 | |
| Pipetman P-1000 | GILSON | 1-6855-06 | |
| Disposable Serolocical Pipettes (10 mL) | SANPLATEC | SAN27014 | |
| Disposable Serolocical Pipettes (25 mL) | SANPLATEC | SAN27015 | |
| Microtube stand | BM Bio | 801-02Y | |
| Vortex | BM Bio | BM-V1 | |
| Corning Costar 96-well microplate with lid (Flat bottom, Clear) | Sigma-Aldrich | Corning, 3370 | |
| Corning Costar reagent reservoir (50 mL) | Sigma-Aldrich | Corning, 4870 | |
| Stericup GV PVDF (250 mL, 0.22 µM) | Merck Millipore | SCGVU02RE | |
| Pipet-Aid XP | DRUMMOND | 4-000-101 | |
| Bioshaker (BR-23UM MR) | TAITEC | 0053778-000 | |
| Disposal cell (1.5 mL) | Kartell | 1938 / 2-478-02 | |
| DU 730 Life Science UV/Vis Spectrophotometer | Beckman Coulter | A23616 | |
| EPOCH2 | BioTek | 2014-EP2-002 / EPOCH2T | |
| Beaker (500 mL) | IWAKI | 82-0008 | |
| BIO clean bench | Panasonic | MCV-B131F | |
| Glass tubes | NICHIDEN RIKA GLASS | P-10M~P-30 /101019 | |
| Silicone rubber stoppers | ShinEtsu Polymer | T-19 | |
| Bacterial strains | Strain bank organization; National Bio Resource Project (NBRP) in Japan |
References
- Kovarova-Kovar, K., Egli, T. Growth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics. Microbiol Mol Biol Rev. 62 (3), 646-666 (1998).
- Soupene, E., et al. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J Bacteriol. 185 (18), 5611-5626 (2003).
- Sezonov, G., Joseleau-Petit, D., D'Ari, R. Escherichia coli physiology in Luria-Bertani broth. J Bacteriol. 189 (23), 8746-8749 (2007).
- Egli, T. Microbial growth and physiology: a call for better craftsmanship. Front Microbiol. 6, 287 (2015).
- Kurokawa, M., Seno, S., Matsuda, H., Ying, B. W. Correlation between genome reduction and bacterial growth. DNA Res. 23 (6), 517-525 (2016).
- Matsumoto, Y., Murakami, Y., Tsuru, S., Ying, B. W., Yomo, T. Growth rate-coordinated transcriptome reorganization in bacteria. BMC Genomics. 14, 808 (2013).
- Nahku, R., et al. Specific growth rate dependent transcriptome profiling of Escherichia coli K12 MG1655 in accelerostat cultures. J Biotechnol. 145 (1), 60-65 (2010).
- Dai, X., et al. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat Microbiol. 2, 16231 (2016).
- Madrid, R. E., Felice, C. J. Microbial biomass estimation. Crit Rev Biotechnol. 25 (3), 97-112 (2005).
- Harris, C. M., Kell, D. B. The estimation of microbial biomass. Biosensors. 1 (1), 17-84 (1985).
- Yates, G. T., Smotzer, T. On the lag phase and initial decline of microbial growth curves. J Theor Biol. 244 (3), 511-517 (2007).
- Kargi, F. Re-interpretation of the logistic equation for batch microbial growth in relation to Monod kinetics. Lett Appl Microbiol. 48 (4), 398-401 (2009).
- Peleg, M., Corradini, M. G. Microbial growth curves: what the models tell us and what they cannot. Crit Rev Food Sci Nutr. 51 (10), 917-945 (2011).
- Sprouffske, K., Wagner, A. Growthcurver: an R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinformatics. 17, 172 (2016).
- Hall, B. G., Acar, H., Nandipati, A., Barlow, M. Growth rates made easy. Mol Biol Evol. 31 (1), 232-238 (2014).
- Hermsen, R., Okano, H., You, C., Werner, N., Hwa, T. A growth-rate composition formula for the growth of E.coli on co-utilized carbon substrates. Mol Syst Biol. 11 (4), 801 (2015).
- Engen, S., Saether, B. E. r- and K-selection in fluctuating populations is determined by the evolutionary trade-off between two fitness measures: Growth rate and lifetime reproductive success. Evolution. 71 (1), 167-173 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved