Method Article
Forskolin-induced Swelling in Intestinal Organoids: An In Vitro Assay for Assessing Drug Response in Cystic Fibrosis Patients
In This Article
Summary
This protocol describes an assay for measuring CFTR function and CFTR modulator responses in cultured tissue from subjects with cystic fibrosis (CF). Biopsy-derived intestinal organoids swell in a cAMP-driven fashion, a response that is defective (or strongly reduced) in CF organoids and can be restored by exposure to CFTR modulators.
Abstract
Recently-developed cystic fibrosis transmembrane conductance regulator (CFTR)-modulating drugs correct surface expression and/or function of the mutant CFTR channel in subjects with cystic fibrosis (CF). Identification of subjects that may benefit from these drugs is challenging because of the extensive heterogeneity of CFTR mutations, as well as other unknown factors that contribute to individual drug efficacy. Here, we describe a simple and relatively rapid assay for measuring individual CFTR function and response to CFTR modulators in vitro. Three dimensional (3D) epithelial organoids are grown from rectal biopsies in standard organoid medium. Once established, the organoids can be bio-banked for future analysis. For the assay, 30-80 organoids are seeded in 96-well plates in basement membrane matrix and are then exposed to drugs. One day later, the organoids are stained with calcein green, and forskolin-induced swelling is monitored by confocal live cell microscopy at 37 °C. Forskolin-induced swelling is fully CFTR-dependent and is sufficiently sensitive and precise to allow for discrimination between the drug responses of individuals with different and even identical CFTR mutations. In vitro swell responses correlate with the clinical response to therapy. This assay provides a cost-effective approach for the identification of drug-responsive individuals, independent of their CFTR mutations. It may also be instrumental in the development of future CFTR modulators.
Introduction
CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that encodes an epithelial anion channel. CF affects approximately 85,000 people worldwide1. Over 2,000 CFTR mutations have been identified (www.genet.sickkids.on.ca). This diversity partially explains a wide spectrum of observed disease phenotypes (www.CFTR2.org)2,3. Six classes of CFTR mutations are defined based on their effect on CFTR protein expression and function: (I) no synthesis, (II) impaired trafficking, (III) defective channel gating, (IV) altered conductance, (V) reduced levels of normally functioning CFTR, and (VI) impaired cell surface stability4. Although the common CFTR mutations are well studied, CFTR function and relation with clinical status remain poorly understood at the level of the individual, particularly for the large group of rare "orphan" mutations (www.CFTR2.org)1,3.
Recently, drugs have been developed that target the CFTR protein in a mutation-specific fashion. Two classes of CFTR protein-targeting drugs are currently in clinical use and have distinct modes of action. Potentiators, such as VX-770, enhance the open-probability of apically-localized mutant CFTR and act directly upon their addition to cells5. Correctors, such as VX-809, restore the trafficking of endoplasmic reticulum-localized misfolded CFTR and require pre-incubation with cells before the effects are observed6. The CFTR potentiator, VX-770, has been registered for subjects with the G551D mutation7,8, as well as for eight other CFTR gating mutations, including S1251N9; together, these mutations are carried by 5% of all CF subjects. Other clinical trials have indicated that VX-770, combined with the corrector VX-809, has limited yet significant effects on lung function and causes a decrease in exacerbation rates in subjects homozygous for the F508del mutation carried by 45-50% of patients10,11.
Conventional clinical trials to identify drug-responsive subjects within the remaining 50% of CF patients are costly and time-consuming and are not feasible for individuals with extremely rare CFTR genotypes. Novel, cost-effective, personalized methods are crucial to match the increasing number of CFTR modulators to individuals carrying any type of CFTR mutation. Until now, the trial inclusion of patient groups carrying specific CFTR mutations has been guided by studies using mutant CFTR gene transfection in heterologous cell systems, followed by electrophysiological studies in Ussing chambers5,6,12. Due to a lack of adequate CF animal models, drug efficacy studies in air-liquid-interface-differentiated bronchial epithelial cells derived from CF lung explant materials have been used for drug development13,14,15. However, the limited availability of lung explant tissues and the invasive procedures to obtain bronchial cells from subjects without end-stage disease hamper the analysis of less common CFTR mutations and prevent drug testing in a personalized fashion. To overcome these limitations, "easy access" tissues, such as colorectal organoids, nasal airway cells, and airway cells derived from induced pluripotent stem cells, are currently being explored for personalized drug treatments.
Previously, we established protocols to culture epithelial stem cells from any gastrointestinal organ in the form of 3D organoids16,17. For the human colon/rectum, the culture conditions involve defined growth factors (Epithelial Growth Factor (EGF), Gastrin, Wnt-3A, R-spondin 3 (Rspo3), and Noggin) combined with small molecules (Nicotinamide, A83-01, and SB202190) in a basement membrane matrix. Under these conditions, single stem cells or small tissue fragments grow out into closed, cystic, 3D structures formed by highly-polarized epithelium with its basal side oriented towards the outside. All cell types typically appear in their normal ratios and positions. Organoids can be expanded over long time periods by weekly mechanical disruption and re-plating. They are genetically and phenotypically stable and can be stored, allowing long-term expansion and bio-banking17. They are amenable to all standard cell-biological/genetic manipulations and analytical techniques developed for 2D cell lines18.
We recently demonstrated that CFTR function can be readily measured in colorectal organoids in a forskolin-induced swelling (FIS) assay19,20. When exposed to forskolin (Fsk) or, alternatively, to cholera toxin, organoids rapidly increase their cyclic adenosine monophosphate (cAMP) levels, which in turn results in the opening of the CFTR channel19. Organoids from healthy individuals, or from subjects with CFTR mutations associated with residual function, will subsequently swell as a consequence of ion and water transport to the organoid lumen, the in vitro equivalent of secretory diarrhea. The FIS response of colorectal organoids was previously shown to be fully CFTR-dependent, as indicated by organoids derived from CFTR-null individuals and by the use of specific pharmacological CFTR inhibitors19. Large subject-specific data sets can be derived within several weeks after taking a biopsy.
For the FIS assay described in detail here, organoids are cultured from rectal biopsies that can be obtained at any age and with only limited discomfort21. Organoids are passaged weekly by mechanical disruption into single crypts that easily reseal and form new organoids. For running the FIS assay, ~30-80 of these disrupted little organoids are plated in each well of a 96-well plate19. At the day of the assay, the organoids are stained with calcein green, a fluorescent cell-permeable dye that it is retained within living cells, facilitating live imaging. Then, Fsk, which raises intracellular cAMP and thereby activates CFTR, is added in order to stimulate organoid swelling. Potentiators that act upon apical CFTR are added simultaneously with the forskolin, whereas correctors that restore CFTR trafficking are added 24 hr before the addition of Fsk. The organoid swelling is quantified by an automated image analysis that calculates the relative increase in the total area of all fluorescent objects for each time point upon forskolin addition.
3D organoid swelling provides advantages and disadvantages over existing electrophysiological CFTR readouts in 2D cultured airway cells in Ussing chambers. A major advantage is the throughput of the swelling assay. Cells are cultured and assayed using a single type of culture medium, and an experienced technician can culture up to 25 organoid samples on a weekly basis while quantifying approximately 1,200 data points per week in 12 patient samples. We conventionally type a single experimental condition by duplicate or triplicate measurements per plate and repeat such measurements at three independent incubation time points. In total, approximately 300-500 single organoid structures are then measured per experimental condition, which leads to very precise measurements of CFTR function with limited technical variability. This precision allows us to clearly define differences in residual function and response to CFTR modulators and allows us to readily pick up genetic background effects between patients carrying identical CFTR mutations19,22,23,24,25. Data quality can be easily assessed from microscope images. While FIS is fully CFTR-dependent, it is an indirect outcome measure for CFTR function, its read-out caused by the coupling of ion transport to fluid transport. This contrasts with direct CFTR function measurements in Ussing chambers, which measure transepithelial ion currents26. Ussing chambers allow the select stimulation of apical or basolateral compartments (which the organoid assay does not allow); by permeabilizing basolateral membranes, the apical CFTR-dependent anion secretion can be selectively measured27.
Protocol
All experimentation using human tissues described herein was approved by the ethical committee at University Medical Center Utrecht (UMCU; TcBio #14-008). Informed consent for tissue collection, generation, storage, and use of the organoids was obtained from the patients at Wilhelmina Children's Hospital (WKZ)-UMCU.
| Equipment | Consumable | Tools |
| Laminar flow hood | 15 and 50 ml conical tubes | Zeiss LSM 800 – Zen 2 (Blue edition) software for measuring images |
| CO2 incubator | Microfuge tubes | Microsoft Excel program |
| Cell culture Microscope (light/optical microscope) | 0.22 µm filters | |
| Centrifuge | Serological pipettes | |
| Rolling Shaker | Micropipette filter tips | |
| 4 °C room or 4 °C fridge for incubator | Cryovials | |
| CoolCell Cell Freezing Container | ||
| Serological Pipettor | ||
| Micropippette (1,000, 200 and 20 µl) | ||
| Viaflo Repeat pipette | ||
| (Live cell) Confocal Microscope | ||
| Computer | ||
| Liquid nitrogen tank | ||
| Multichannel (200 µl) |
1. Preparing Reagents for Culture
NOTE: All steps are performed inside a biosafety cabinet and following standard guidelines for working with cell cultures. In order to facilitate the formation of nice drops of basement membrane matrix, maintain a pre-warmed stock of 96-, 24-, and 6-well plates in the incubator at 37 °C.
- Basal Medium Preparation
NOTE: Basal medium (BM) refers to Advanced Dulbecco's Modified Eagle Medium with Ham's Nutrient Mixture F-12 (Ad-DF) supplemented with 4-(2-hydroxyethil)-1piperazineethanesulfonic acid (HEPES), glutamine, and penicillin/streptomycin (Pen/Strep).- In a 500 ml Ad-DF medium bottle, add 5 ml of 200 mM glutamine, 5 ml of 1 M HEPES, and 5 ml of pen/strep solutions (10,000 U/ml, 10,000 µg/ml).
- Store BM in the refrigerator at 4 °C for at least 4 weeks.
- Wnt-3A-conditioned Medium Preparation
NOTE: Wnt-3A-conditioned medium is made in-house using the cell line L-Wnt-3A according to the manufacturer's instructions.- For harvesting the Wnt-3A-conditioned medium, collect the medium exposed to the cells for one week and spin it down for 5 min at 450 x g to remove floating cells.
- Filter the Wnt-3A-conditioned medium through a 0.22 µm filter and divide it into 40 ml aliquots in 50 ml conical tubes. Store them at 4 °C for at least 4 months without a loss in activity.
- Test the activity of the Wnt-3A-conditioned medium in a TOP/FOP assay using human embryonic kidney (HEK)-293 cells transfected with TOP- and FOP-luciferase and TK-Renilla and measured with a Renilla-luciferase assay kit according to the manufacturer's instructions.
NOTE: TOP-luciferase is a reporter plasmid that contains wild-type TCF binding regions. If the Wnt-3A-conditioned medium is active, the canonical Wnt signaling is activated. Beta-catenin translocates into the nucleus to associate with TCF/LEF transcription factors and activates transcription of the luciferase reporter, inducing an increase in relative luciferase activity when the substrate is added. The FOP-luciferase is used as a negative control because the TCF binding regions upstream of the luciferase gene are mutated.
- Colon Medium Preparation
NOTE: Prepare and dilute all growth factors and reagents according to the manufacturers' recommendations. Use small-size aliquots an avoid freeze-thaw cycles. Functional growth factors are essential for the results.- Prepare colon medium (CM) by supplementing BM with 1x B27, 1.25 mM N-Acetylcysteine, 50 ng/ml hEGF, 5 nM gastrin, 10 mM nicotinamide, 300 ng/ml hRspo3, 100 ng/ml hNoggin, 500 µM A83-01, 10 µM SB202190, and 100 µg/ml of an antimicrobial for primary cells.
- Divide the CM into aliquots and freeze them at -20 °C for up to 4 months. Thaw the aliquots to prepare full colon medium (FCM) by adding 50% Wnt-3A-conditoned medium to the CM. Store FCM up to 7 days at 4 °C without a loss in activity.
- For the establishment of an organoid culture from rectal biopsies, supplement FCM with 50 µg/ml vancomycin, 50 µg/ml gentamycin, and 10 µM rho-associated coiled-coil-forming protein serine/threonine kinase inhibitor (RhoKi). Use this medium, called isolation medium (IM), only for the first two to three days in culture.
- EDTA Stock Solution Preparation
- Prepare 0.5 M ethylenediaminetetraacetic acid (EDTA), pH 8, in ultrapure H2O, sterilized with a 0.22 µm filter.
- Manipulation of the Basement Membrane Matrix
- Prepare the basement membrane matrix (BMM) according to the manufacturer's recommendation.
- Thaw BMM overnight on ice.
- When transferring the BMM from the bottle to a 15 ml conical tube, use a 5 ml pipette and a 15 ml conical tube pre-cooled at -20 °C.
- Once thawed, store the BMM in a refrigerator at 4 °C and incubate on ice for at least 30-60 min before use.
NOTE: In order to obtain the best results, the BMM must be cold and properly mixed before embedding crypts or organoids.
2. Establishing Colon Organoids from a CF Patient Biopsy
NOTE: After tissue collection, it is important to maintain the sample on ice in saline, Dulbecco's Phosphate-Buffered Saline without Ca2+ and Mg2+ (DPBS), or BM. Rapid processing of the biopsies is recommended, but it has proven possible to establish organoid cultures from biopsies stored on ice for up to 7 days.
- Let the biopsies settle at the bottom of a 15 ml conical tube and remove the supernatant.
- Add 10 ml of DPBS to the biopsies and pipette up and down 10-20 times using a 10 ml pipette.
NOTE: The pipette needs to be pre-wetted with BM before pipetting the biopsy. - Let the biopsies settle at the bottom and remove the supernatant.
- Repeat steps 2.2 and 2.3 4-5 times until the supernatant is clear.
- Add 10 ml of DBPS and 200 µl EDTA (0.5 M) to the biopsies and place the tube on a rocking tube platform for 60-120 min at 4 °C.
NOTE: Incubation time may differ per patient. If crypts are released, DPBS becomes cloudy. EDTA incubation can be finalized when crypts can be observed under a microscope. - Allow the crypts to settle. Discard the supernatant.
- Take up 2 ml of BM and add it to a new 15 ml conical tube. Shake this new tube manually in such a way that the inside is covered with BM.
- Using a pipette pre-wetted with BM, add 2 ml of DPBS to the tube containing the biopsies and pipette up and down 10-20 times.
- Allow the biopsies to settle and transfer the DPBS with the crypts to the new tube containing the 2 ml of BM that was prepared in step 2.7.
- Repeat steps 2.8 and 2.9 until no more crypts are released.
- Spin the crypts down at 130 x g for 5 min at 8 °C.
- In the meantime, transfer IM to the safety cabinet at room temperature (RT).
- Discard the supernatant and add 10 ml of BM to the crypt pellet and repeat step 2.11.
- Resuspend the pellet in 1 ml of BM. Take 5-10 µl and count the number of crypts by eye under a microscope.
- Spin the crypts down at 130 x g for 5 min at 8 °C.
- Remove the supernatant and resuspend the pellet in 55% BMM (v BMM to v IM).
NOTE: The crypts are resuspended in the corresponding volume of 55% BMM at 1 crypt per µl. If there are not enough crypts, the minimum volume of BMM is 40 µl. - For seeding, pipette 35 µl per well (in a 24-well plate). Divide the 35 µl of BMM into 3-5 drops separately in order to improve the diffusion of growth factors into the BMM. Do not create bubbles.
- Place and leave the plate upside down in the incubator at 37 °C for at least 20 min for the BMM to solidify.
- Add 500 µl of IM per well and keep it in the incubator at 37 °C and 5% CO2.
- Refresh the medium every 2-3 days. Remove the old medium by pipetting out with a P1000 pipette, leaving the BMM drops intact. Carefully add FCM by pipetting it in the side of the well, not directly on the BMM drops.
NOTE: If no crypts are released in the isolation protocol, centrifuge the supernatant, wash the pellet 2-3 times with BM, and resuspend it in BMM. Take the leftover tissue and cut it into little pieces with a razor. Collect these into a 15 ml conical tube, centrifuge them, and resuspend them in the same BMM. If any epithelial stem cells are present, these will also generate organoids.
3. Passaging of Colon Organoids for Maintenance, Freezing, and Forskolin-induced Swelling Assay (FIS)
NOTE: Every organoid culture has its own doubling time. Normally, colorectal CF organoids can be expanded 1:3-1:5 times every 7-10 days. It is a good sign if budding structures are observed. Colorectal CF organoids are less cystic (Figure 2A-2C) than colorectal normal organoids (Figure 2D). For establishment and maintenance, organoids are cultured in 24-well plates; for freezing, in 6-well plates; and for the FIS assay, in 96-well pates.
- Passaging of Organoids
- Keep the BMM on ice for at least 30-60 min before using it.
- Keep the FCM at RT for at least 1 hr before using it.
- Label one 15 ml conical tube with the sample name and another tube as "washed."
- Carefully aspirate the medium from the wells using a P1000 pipette without disturbing the BMM drops.
- Add 1 ml of BM to 1 well and break up the BMM drops by using a P1000 pipette. Transfer this 1 ml into the 15 ml conical tube with the sample name.
- Wash the well with another 1 ml of BM and transfer it to the same 15 ml tube.
- Repeat steps 3.1.5 and 3.1.6 with 5 more wells (up to 6 wells of a 24-well plate will be washed in one 15 ml conical tube).
- Fill the tube up to 12 ml with BM. Pipette up and down using a pre-wetted 5 ml pipette.
- Spin at 85 x g for 5 min at 8 °C.
- Discard the supernatant and add 1 ml of BM to the pellet. With the same P1000 pipette tip, take up a P10 tip without a filter, and pipette up and down 20 times. Discard both P1000 and P10 tips.
- Add 4 ml of BM with a 5 ml pipette and hold the tube tilted at about 70° from the vertical to one side. Pre-wet a new P1000 tip and mix vigorously 2-3 times with the P1000 (1 ml volume).
- Count to 10 while remaining in the tilted position, collect the uppermost layer carefully with the P1000 pipette, and transfer 4 x 1 ml into the tube labelled as "washed."
- Observe the organoids settling to the bottom in the tilted tube; undisrupted organoids and bigger chunks will sink to the bottom. Centrifuge the 15 ml "washed" tube for 5 min at 85 x g and 8 °C.
- Discard the supernatant and add the required amount of medium and BMM to the organoid pellet to a final concentration of 55% BMM. Mix by pipetting up and down without creating bubbles (Figure 3B).
NOTE: A good density is achieved by seeding 25-30 organoids in 10 µl of BMM. - Follow steps 2.17-2.19.
- Passaging for Freezing
- Keep the BM in ice for at least 30-60 min before use. Keep the FCM at RT for at least 1 hr before use.
- Prepare FCM supplemented with RhoKi (Step 1.3.3).
- Take trypsin from the refrigerator and leave it at RT for at least 30 min before use.
- Follow steps 3.1.5-3.1.10.
- Remove as much supernatant as possible, add 4 ml of trypsin, and vortex for 30 sec.
- Put the tube in a warm water bath at 37 °C for 1 min and vortex vigorously for 30 sec.
- Inspect the solution in the tube. If intact organoids are still visible under the microscope, repeat step 3.2.7.
- When the organoids are disrupted, add 8 ml of BM to neutralize the trypsin and pipette 10 times.
- Spin for 3 min at 450 x g at 8 °C.
- Discard the supernatant and add the required amount of medium and BMM to the organoids to reach a 55% BMM solution. Mix by pipetting up and down without creating bubbles.
NOTE: For freezing, organoids must be seeded in a 1:1 ratio after trypsinization. - Seed 250 µl in a single well of a pre-warmed 6-well tissue culture plate, producing tiny, separated droplets of ~10 µl. Place the plate upside down in the incubator at 37 °C and leave the BMM to solidify for 20-30 min.
- Add 2.5 ml of fresh FCM + RhoKi in each 6-well plate well and transfer the plate to the incubator (Figure 3C).
- Passaging Organoids for the FIS Assay
NOTE: Depending on the number and size of the organoids, between 4 and 6 wells of a 24-well plate are enough for seeding 27 wells of a 96-well plate.- Process the organoids as described from steps 3.1-3.1.13.
- Resuspend the pellet in 120 µl of 50% BMM.
- Confirm the number of organoids (30-50) in a 3 µl BMM drop under the microscope.
- Plate the organoids using a single drop of 3 µl placed in the middle of each well of a 96-well pate.
- Place the plate in the 37 °C incubator for 15 min for the BMM to solidify; further steps are described in step 6.1.1.
4. Freezing Colon CF Organoids
NOTE: Organoids are ready to be frozen down 1-2 days after trypsinization and culturing, so organoids will still be small, which increases the efficiency of survival after thawing.
- Add 1 ml of BM and break up the BMM drops using a P1000 pipette. Transfer to a 15 ml conical tube.
- Wash the well with another 1 ml of BM and transfer to the same 15 ml tube.
- Repeat steps 4.1 and 4.2 with all wells.
NOTE: To avoid excess BMM, 3 wells of a 6-well plate are pooled in one 15 ml tube. - Fill up the 15 ml tube containing the organoids with 12 ml of cold BM and pipette up and down with a 5 ml pipette. Leave the tube on ice for 5 min.
- Spin for 3 min at 450 x g and 8 °C and remove the supernatant.
- Dissolve the pellet of organoids with freezing medium and pipette up and down to properly resuspend the organoids.
NOTE: 500 µl of freezing medium is used for each 100 µl of BMM. - Using a 5 ml pipet, transfer 0.5 ml of organoids resuspended in freezing medium to sterile cryogenic vials.
- Transfer the vials to a cell freezing container and put it at -80 °C.
- After 24 hr, transfer the vials for storage in liquid nitrogen.
5. Establishing Cultures from Frozen Organoids
NOTE: Thaw the BMM on ice and keep it on ice. Let the BM reach RT, and warm a 10 ml aliquot to 37 °C before starting the procedure of thawing one cryovial.
- Thaw the vial rapidly by agitation in a 37 °C water bath until there is still a little frozen material.
- Transfer the thawed organoids to a 15 ml conical tube using a P1000 pipette.
- Immediately after, add 1 ml of warm BM drop by drop while shaking the bottom of the tube. Once the 1 ml of medium is added, mix carefully by pipetting up and down a few times to dilute the freezing medium.
- Slowly (drop by drop) add 9 ml of warm BM to the conical tube containing the organoids. Invert a few times.
- Spin the cell suspension for 3 min at 85 x g and 8 °C.
- Discard the supernatant carefully without disturbing the pellet. Resuspend the organoid in 90 µl of FCM supplemented with RhoKi (step 1.3.3). Then, add 110 µl of BMM.
- Add 35 µl to each well of a pre-warmed 24-well plate, making tiny, separated droplets.
- Transfer the plate to the 37 °C incubator, leaving the plate upside down for 20-30 min.
- Add 500 µl of FCM with RhoKi to each well and transfer the plate back to the incubator (Figure 4A-4C).
- Once organoids have recovered properly from thawing, dilute in more BMM so each 10 µl of BMM contains 25-30 organoids. This procedure can be done 2-3 days after thawing (Figure 4D-4G) and ensures the proper growth of the organoid.
6. Forskolin-induced Swelling Assay (FIS)
NOTE: Fsk titration allows for the measurement of CFTR residual function. For CFTR modulation, organoids are exposed to a given corrector (e.g., VX-809) and/or potentiator (e.g., VX-770), depending on genotype. Usually, VX-809 is added 18-24 h before the measurement, while VX-770 and Fsk are added right before starting the measurements. Be aware that some modulators (correctors/potentiators) can bind to the plastic surface of the assay plate.
- Plating for the Assay
NOTE: VX-809 stocks are aliquoted at 20 mM and stored at -80 °C. When thawing, leave at RT and protect from light.- Process the organoids as described in section 3.3.
- If testing the VX-809 corrector, prepare a dose-response curve in triplicates with the following concentrations: 0.0003, 0.003, 0.03, 0.3, 3.0, and 30 µM. Prepare the dilutions in FCM.
- To the 96-well plate, add either 100 µl of FCM with the respective VX-809 concentration or 100 µl of FCM only and incubate the plate.
- Measuring the Assay
NOTE: VX-770 stocks are aliquoted at 20 mM, while Fsk is at 10 mM; both are stored at -80 °C. When thawing, leave at room temperature and protect from light.- Take a vial of calcein and DMSO and leave at RT for 15 min.
- Add 5.1 µl of DMSO to the vial where the calcein is provided as a powder, if unopened. Otherwise, use an already resuspended calcein vial containing 2.5 µl of calcein.
- Add 2.5 µl of calcein to 580 µl of BM in a 1.5 ml tube and label it.
- Add 10 µl of this dye solution (BM + calcein) to each well using a repeat pipette.
- Resuspend once with a multichannel to ensure that the dye is mixed well.
- Incubate the plate in a 37 °C incubator for 30 min.
- During the calcein incubation, start preparing the Fsk and VX-770 solutions, if needed.
- If using a VX-770 potentiator, prepare a dose-response curve in triplicate with the following concentrations: 0.0003, 0.003, 0.03, 0.3, 3.0, and 30 µM. In case of an Fsk titration, the concentrations are: 0, 0.008, 0.02, 0.05, 0.12, 0.32, 0.8, 2.0, and 5.0 µM. Prepare the dilutions in BM.
NOTE: For Fsk, VX-770, or any other drug added on the second day, the dilutions must be prepared at 2x final concentration, since 100 µl of Fsk/drug titration solution will be added to each well, which already contain 100 µl of FCM. - Transfer the Fsk and VX-770 solutions, a p200 pipette, and tips to the microscope room.
- For imaging the FIS assay, use a live cell imaging confocal microscope equipped with an automated stage, a heated chamber, and CO2 flow.
NOTE: The following steps refer to a specific microscope. Different setups should apply to other microscopes following manufacturer's instructions. - Put the 96-well plate in the plate holder.
- Enter the confocal settings for imaging.
- Preset the live imaging tool to 37 °C and 5% CO2 and let the chamber pre-incubate for a minimum of 30 min prior to measurement.
- Use the "Smart setup" option to select the Alexa fluor-488 track.
- Set the 5X lens and scan area to 0.6X to zoom out and capture the entire well.
- Adapt the laser power and detector sensitivity to enable the optimal detection of calcein-green labeled organoids over the background. The pinhole can be increased to 130 µm and the image averaging can be set at 2.
- Set the bit depth at 8 and the frame size to 512 x 512.
- Use the time series option to set the measurement time, frequency, and intervals.
NOTE: Suggested for regular measurements: 1 hr with 10 min interval: cycles = 7 (Cycle 1 is T = 0). For measurements of reduced swelling, organoids can be imaged for up to 3 hr. - Use the tile option to manually determine the individual well positions.
- Add 100 µl of the Fsk and/or VX-770 solutions to the corresponding wells, following the same order as the measurement. Start adding the solutions in the first imaged well and continue with the imaging order.
- Start the measurement.
- After the experiment is finished, save the file.
- Analysis of FIS Assay Data
- Create an "organoid analysis" macro for the data analysis of the FIS assay using the analysis tool in an image analysis software that recognizes all structures imaged through the Alexa-488 track and fills the identified structures to calculate the increase of total organoid area over the different time points.
- Open the data file with the acquired images in the image analysis program.
- Select the macro for organoid analysis.
- Set the threshold to balance the signal-to-noise ratio in one well at time point 1 and ensure that all organoid structures are recognized and filled.
- Check in 4-6 wells to see if all structures are also recognized at time point 7. Slightly modify the threshold to ensure that the background signals at time point 7 are not recognized as structures (the specific threshold may change somewhat between experiments).
- Set the criteria of minimal area size of recognized objects to 1,000 µm2.
- Press "analyze" to start. The analysis takes approximately 3 min, depending on the software used.
- When the software is finished, go to "create table" and select the following data: well numbers (table should increase in this order), time points, and total area per well.
- Save file as an .xlm to export to a spreadsheet program.
- In this program, calculate the relative increase in area per well by setting the area of time point 1 at 100%. Calculate the area under the curve (AUC) from time points 1-7 per condition; the lower limit of the area (Y-value) is set at 100%. Fsk or drug titrations (X-axis) are plotted against the AUC (Y-axis).
Results
Figure 1A shows a representative fresh isolation of crypts embedded on BMM. The crypts are from a colorectal biopsy of a CF subject. Usually, an organoid is generated from each crypt (Figure 1A-C). Due to the dysfunction of the CFTR, most of the colon CF organoids are not cystic, but rather are compact and with projections and buddings (Figure 2A-2B). However, some CF organoid cultures, especially with high residual function, have a few organoids with a cystic shape (Figure 2C), like wild-type organoid cultures (Figure 2D).
Before passaging the organoids for expanding the culture or seeding a FIS assay, is important that the organoids have a big size with multiple buddings, as shown in Figure 3A. If the organoids are small and without numerous buddings when processed for passaging, the organoid culture is negatively affected. Depending on the quality and activity of essential growth factors like EGF, Wnt-3A, and Rspo3, the organoids reach the desired size between 7 and 12 days (Figure 3A). After mechanical disruption, the buddings are disrupted and used to generate new organoids in the following passage (Figure 3B). If processing the organoids for freezing, it is important that they are trypsinized to a small size, as represented in Figure 3C. After trypsinization, the organoids are plated for 1 or 2 days in order to let them recover from the stress of the manipulation. This step, together with the small size of the organoids, ensures a 98% cell recovery after thawing (Figure 4A).
When the organoids are thawed, it is important to have them in high density, so normally, they are thawed in a small volume (Figure4A). After one or two days in culture, and after observing that the organoids are increasing size (compare Figure 4A with Figure 4B-4C), the organoids are diluted in a ratio of 20-30 organoids per 10 µl of BMM, providing the right density to grow into a bigger size. If the organoids are too diluted, they can slow growth and can even differentiate and die. If the organoids are too crowded, the lack of space or the shortage of growth factors also slows the growth of the organoids and reduces the number of buddings.
The plating of organoids from viable cultures with limited cellular debris should result in conditions in which >95% of the organoids swell upon Fsk stimulation, provided that sufficient CFTR function is present (Figure 5A-5B). Organoids swell in a genotype- and drug-dependent fashion, as illustrated by representative images of organoids with two F508del alleles (Figure 5A) and organoids compound-heterozygous for F508del and S1251N (Figure 5B), a CFTR gating mutation associated with some residual function.
To quantitate swelling, total calcein-green surface areas are selected for each time point and expressed as percentage of T = 0 (set at 100%; Figure 6A). Debris and small, non-swelling structures are typically below 5% of the total surface area. The relative area increase is expressed per 10 min time interval, and AUC (arbitrary units) measurements are generated for each condition (T = 0 to 60 min, baseline threshold set at 100%; Figure 6B). We found AUC to be the most robust, as it incorporates some variation in initiation of swelling, as well as a curvilinear relationship beyond 30-40 min of Fsk at high CFTR function levels.
Residual function measurements in F508del/F508del organoids typically reach 0-200 AUC units (100-110% of surface area increase after 60 min of 5 µM Fsk treatment), which can increase to ~2,500-3,500 AUC units upon incubation with CFTR modulators (VX770+VX809; Figure 6C). Organoids associated with CFTR residual function can reach up to 4,500 AUC units after 60 min of 5 µM Fsk stimulation in the absence of CFTR modulators (200-220% surface area of pre-Fsk conditions). Organoid swelling reaches a ceiling at ~4,500 AUC units, presumably due to the physical limitations of the organoid structures, the rate-limiting impact of basolateral ion transport, and the establishment of ion- and fluid-transport homeostasis under "stretched" conditions. Fsk can be titrated to further expand the dynamic range of the assay at these high CFTR residual function levels to better quantitate residual function or highly-effective compound treatment (e.g., as indicated by the higher activity of VX770 on the swelling of S1251N organoids upon stimulation with lower Fsk concentrations; Figure 6D).

Figure 1: Establishment of CF colorectal organoids from biopsies. (A) Representative images of isolated material from a rectal biopsy after being embedded in BMM on the isolation day (passage 0, day 0). Enlargement of the indicated crypt is shown on the lower right panel. (B) The same crypts were followed after 7 days in culture (passage 0, day 7). Enlargement of the same crypt presented in panel A is shown. (C) Representative image of the same organoid culture 11 days after being split (passage 1, day 11). Scale bars = 100 µm.
Please click here to view a larger version of this figure.

Figure 2: Representative images of CF colorectal organoids derived from subjects with different CFTR mutations. Representative images of CF colorectal organoids from subjects with F508del/F508del (A), F508del/R117H-7T (B), and F508del/S1251N (C) mutations. (D) Representative image of a colorectal organoid culture from a wildtype CFTR subject. Scale bars = 100 µm. These images were obtained with no Fsk stimulation. Please click here to view a larger version of this figure.

Figure 3: Processing CF colon organoids for maintenance and assay or for freezing. (A) Representative images of a CF colon organoid culture ready to be processed for either passaging (B) or freezing (C). (B) After their mechanical disruption, little pieces are formed, which will generate the new organoids in the next passage. (C) After trypsinization, very small, round organoids are generated, facilitating their viability during the freezing process. Scale bars = 100 µm. Please click here to view a larger version of this figure.
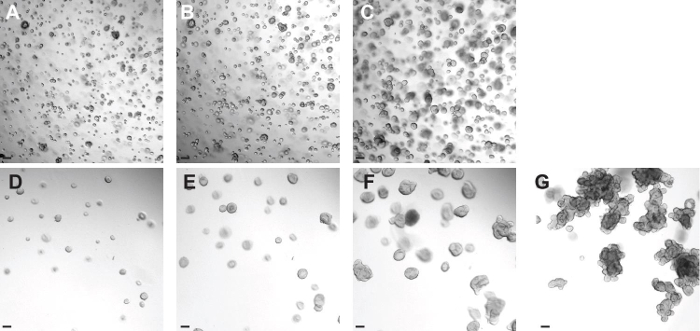
Figure 4: Establishing a CF colorectal organoid culture from frozen organoids. (A-C) Representative images of a CF colorectal organoid culture thawed on day 0 (A). Images from the same well were taken one (B) and two (C) days after. (D-G) Representative images of the dilution step described in section 5.9. Once the organoids have recovered properly from thawing (day 3 after thawing), they are diluted so they have space to grow (D). Images from the same well were taken six (E), ten (F), and fifteen (C) days after thawing. Scale bars = 100 µm. Please click here to view a larger version of this figure.

Figure 5: Forskolin-induced swelling of CF organoids. (A and B) Representative images of F508del/F508del (A) or F508del/S1251N (B) colorectal organoids before or after 60 min of stimulation with Fsk (5 µm) in the absence or presence of VX-770 (3 µm]) or VX-770 in combination with VX-809 (3 µm). Scale bars = 200 µm. Please click here to view a larger version of this figure.
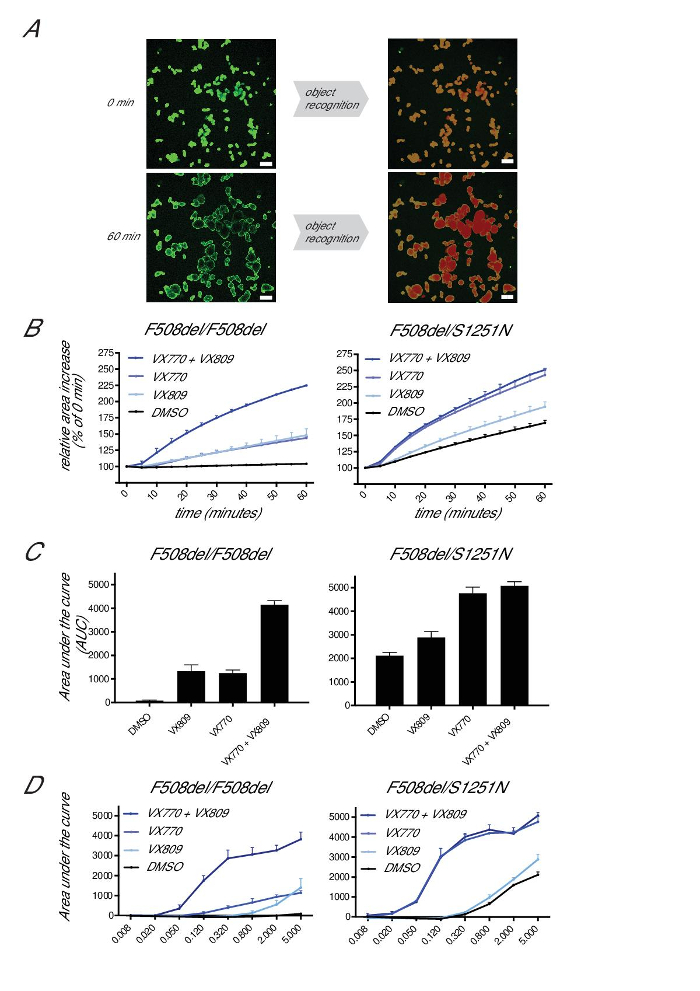
Figure 6: Quantifying Forskolin-induced swelling. (A) Representative images of organoid area selection by an image analysis software of F508del/S1251N organoids before (0 min) and after 60 min of stimulation with Fsk (5 µM) and VX770 (3 µM). Scale bars = 200 µm. (B) Representative images of the relative area increase of F508del/F508del or F508del/S1251N organoids during 60 min of Fsk (5 µm) stimulation in the absence or presence of VX770 (3 µm) and/or VX809 (3 µm). (C) Area under the curve measurements of conditions indicated in (B) (T = 60 min, baseline threshold = 100%). (D) Area under the curve measurements of F508del/F508del or F508del/S1251N organoids at different concentrations of Fsk. Data represent the averages ± SD from single representative experiments. Please click here to view a larger version of this figure.
Discussion
Here, we provide a complete protocol for the generation, expansion, freezing, and thawing of human colorectal organoids. While we have established human organoid cultures some time ago17, it has sometimes proven difficult to establish the technology in other labs without hands-on training. We anticipate that these protocols will replace such training.
Wnt-3A-conditioned medium is one of the most crucial reagents in order to succeed in establishing and maintaining a long-term organoid culture. Indeed, organoid cultures might "crash" when exposed to Wnt-3A-conditoned medium with low activity. Since Wnt-3A-conditioned medium is made in-house, there are several aspects that must be taken into account when generating an active medium. Active Wnt-3A can only be produced in medium with high-quality fetal bovine serum (FBS) that provides the proper growth signals for L cells and the stabilization of hydrophobic Wnt-3A molecules. When Wnt-3A activity is low (i.e., low TOP/FOP ratios, see below), it is usually the FBS that is causing the problem.
Commercial recombinant Wnt works poorly, so we do not recommend using it as positive control for the TOP/FOP Wnt reporter assay. The ratio between TOP/Renilla and FOP/Renilla is an indication of Wnt activity of the conditioned medium, so a good batch of Wnt-3A-conditioned medium gives a TOP/FOP ratio higher than 25, compared to the TOP/FOP value from the negative control: medium not exposed to Wnt-3A-producing cells. Recombinant Noggin and R-spondin can be replaced by Noggin and R-spondin conditioned-medium28,29.
The current protocol also describes a functional test for CFTR-the FIS assay. We have recently demonstrated the relationship between the CFTR genotype and FIS and how this reflects published CFTR genotype-phenotype relationships obtained from clinical registries(www.CFTR2.org)25. Using this assay, two individuals with extremely rare CFTR mutations were identified whose organoids were responsive to ivacaftor (VX-770)25. Subsequent prescription of this drug resulted in clear-cut clinical response, highlighting the value of the FIS assay to match specific drugs with patients with rare mutations in the absence of clinical trial data. Essential for the quantification of FIS are optimal growth and assay conditions, indicated only by a limited fraction of non-swelling organoids (usually below 5%). High levels of debris lead to the underestimation of swelling, as a larger proportion of non-viable, non-swelling structures are recognized by the image analysis software.
Correlations between in vitro FIS responses and previously-established CFTR-dependent biomarkers (sweat chloride concentration and intestinal current measurements) at the personalized level were readily demonstrable in our previous studies19,25. This supported the notion that residual function measurements using FIS on subjects with CF may complement current approaches as a diagnostic or prognostic marker in a personalized fashion. The throughput of the FIS assay compared to other biomarkers of CFTR function, such as sweat chloride concentration measurements in vivo and intestinal current measurements in ex vivo rectal biopsies, allows for better control of technical variability. It also provides a fully CFTR-dependent readout and a large dynamic range by using a titration of Fsk that accurately types residual CFTR function.
However, the FIS assay reflects only the impact of the individual genotype on the variability of drug efficacy, while modulation of CF disease is affected by more than only CFTR function. For drug response, the assay is reflective of a potential tissue response. Although very precise in CFTR measurement, human variation in pharmacokinetics is not incorporated, as opposed to other in vivo7,8 or direct ex vivo CFTR measurements32. As discussed previously, non CF modifiers (both genetic and environmental) are also influencing phenotype, causing a disconnect between in vitro observations or CFTR biomarkers and clinical phenotype2. Further, the identification of potential responsive mutations using cell lines33 is associated with less impact on patients who need to get a rectal biopsy for the establishment of organoids.
Multiple CFTR-targeting drugs are currently in development1. It is anticipated that clinical trials will not allow a correct prediction of clinical efficacy based on CFTR mutational status alone. As the FIS assay measures CFTR function and drug response functionally, it may become the method of choice to determine which drug works best for which patient. The FIS assay may also be useful for the identification of patient groups to be included in registration trials and for the development of novel drug modalities. Stimulation of colorectal organoids with plasma before and during treatment may further help to quantitate functional amounts of circulating CFTR modulators in blood, potentially facilitating personalized dosing and the selection of drug dosages in clinical trials30,31. Finally, FIS may be useful for a better basic understanding of CFTR function and its interactions with other -so far largely unknown- genetic modifiers of its function.
Disclosures
Hans Clevers is an inventor on patents for organoid culture and the Forskolin-induced swelling assay. Jeffrey M Beekman is an inventor on a patent for the Forskolin-induced swelling assay. Sylvia F Boj and Robert Vries are employed by the Foundation Hubrecht Organoid Technology (HUB), which holds the exclusive license to the Organoid Technology. Otherwise, the authors have nothing to disclose.
Acknowledgements
This work was supported by the HIT-CF program of the Dutch CF foundation (NCFS), ZonMW (40-00812-98-14103), the Wilhelmina Children's Hospital Research Fund and CZ, and Zilverenkruis/Achmea. We would like to thank S. Heida-Michel, M. Geerdink, K.M. de Winter-de Groot, and G. Berkers (Department of Pediatric Pulmonology, Wilhelmina Children's Hospital, UMC Utrecht), and R.H.J. Houwen (Department of Pediatric Gastroenterology, Wilhelmina Children's Hospital, UMC Utrecht) for approaching the patients and getting the biopsies for the generation of a CF Biobank.
Materials
| Name | Company | Catalog Number | Comments |
| Advanced Dulbecco’s Modified Eagles Medium with Nutrient Mixture F-12 Hams (Ad-DF) 500 ml | Thermo Fisher Scientific: Invitrogen | #12634 | stored at 4 °C |
| GlutaMax | Thermo Fisher Scientific: Invitrogen | #35050 | stored at 4 °C |
| Hepes | Thermo Fisher Scientific: Invitrogen | # 15630-056 | stored at 4 °C |
| Penicillin/Streptomycin | Thermo Fisher Scientific: Invitrogen | #15140-122 | stored at -20 °C |
| 96 well culture plate | Cellstar | #655180 | |
| 24 well culture plate | Cellstar | #662160 | |
| 6 well culture plate | Cellstar | #657160 | |
| Dulbecco's Phosphate Buffered Saline (-) CaCl2 (-) MgCl2) (DPBS) | Life Technologies: Gibco | #14190-094 | stored at 4 °C |
| Dulbecco’s Modified Eagles Medium (DMEM) 500 ml | Thermo Fisher Scientific: Invitrogen | #31966-021 | For Wnt-3A Conditioned Medium Production. Stored at 4 °C |
| Fetal Bovine Serum (FBS) | Bovogen | #SFBS LOT#11113 | For Wnt-3A Conditioned Medium Production. Stored at -20 °C |
| L Wnt3A cell line | ATCC | #CRL-2647 | For Wnt-3A Conditioend Medium Production. |
| TOP/FOP plasmids | Millipore | #17-285 | For measuring Wnt activity |
| pTK-Renilla | Promega | #E2241 | For measuring Wnt activity |
| HEK-293 | ATCC | #CRL-1573 | For measuring Wnt activity |
| Dual-Luciferase Reporter Assay System | Promega | #E1910 | For measuring Wnt activity |
| Zeocin | Thermo Fisher Scientific: Invitrogen | #R250-01 | For Wnt-3A Cell line selection |
| B27 supplement | Thermo Fisher Scientific: Invitrogen | #17504-044 | stored at -20 °C |
| N-Acetylcysteine | Sigma Aldrich | #A9165-5G | stored at -20 °C |
| Nicotinamide | Sigma Aldrich | #N0636 | stored at -20 °C |
| Human Epithelial Growth Factor (hEGF) | PrepoTech | #AF-100-15 | stored at -20 °C |
| Gastrin | Sigma Aldrich | #G9145 | stored at -20 °C |
| TGFb type I Receptor inhibitor (A83-01) | Tocris | #2939 | stored at -20 °C |
| Y-27632 dihydrochloride (RhoKi) | Selleckchem | #S1049 | stored at -20 °C |
| p38 MAPK inhibitor (p38i) (SB202190) | Sigma Aldrich | #S7067 | stored at -20 °C |
| Primocin | InvivoGen | #ant-pm-1 | stored at -20 °C |
| Human Noggin (hNoggin) | PrepoTech | #120-10C | stored at -20 °C |
| Human R-spondin 3 (hRspo-3) | R&D Systems | #3500-RS/CF | stored at -20 °C |
| Vancomycin | Sigma Aldrich | #861987- 250mg | stored at -20 °C |
| Gentamycin | Life Technologies: Gibco | #15710-049 | stored at -20 °C |
| Ethylenediamine tetraacetic acid (EDTA) | Sigma Aldrich | #431788 | Stored at 4 °C |
| Matrigel | Corning | #354230 | stored at -80 °C |
| TryplE Express | Life Technologies: Gibco | #12605-010 | for trypsinizing organoids for freezing |
| Recovery Cell Culture Freezing Medium | Life Technologies: Gibco | #12648010 | for freezing |
| Calcein | Life Technologies: Gibco | #C3100MP | stored at -20 °C |
| Forskolin | R&D Systems | #1099-50 mg | stored at -80 °C |
| Lumacaftor (VX-809) | Selleckchem | #s1565 | stored at -80 °C |
| Ivacaftor (VX-770) | Selleckchem | #s1144 | stored at -80 °C |
| Name of Reagents/Material | Solvent | Stock Concentration | Final Concentration |
| GlutaMax | 200 mM | 2 mM | |
| Hepes | 1 M | 10 mM | |
| Penicillin/Streptomycin | 10K U/ml 10K µg/ml | 100 U/ml 100 µg/ml | |
| Zeocin | 100 mg/ml | 125 µg/ml | |
| B27 supplement | 100x | 1x | |
| N-Acetylcysteine | MiliQ H2O | 500 mM | |
| Nicotinamide | DPBS | 1 M | 10 mM |
| Human Epithelial Growth Factor (hEGF) | DPBS 0.1% BSA | 0.5 mg/ml | 50 ng/ml |
| Gastrin | DPBS | 100 µM | 10 nM |
| TGFb type I Receptor inhibitor (A83-01) | DMSO | 5 mM | 500 nM |
| Y-27632 dihydrochloride (RhoKi) | DMSO | 10 mM | 10 µM |
| p38 MAPK inhibitor (p38i) (SB202190) | DMSO | 30 mM | 10 µM |
| Primocin | 50 mg/ml | 100 µg/ml | |
| Human Noggin (hNoggin) | DPBS 0.1%BSA | 100 µg/ml | 100 ng/ml |
| Human R-spondin 3 (hRspo-3) | varies per lot | 300 ng/ml | |
| Vancomycin | 10 mg/ml | 50 µg/ml | |
| Gentamycin | 10 mg/ml | 50 µg/ml | |
| Ethylenediamine tetraacetic acid (EDTA) | MiliQ H2O | 0.5 M | 2 mM |
| Calcein | DMSO | 10 µg/ml | 3.3 ng/ml |
| Forskolin | DMSO | 10 mM | variable |
| Lumacaftor (VX-809) | DMSO | 20 mM | variable |
| Ivacaftor (VX-770) | DMSO | 20 mM | variable |
References
- De Boeck, K., Amaral, M. D. Progress in therapies for cystic fibrosis. Lancet Respir Med. 4 (8), 662-674 (2016).
- Cutting, G. R. Cystic fibrosis genetics: from molecular understanding to clinical application. Nature Rev Genet. 16 (1), 45-56 (2015).
- Sosnay, P. R., Siklosi, K. R., et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nature Genet. 45 (10), 1160-1167 (2013).
- Zielenski, J. Genotype and phenotype in cystic fibrosis. Respiration. 67 (2), 117-133 (2000).
- Van Goor, F., Hadida, S., et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U.S.A. 106 (44), 18825-18830 (2009).
- Van Goor, F., Hadida, S., et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U.S.A. 108 (46), 18843-18848 (2011).
- Accurso, F. J., Rowe, S. M., et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 363 (21), 1991-2003 (2010).
- Ramsey, B. W., Davies, J., et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 365 (18), 1663-1672 (2011).
- De Boeck, K., Munck, A., et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 13 (6), 674-680 (2014).
- Boyle, M. P., Bell, S. C., et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2 (7), 527-538 (2014).
- Wainwright, C. E., Elborn, J. S., et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N Engl J Med. 373 (3), 220-231 (2015).
- Van Goor, F., Yu, H., Burton, B., Hoffman, B. J. Effect of ivacaftor on CFTR forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 13 (1), 29-36 (2014).
- Neuberger, T., Burton, B., Clark, H., Van Goor, F. Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol. 741, 39-54 (2011).
- Randell, S. H., Fulcher, M. L., O'Neal, W., Olsen, J. C. Primary epithelial cell models for cystic fibrosis research. Methods Mol Biol. 742, 285-310 (2011).
- Karp, P. H., Moninger, T. O., et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol. 188, 115-137 (2002).
- Sato, T., Vries, R. G., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244), 262-265 (2009).
- Sato, T., Stange, D. E., et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 141 (5), 1762-1772 (2011).
- Clevers, H. Modeling development and disease with Organoids. Cell. 165 (7), 1586-1597 (2016).
- Dekkers, J. F., Wiegerinck, C. L., et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nature Med. 19 (7), 939-945 (2013).
- Dekkers, J. F., van der Ent, C. K., Beekman, J. M. Novel opportunities for CFTR-targeting drug development using organoids. Rare Diseases. 1 (1), e27112 (2014).
- Servidoni, M. F., Sousa, M., et al. Rectal forceps biopsy procedure in cystic fibrosis: technical aspects and patients perspective for clinical trials feasibility. BMC gastroenterology. 13, 91 (2013).
- Ommen, D. D. Z. -. V., Vijftigschild, L. A. W., et al. Limited premature termination codon suppression by read-through agents in cystic fibrosis intestinal organoids. J Cyst Fibros. 15 (2), 158-162 (2016).
- Dekkers, J. F., Gogorza Gondra, R. A., et al. Optimal correction of distinct CFTR folding mutants in rectal cystic fibrosis organoids. Eur Respir J. , (2016).
- Dekkers, J. F., Van Mourik, P., et al. Potentiator synergy in rectal organoids carrying S1251N, G551D, or F508del CFTR mutations. J Cyst Fibros. , (2016).
- Dekkers, J. F., Berkers, G., et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 8 (344), 344ra84 (2016).
- Li, H., Sheppard, D. N., Hug, M. J. Transepithelial electrical measurements with the Ussing chamber. J Cyst Fibros. 3, 123-126 (2004).
- Sheppard, D. N., Carson, M. R., Ostedgaard, L. S., Denning, G. M., Welsh, M. J. Expression of cystic fibrosis transmembrane conductance regulator in a model epithelium. Am J Physiol. 266 (4 Pt 1), L405-L413 (1994).
- Farin, H. F., van Es, J. H., Clevers, H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 143 (6), 1518-1529 (2012).
- Drost, J., et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 11 (2), 347-358 (2016).
- Vijftigschild, L. A. W., Berkers, G., et al. β2-adrenergic receptor agonists activate CFTR in organoids and subjects with cystic fibrosis. Eur Respir J. 48 (3), 768-779 (2016).
- Dekkers, R., et al. A bioassay using intestinal organoids to measure CFTR in human plasma. J Cyst Fibros. 14 (2), 178-181 (2015).
- Graeber, S. Y., et al. Intestinal Current Measurements Detect Activation of Mutant CFTR in Patients with Cystic Fibrosis with the G551D Mutation Treated with Ivacaftor. Am J Respir Crit Care Med. 192 (10), 1252-1255 (2015).
- Van Goor, F., et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. Journal of Cystic Fibrosis: Official Journal of the Eur Cyst Fibrosis. 11 (3), 237-245 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved