Method Article
A Rat Tibial Growth Plate Injury Model to Characterize Repair Mechanisms and Evaluate Growth Plate Regeneration Strategies
In This Article
Summary
The growth plate is a cartilaginous region in children's long bones where longitudinal growth occurs. When injured, bony tissue can form and impair growth. We describe a rat model of growth plate injury that leads to bony repair tissue, allowing the study of repair mechanisms and growth plate regeneration strategies.
Abstract
A third of all pediatric fractures involve the growth plate and can result in impaired bone growth. The growth plate (or physis) is cartilage tissue found at the end of all long bones in children that is responsible for longitudinal bone growth. Once damaged, cartilage tissue within the growth plate can undergo premature ossification and lead to unwanted bony repair tissue, which forms a "bony bar." In some cases, this bony bar can result in bone growth deformities, such as angular deformities, or it can completely halt longitudinal bone growth. There is currently no clinical treatment that can fully repair an injured growth plate. Using an animal model of growth plate injury to better understand the mechanisms underlying bony bar formation and to identify ways to inhibit it is a great opportunity to develop better treatments for growth plate injuries. This protocol describes how to disrupt the rat proximal tibial growth plate using a drill-hole defect. This small animal model reliably produces a bony bar and can result in growth deformities similar to those seen in children. This model allows for investigation into the molecular mechanisms of bony bar formation and serves as a means to test potential treatment options for growth plate injuries.
Introduction
Growth plate injuries account for 30% of all pediatric fractures and can result in impaired bone growth1. In addition to fractures, growth plate injuries may be caused by other etiologies, including osteomyelitis2, primary bone tumors3, radiation and chemotherapy4, and iatrogenic damage5. The growth plate (or physis) is a cartilage region at the end of children's long bones that is responsible for longitudinal bone growth. It drives bone elongation through endochondral ossification; chondrocytes undergo proliferation and hypertrophy and are then remodeled by incoming osteoblasts to form trabecular bone6. The growth plate is also a weak area of the developing skeleton, making it prone to injury. The major concern with growth plate fractures or injuries is that the damaged cartilage tissue within the growth plate can be replaced with unwanted bony repair tissue, also known as a "bony bar." Depending on its size and location within the growth plate, the bony bar can lead to angular deformities or complete growth arrest, a devastating sequela for young children that have not yet reached their full height7.
There is currently no treatment that can fully repair an injured growth plate. Once the bony bar forms, the clinician must decide whether or not to surgically remove it8. Patients with at least 2 years or 2 cm of skeletal growth remaining and with a bony bar that spans less than 50% of the growth plate area are usually candidates for bony bar resection8. Surgical removal of the bony bar is often followed by interposition of an autologous fat graft to prevent reformation of the bony tissue and to allow the surrounding uninjured growth plate to restore growth. However, these techniques are problematic and often fail, leading to bony bar recurrence and continued negative effect on growth9. There is a critical need to develop effective treatments that not only prevent bony bar formation, but also regenerate the growth plate cartilage, thus restoring normal bone elongation.
The molecular mechanisms underlying bony bar formation have yet to be fully elucidated. A greater understanding of these biological mechanisms could lead to more effective therapeutic interventions for children suffering from growth plate injuries. Since studying these mechanisms in humans is difficult, animal models have been used, especially the rat model of growth plate injury10,11,12,13,14,15,16. The method presented in this paper describes how a drill-hole defect in the rat tibial growth plate leads to predictable and reproducible repair tissue that begins ossification as early as 7 days after injury and forms a fully mature bony bar with remodeling at 28 days after injury10. This provides a small animal in vivo model in which to study the biological mechanisms of bony bar formation, as well as to evaluate novel therapies that could prevent the bony bar and/or regenerate the growth plate cartilage. For example, this model can be used to test chondrogenic biomaterials that can regenerate growth plate cartilage and offer valuable treatment for children suffering from growth plate injuries. The techniques presented in this paper will describe the surgical methods used to produce the growth plate injury and the subsequent delivery of biomaterials to the injury site. We will also discuss methods to assess bony bar formation and repair tissue.
Protocol
All animal procedures must be approved by the local Institutional Animal Care and Use Committee (IACUC). The animal protocol for the following procedure was approved by the University of Colorado Denver IACUC.
1. Obtain Rats
NOTE: Unless genetically modified animals are desired, 6-week-old, skeletally immature Sprague-Dawley rats are needed at the time of surgery. Other strains could potentially be used; however, the majority of published studies have been performed on Sprague-Dawley rats.
2. Preparation of Surgical Supplies
- Autoclave surgical supply packs that include one each of the following: #3 scalpel handle, needle holder, Adson forceps, and iris scissors.
- Autoclave the keyless drill chucks. Drill chucks may be bead sterilized between animal surgeries when operating on multiple animals.
NOTE: Local IACUC rules pertaining to the use of sterile surgical tools on multiple animals must be adhered to. For example, the University of Colorado Denver IACUC allows for one surgical tool set to be used on up to 5 animals before their discontinuation. Furthermore, surgical tools must be heat sterilized using a bead sterilizer between animals. Additional sterile surgical packs must be used for any additional animals. - Autoclave 5-cm Steinmann pins, one for each animal.
NOTE: To reduce the risk of infection, the Steinmann pins must not be used for multiple animals. - Autoclave 1.8-mm dental burs, one for each animal.
NOTE: To reduce the risk of infection, the dental burs must not be used for multiple animals. - Autoclave a wound clip applicator, if applicable. Alternatively, buried sutures may be used to close the cutaneous layer. See step 7.3.
- If possible, sterilize a rotary drill using irradiation or gas sterilization.
- Collect the following additional supplies: electric shaver, sterile 3-0 polyglycolic acid sutures, sterile gauze, povidone-iodine, sterile saline, sterile 10-mL syringes, sterile 23-gauge needles, isopropyl alcohol swabs, isoflurane, calipers, post-surgical analgesics (e.g., NSAIDs and buprenorphine), sterile surgical drapes, sterile surgical gloves, sterile #15 scalpel blades, sterile wound clips, anesthesia machine, bead sterilizer, warming pad, and absorbent underpads.
3. Anesthesia and Preparation of Animals
- Anesthetize the animal by introducing it to a 1- to 2-L induction chamber receiving 1 L/min oxygen flow with 5% isoflurane from a vaporizing system with a passive scavenging system.
NOTE: Exposure to 5% isoflurane should anesthetize 6-week-old rats within 5 min. - Move the animal to the surgical site and maintain the animal under anesthesia with 2 - 3% isoflurane using a nose cone for the remainder of the procedure. Place the animal supine on a warming pad and an absorbent underpad.
NOTE: The animal does not need to be fixed to the surgical table. Holding the leg as specified in the steps below is a sufficient method of stabilization.
NOTE: All subsequent procedures are to be done with the animal under anesthesia. 2 - 3% isoflurane should be sufficient to maintain anesthesia in rats at this age. This can be confirmed by testing the bipedal withdrawal reflex. - Administer intraoperative analgesics in accordance with institutionally approved policies (e.g. buprenorphine at 0.05 mg/kg and carprofen at 5 mg/kg).
4. Preparation of the Tibia for Surgery
- Shave the entire hind leg(s) from the medial malleolus to the pelvis with an electric shaver.
- Measure and record the tibial length from the anterior tibial plateau to the inferior side of the medial malleolus using calipers. Alternatively, measure the whole tibial length using X-ray or microCT11,12,14. Optionally, measure growth plate dimensions prior to surgery using X-ray or microCT.
- Clean the surgical site by wiping the entire leg(s), abdomen, and genitalia with alcohol swabs and then with povidone-iodine-soaked gauze.
NOTE: To minimize the risk of infection, all subsequent procedures, until the animal is removed from anesthesia (step 7.4), must be done under sterile conditions. All of the surgical materials must be accessed using sterile technique. The use of a surgical assistant is highly recommended to maintain sterility throughout the surgery. - Wearing sterile surgical gloves, place a fenestrated sterile surgical drape over the animal, leaving the leg(s) exposed through the central fenestration.
5. Surgical Procedure to Access the Growth Plate

Figure 1:Overview of the Surgical Procedure.
A) Location of several anatomical markers used to create a successful growth plate injury. The knee capsule is immediately posterior to the kneecap (white), separating the tibia from the femur. The tibial growth plate (dark red) can be seen inferior to the kneecap and circumventing the tibia. The proximal growth plate is a mostly flat plane, except for the anterior quarter that forms a diagonal plane. The intersection of these two planes forms the growth plate angle, which is used for appropriate drill angulation. The semitendinosus insertion is where the quadriceps muscle inserts in the posterior tibia. B) Incision through the anterior-medial aspect of the tibial soft tissues to access the cortical bone. C) Location of the cortical window using alignment with the distal semitendinosus insertion as a reference point. D) Evaluating the depth of the injury by aligning the bevel on the dental bur with the cortical window.
- Make a ~1-cm incision through the skin along the medial-anterior aspect of the proximal tibia using a #3 scalpel handle and a #15 blade, starting at the distal end of the medial femoral condyle (Figure 1A).
- Pull the skin tight against the underlying bone and hold the leg firmly while making the incision.
NOTE: This will keep the skin incision at the desired location and will aid in the creation of a clean incision. Do not press too firmly with the scalpel to avoid puncturing the knee capsule, which would result in profuse bleeding and will make the remaining steps difficult.
- Pull the skin tight against the underlying bone and hold the leg firmly while making the incision.
- Make note of important anatomical markers, including: 1) the growth plate, 2) the growth plate angle, 3) the knee capsule, and 4) the semitendinosus insertion (Figure 1A).
- Using the scalpel, make a ~ 0.5-cm incision through the fascia and soft tissues on the medial-anterior aspect of the proximal tibia, from the growth plate to the bottom of the skin incision (Figure 1B).
- Gently dissect or scrape away the fascia and soft tissues from the tibia using the scalpel (Figure 1B).
NOTE: It is important to remove or scrape as much soft tissue from the tibia as possible so as not to interfere with the drilling steps. - Drill a cortical window through the tibial cortical bone at the diaphysis with a Steinmann pin attached to a rotary tool at 10,000 RPM (low speed of the rotary tool specified in the materials section). Create the cortical window such that it aligns with the distal semitendinosus insertion (Figure 1C).
- Hold the drill perpendicular to the tibial diaphysis and drill slowly, being careful not to drill through the other side of the diaphysis; the cortical window needs to be only ~ 2 mm in depth and will be made when no resistance is felt.
- As above, hold the leg firmly with the other hand.
NOTE: A dental bur may be used for this step. However, if a dental bur is used, the leg must be held very firmly to make a clean cortical window and to ensure that the bur grabs and cuts the bone at the desired location. A Steinmann pin is recommended for this step, given its far superior cutting ability.
- Dab the cortical window with gauze, as light bleeding is expected.
6. Creating the Growth Plate Injury
- Create a drill hole injury through the central growth plate using a 1.8-mm dental bur attached to a rotary tool.
NOTE: The proper depth, angle, and direction are critical in disrupting the central growth plate (Figure 1C and D). Instructions for attaining the appropriate depth, angle, and direction are given below.- To measure the appropriate depth using the dental bur, begin by aligning the end of the dental bur with the proximal tibia, where the semitendinosus crosses the knee capsule (Figure 1C).
- With the end of the dental bur at the knee capsule, follow the bur shaft along the semitendinosus and make note of where the bur aligns with the cortical window. This is the appropriate depth for the bur to fully disrupt the growth plate without disrupting the articular surface (Figure 1C).
NOTE: The dental bur is used to measure the appropriate depth. The bur may be marked with a permanent marker at the location where it aligns with the cortical window to reference the depth during drilling. However, if the anatomical markers and the above protocol are closely referenced, the first bevel on the dental burs specified here (FG6) will align appropriately with the cortical window (as seen in Figure 1C). - To attain the appropriate drill angle, hold the rotary tool at an angle of less than 30° with respect to the tibial diaphysis.
NOTE: This is a visual approximation. - To attain the appropriate drill direction, aim for the growth plate angle (Figure 1C). Draw a visual line along the dental bur to the growth plate angle to aid in creating a central defect.
- Turn on the rotary tool to 10,000 RPM (low speed of the rotary tool specified in the materials section) before entering the cortical window.
- With the rotary tool at an appropriate angle and direction, enter the cortical window and push the rotary tool until the bur marker aligns with the cortical window. Once the proper depth is attained, remove the rotary tool.
NOTE: Perform the growth plate disruption in one, quick motion, using minimal time with the bur in the growth plate in order to create a clean injury. This is important for data analysis.
- Dab the cortical window with gauze for ~ 30 s, as bleeding is expected.
- Ensure the appropriate depth of the injury by again measuring the bur length (step 6.1.2).
- Insert the bur into the drill track (with the rotary tool off) and align the marked bur with the cortical window (Figure 1D).
- If the depth is inadequate, turn the rotary tool on and push to the desired depth.
NOTE: Although a second round of drilling is not ideal, fully disrupting the growth plate is paramount to the development of the bony bar. - Rinse the drill track with ~ 3 mL of sterile saline using a 10-mL syringe and a 23-gauge needle.
- Dry the wound with gauze.
7. Post-Injury Procedures
- If evaluating a biomaterial-based growth plate treatment, inject the biomaterial through the drill track into the injury site using an appropriately sized needle (18- to 26-gauge, depending on the biomaterial viscosity).
NOTE: The volume of the growth plate injury is ~ 3 µL, and the volume of the drill track is ~ 20 µL. The maximum volume of material that can be injected into the growth plate injury and drill track is between 20 and 25 µL. - Close the wound by suturing the fascia with 3-0 polyglycolic acid sutures. Apply bone wax over the cortical window to isolate the underlying bone (optional).
- Close the skin incision with buried sutures or wound clips.
NOTE: Wound clips are recommended, as the animal will scratch at the injury site and may open the wound. - Remove the animal from isoflurane anesthesia, place it on a warming blanket, and monitor it until it is awake.
- To reduce the risk of infection, place the animal in a new cage containing dry, autoclaved bedding.
- Allow the animal to bear weight post-operatively.
- Monitor the animal every 12 h for 72 h after surgery to check for signs of infection, to ensure that the wound clips remain in place, and to administer postoperative analgesics in accordance with institutionally approved policies (e.g. buprenorphine at 0.05 mg/kg every 12 h for 36 h and carprofen at 5 mg/kg every 24 h for 72 h).
- Remove the wound clips 10 - 14 days post-surgery under anesthesia.
Results
Successful growth plate injury using this method involves the disruption of the center of the tibial growth plate without disrupting the articular cartilage surface. Bony repair tissue has been reported to begin at approximately 7 days post-injury and becomes fully developed by 28 days post-injury13, as visualized by micro computed tomography (micro CT) (Figure 2). Although these timepoints were chosen here to display the beginning and maturation of bone formation based on previously published data, other timepoints can be used to investigate the various stages of the repair process, from day 1 to 6 months post-surgery17. Table 1 gives an overview of bone volume formation within surgically injured rat growth plates 28 days post-surgery from three independent runs by providing (1) the bone volume fraction within the entire growth plate and (2) the bone volume fraction within the repair tissue area only15. The data are reported as the mean percent ± the standard deviation and indicate that similar results were obtained between the independent runs. Variance among the different runs was analyzed by a one-way analysis of variance (ANOVA) and shows no statistically significant difference between the runs, suggesting the reproducibility of the model. Alcian blue hematoxylin (ABH) with Orange G/Eosin counterstain18 was used to histologically show a variety of repair tissues at different stages of bony bar formation (Figure 2). Using this histological stain, different types of repair tissue, including mesenchymal, cartilaginous, bony trabeculae, and bone marrow, can be identified and quantified16.
Several problems may arise from incorrectly following the above procedures. An insufficient drill depth will not disrupt the growth plate, which will result in little or no bony bar formation. Disruption of the articular cartilage surface creates a larger injury that can introduce articular cartilage into the growth plate injury site, complicating the healing process (Figure 3A). Disrupting the growth plate at an inappropriate angle or direction results in a non-central injury (Figure 3B). In this case, bony bar formation will still occur, although it will be lateral or medial to the desired location. Overall, repair tissue formed after growth plate injury may be analyzed in a variety of ways, including microCT, quantitative PCR, histological staining, and immunohistochemistry. In addition to histological and molecular measurements, limb length and growth plate measurements provide an important measure of whole bone growth. Affected limbs have been reported to experience growth reduction compared to uninjured control limbs13. Limb length can be measured at different timepoints throughout the course of the study using microCT images to investigate limb length discrepancies14. Examples of timepoints previously used include 28 days and 56 days post-injury. Growth plate measurements, including overall height, zonal heights, and tether formation, can also provide important information on the tissue repair process13,14,15. Ideally, one should take limb lengths and growth plate measurements before surgery to have a baseline value. To further elucidate biological mechanisms or to test the efficacy of a treatment, appropriate control groups should be designed and include unaffected limbs and limbs that underwent surgery but are left untreated.
Biomaterials may also be tested in this growth plate injury model. As an example, a chitosan microgel19 was injected into the growth plate injury site, as described in step 7.1, and it is clearly seen at the injury site in Figure 4. Subsequent analysis may involve determining the effects of the biomaterial on repair tissue composition, limb length, and growth plate measurements, as discussed earlier.
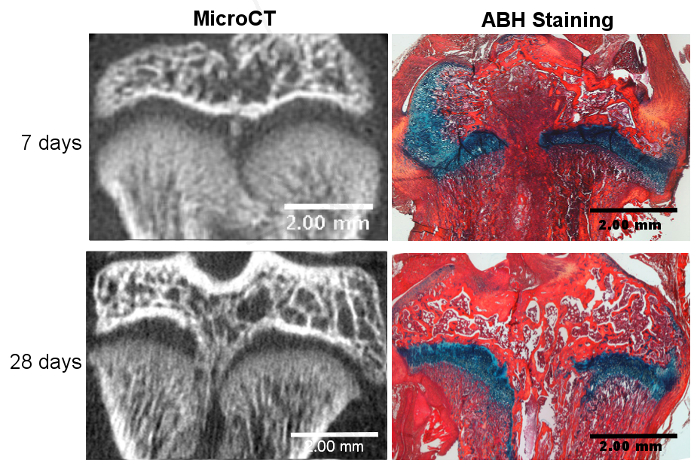
Figure 2. Successful Growth Plate Disruption and Bony Bar Formation.
Bony bar formation is seen at 7 days post-injury with microCT and confirmed through Alcian blue hematoxylin (ABH) staining. The bony bar is fully mature by day 28 post-injury, as seen with microCT and ABH staining. Please click here to view a larger version of this figure.
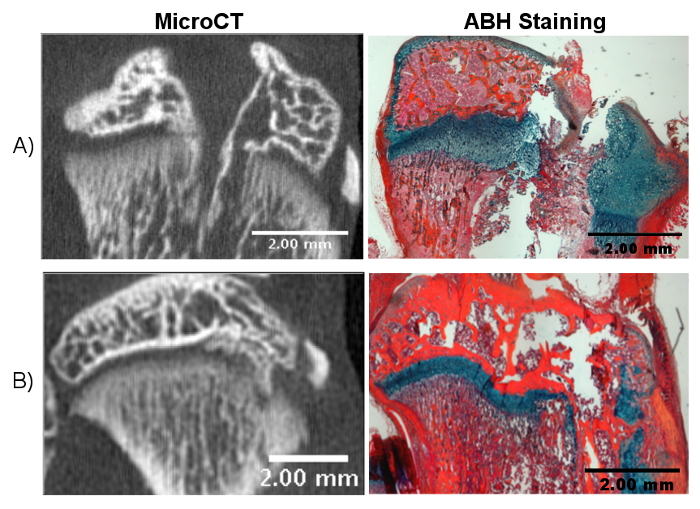
Figure 3. Potential Outcomes of Incorrect Drilling.
A) Drilling too far through the tibia can disrupt the articular surface, which complicates the healing process and may lead to inconclusive results. B) Incorrect angulation of the drill can lead to a non-central growth plate injury. Please click here to view a larger version of this figure.
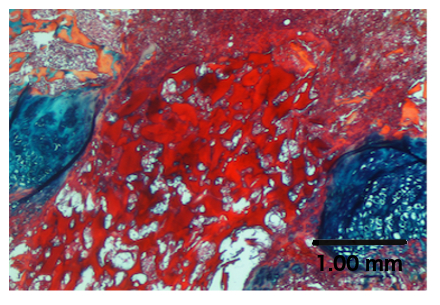
Figure 4. Treatment of a Growth Plate Injury with a Biomaterial.
ABH staining shows the chitosan microgel in the injured growth plate.
| Metric | Run 1 | Run 2 | Run 3 | P-value |
| Bone volume fraction within the whole growth plate | 9.76 +/- 3.81% | 10.52 +/- 4.06% | 11.93 +/- 2.04% | 0.5493 |
| Bone volume fraction within the repair tissue area | 41.5 +/- 8.33% | 46.08 +/- 10.12% | 46.77 +/- 8.14% | 0.5128 |
Table 1. Bone Volume Fraction Data.
Data was from micro CT images at 28 days post-injury on untreated rats from three independent runs.
Discussion
A growth plate injury animal model greatly adds to our understanding of the biological mechanisms of this injury, thus potentially leading to more effective therapeutic interventions for children suffering from growth plate injuries. To successfully create a bony bar and to study its formation in vivo using the model presented in this work, it is critical to disrupt the growth plate by drilling to a sufficient depth, without disrupting the articular cartilage. Variation in surgical implementation among animals and, to a lesser extent, variation in anatomical markers may lead to problematic results. We recommend practicing the procedures outlined above on cadaveric animals to ensure successful growth plate injury before performing the procedure for live animal studies. While cadaveric animals lack tissue pliability and will not bleed, the growth plate injury procedure and anatomical features on these animals will be similar to those of live animals. Furthermore, the cadaveric tibial growth plate can be dissected easily, as the epiphysis separates from the metaphysis through the application of light force, and the location of the drill hole can be observed. This quick analysis allows for technique modifications to learn the proper drill depth and angulation on cadaveric animals, without the need for imaging.
It should be noted that other animal models of growth plate injury exist. A similar transphyseal defect has been performed in the mouse and led to bony bar formation20. Despite its smaller size, it can also be used to study the mechanisms involved in bony bar formation. Coleman et al. reported on another valid rat model of growth plate injury, where a central transphyseal defect was created in the distal femur by drilling through the articular cartilage21. This approach also led to the formation of a bony bar and limb length inequalities, as in the model presented here. Other animal models of growth plate injury and treatment have included rabbits22, pigs23, and sheep24. While larger animal injury models may more closely represent clinical injuries, the rat model is useful for research on the biological mechanisms of physeal injuries. For example, the rat model presented here has been used extensively to investigate molecular mechanisms of physeal injury and the bony bar formation process10,11,12,13,14,15,16. Furthermore, the rat model can be used to test various physeal treatments before moving to larger animal models. However, a challenge of this rat model of growth plate injury is that the drilling is done inside the bone, making it impossible to observe where the drill hole is located within the growth plate. Thus, successful disruption of the growth plate on live animals can only be confirmed using imaging techniques at the time of surgery or by assessing bony bar formation between 7 to 28 days post-surgery. With practice, a high degree of success in obtaining bony bar formation can be achieved, but early studies can result in a number of animals that lack the formation of a bony bar, due either to an uninjured growth plate or to insufficient disruption of the growth plate.
Another limitation of this model is that drill hole injuries do not represent normal growth plate injuries in children, which usually occur due to fracture25. Fractures within the growth plate can be classified using the Salter-Harris classification system26. Type III and type IV growth plate fractures most commonly contribute to the physeal injuries that lead to bony bar formation. The growth plate injury type presented here most closely relates to a type VI growth plate injury, a rare class of injury in which the physis is removed by a trauma or puncture wound. However, since the pathophysiological mechanisms underlying bony bar formation after growth plate injury remain elusive, the rat model remains important to uncover this process in order to develop novel treatment options for children suffering from all types of growth plate injuries. The method described here reliably creates a bony bar and can be used to study multiple aspects of the growth plate injury repair process in vivo17,27,28,29,30,31,32. It has also been shown that this rat model results in reduced tibial growth after growth plate injury13, which makes it an even more interesting animal model to test novel treatment options that lead to growth plate regeneration and the potential restoration of bone elongation.
In conclusion, this paper details the methods to create a growth plate injury model with which to investigate bony bar formation and potential treatments for growth plate injuries in vivo. This rat model allows for relatively inexpensive and quick studies, given that a bony bar is fully mature 28 days after growth plate injury. In addition to developing our understanding of the molecular mechanisms of bony bar formation in vivo, this model may be used to test biomaterials that inhibit bony bar formation and encourage growth plate cartilage regeneration.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge funding support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) under award number R03AR068087, the Academic Enrichment Fund of the University of Colorado School of Medicine, and the Gates Center for Regenerative Medicine. This work was also supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082. The contents are the authors' sole responsibility and do not necessarily represent official NIH views.
Materials
| Name | Company | Catalog Number | Comments |
| Scalpel handle | McKesson | MCK42332500 | |
| Needle holder | Stoelting | RS-7824 | |
| Adson tissue forceps | Sklar | 50-3048 | |
| Iris Scissors | Sklar | 47-1246 | |
| Rotary Tool | Dremel | 7700 | Variable speed rotary tool |
| Keyless Rotary Tool Chuck | Dremel | 4486 | |
| Dental Burs | Dental Burs USA | FG6 | Round carbide bur, ≤2mm |
| Steinmann pins | Simpex Medical | T-078 | |
| Hair clippers | Wahl | 5537N | |
| 3-0 PGA surutes | Oasis | MV-J398-V | |
| Sterile gauze 2x2" | Covidien | 441211 | |
| Povidone Iodine | McKesson | 922-00801 | |
| Sterile saline | Vetone | 510224 | |
| 10 ml luer lock syringe | Becton Dickinson | 309604 | |
| 23 gauge needle | Becton Dickinson | 305145 | |
| Isopropyl alcohol pads | Dynarex | 1113 | |
| Isoflurane | IsoFlo | 30125-2 | |
| Caliper | Mitutoyo | 500-196-30 | |
| Carprofen | Rimadyl | 27180 | |
| Buprenorphine | Par Pharmaceuticals Inc | NDC 42023-179 | |
| Fenestrated Surgical Drape | McKesson | 25-517 | |
| Surgical Gloves | Uline | S-20204 | |
| #15 Scalpel Blade | Aven | 44044 | |
| 9mm wound clips | Fine Science Tools | 12032-09 | |
| Reflex clip applier | World Precision Instruments | 500345 | |
| Absorbant underpads | McKesson | MON 43723110 | |
| Tec 3 Iso Vaporizer | VetEquip | 911103 | |
| Germinator 500 | Braintree Scientific | GER 5287-120V | |
| Warm water recirculator | Kent Scientific | TP-700 | |
| Absorbent Underpads | Medline Industries | MSC281230 |
References
- Mann, D. C., Rajmaira, S. Distribution of physeal and nonphyseal fractures in 2,650 long-bone fractures in children aged 0-16 years. J Pediatr Orthop. 10 (6), 713-716 (1990).
- Browne, L. P., et al. Community-acquired staphylococcal musculoskeletal infection in infants and young children: necessity of contrast-enhanced MRI for the diagnosis of growth cartilage involvement. AJR Am J Roentgenol. 198 (1), 194-199 (2012).
- Weitao, Y., Qiqing, C., Songtao, G., Jiaqiang, W. Epiphysis preserving operations for the treatment of lower limb malignant bone tumors. Eur J Surg Oncol. 38 (12), 1165-1170 (2012).
- Butler, M. S., Robertson, W. W., Rate, W., D'Angio, G. J., Drummond, D. S. Skeletal sequelae of radiation therapy for malignant childhood tumors. Clin Orthop Relat Res. (251), 235-240 (1990).
- Shapiro, F. Longitudinal growth of the femur and tibia after diaphyseal lengthening. J Bone Joint Surg Am. 69 (5), 684-690 (1987).
- Kronenberg, H. M. Developmental regulation of the growth plate. Nature. 423 (6937), 332-336 (2003).
- Dodwell, E. R., Kelley, S. P. Physeal fractures: basic science, assessment and acute management. Orthopaedics and Trauma. 25 (5), 377-391 (2011).
- Khoshhal, K. I., Kiefer, G. N. Physeal bridge resection. J Am Acad Orthop Surg. 13 (1), 47-58 (2005).
- Hasler, C. C., Foster, B. K. Secondary tethers after physeal bar resection: a common source of failure. Clin Orthop Relat Res. (405), 242-249 (2002).
- Xian, C. J., Zhou, F. H., McCarty, R. C., Foster, B. K. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res. 22 (2), 417-426 (2004).
- Chen, J., et al. Formation of tethers linking the epiphysis and metaphysis is regulated by vitamin d receptor-mediated signaling. Calcif Tissue Int. 85 (2), 134-145 (2009).
- Coleman, R. M., Schwartz, Z., Boyan, B. D., Guldberg, R. E. The therapeutic effect of bone marrow-derived stem cell implantation after epiphyseal plate injury is abrogated by chondrogenic predifferentiation. Tissue Eng Part A. 19 (3-4), 475-483 (2013).
- Chung, R., Foster, B. K., Xian, C. J. The potential role of VEGF-induced vascularisation in the bony repair of injured growth plate cartilage. J Endocrinol. 221 (1), 63-75 (2014).
- Coleman, R. M., et al. Characterization of a small animal growth plate injury model using microcomputed tomography. Bone. 46 (6), 1555-1563 (2010).
- Macsai, C. E., Hopwood, B., Chung, R., Foster, B. K., Xian, C. J. Structural and molecular analyses of bone bridge formation within the growth plate injury site and cartilage degeneration at the adjacent uninjured area. Bone. 49 (4), 904-912 (2011).
- Su, Y. W., et al. Neurotrophin-3 Induces BMP-2 and VEGF Activities and Promotes the Bony Repair of Injured Growth Plate Cartilage and Bone in Rats. J Bone Miner Res. , (2016).
- Zhou, F. H., Foster, B. K., Sander, G., Xian, C. J. Expression of proinflammatory cytokines and growth factors at the injured growth plate cartilage in young rats. Bone. 35 (6), 1307-1315 (2004).
- Sayers, D., Volpin, G., Bentley, G. The demonstration of bone and cartilage remodelling using alcian blue and hematoxylin. Biotechnic & Histochemistry. 63 (1), 59-63 (1988).
- Riederer, M. S., Requist, B. D., Payne, K. A., Way, J. D., Krebs, M. D. Injectable and microporous scaffold of densely-packed, growth factor-encapsulating chitosan microgels. Carbohydrate Polymers. 152, 792-801 (2016).
- Lee, M. A., Nissen, T. P., Otsuka, N. Y. Utilization of a murine model to investigate the molecular process of transphyseal bone formation. J Pediatr Orthop. 20 (6), 802-806 (2000).
- Coleman, R. M., et al. Characterization of a small animal growth plate injury model using microcomputed tomography. Bone. 46 (6), 1555-1563 (2010).
- Lee, S. U., Lee, J. Y., Joo, S. Y., Lee, Y. S., Jeong, C. Transplantation of a Scaffold-Free Cartilage Tissue Analogue for the Treatment of Physeal Cartilage Injury of the Proximal Tibia in Rabbits. Yonsei Med J. 57 (2), 441-448 (2016).
- Planka, L., et al. Nanotechnology and mesenchymal stem cells with chondrocytes in prevention of partial growth plate arrest in pigs. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 156 (2), 128-134 (2012).
- Hansen, A. L., et al. Growth-plate chondrocyte cultures for reimplantation into growth-plate defects in sheep. Characterization of cultures. Clin Orthop Relat Res. (256), 286-298 (1990).
- Cepela, D. J., Tartaglione, J. P., Dooley, T. P., Patel, P. N. Classifications In Brief: Salter-Harris Classification of Pediatric Physeal Fractures. Clin Orthop Relat Res. , (2016).
- Salter, R. B., Harris, W. R. Injuries Involving the Epiphyseal Plate. The Journal of Bone & Joint Surgery. 83 (11), 1753 (2001).
- Chung, R., Foster, B. K., Zannettino, A. C., Xian, C. J. Potential roles of growth factor PDGF-BB in the bony repair of injured growth plate. Bone. 44 (5), 878-885 (2009).
- Fischerauer, E., Heidari, N., Neumayer, B., Deutsch, A., Weinberg, A. M. The spatial and temporal expression of VEGF and its receptors 1 and 2 in post-traumatic bone bridge formation of the growth plate. J Mol Histol. 42 (6), 513-522 (2011).
- Chung, R., Cool, J. C., Scherer, M. A., Foster, B. K., Xian, C. J. Roles of neutrophil-mediated inflammatory response in the bony repair of injured growth plate cartilage in young rats. J Leukoc Biol. 80 (6), 1272-1280 (2006).
- Chung, R., et al. Roles of Wnt/beta-catenin signalling pathway in the bony repair of injured growth plate cartilage in young rats. Bone. 52 (2), 651-658 (2013).
- Zhou, F. H., Foster, B. K., Zhou, X. F., Cowin, A. J., Xian, C. J. TNF-alpha mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J Bone Miner Res. 21 (7), 1075-1088 (2006).
- Arasapam, G., Scherer, M., Cool, J. C., Foster, B. K., Xian, C. J. Roles of COX-2 and iNOS in the bony repair of the injured growth plate cartilage. J Cell Biochem. 99 (2), 450-461 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved