Method Article
Fabricating and Labeling Microbubbles with Fluorescent and Radioactive Tracers
In This Article
Summary
This protocol outlines the fabrication of lipid microbubbles and a compatible one-pot microbubble radiolabeling method with purification-free >95% labeling efficiency that conserves microbubble physicochemical properties. This method is effective across diverse lipid microbubble formulations and can be tailored to generate radioactive and/or fluorescent microbubbles.
Abstract
Microbubbles are lipid-shelled, gas-filled particles that have evolved from vascular ultrasound contrast agents into revolutionary cancer therapy platforms. When combined with therapeutic focused ultrasound (FUS), they can safely and locally overcome physiological barriers (e.g., blood-brain barrier), deliver drugs to otherwise inaccessible cancers (e.g., glioblastoma and pancreatic cancer), and treat neurodegenerative diseases. The therapeutic arsenal of microbubble-FUS is advancing in new directions, including synergistic combination radiotherapy, multimodal imaging, and all-in-one drug loading and delivery from microbubble shells.
Labeling microbubbles with radiotracers is key to establishing these expanded theranostic capabilities. However, existing microbubble radiolabeling strategies rely on purification methodologies known to perturb microbubble physicochemical properties, use short-lived radioisotopes, and do not always yield stable chelation. Collectively, this creates ambiguity surrounding the accuracy of microbubble radioimaging and the efficiency of tumor radioisotope delivery.
This protocol describes a new one-pot, purification-free microbubble labeling methodology that preserves microbubble physicochemical properties while achieving >95% radioisotope chelation efficiency. It is versatile and can be applied successfully across custom and commercial microbubble formulations with differing acyl lipid chain length, charge, and chelator/probe (porphyrin, DTPA, DiI) composition. It can be adaptively applied during ground-up microbubble fabrication and to pre-made microbubble formulations with modular customizability of fluorescence and multimodal fluorescence/radioactive properties. Accordingly, this flexible method enables the production of tailored, traceable (radio, fluorescent, or radio/fluorescent active) multimodal microbubbles that are useful for advancing mechanistic, imaging, and therapeutic microbubble-FUS applications.
Introduction
Microbubbles are micron-sized supramolecular theranostic agents with a gas core stabilized by a protein, polymer, or, in most cases, a lipid shell (Figure 1A). When injected into the bloodstream, microbubbles maintain gas/liquid interfaces that are detectable by ultrasound for minutes-long timeframes prior to the dissolution of their gas cores1,2. Consequently, the first clinical use of microbubbles was as real-time ultrasound imaging contrast agents3. The invention of therapeutic focused ultrasound (FUS) expanded microbubble clinical utilities. When stimulated by low-frequency FUS, microbubbles oscillate and generate targeted, tunable mechanical forces ranging from transient vascular permeabilization to focal tissue ablation4,5. As a result, over the last 20 years, microbubble-FUS has been explored for blood-brain barrier (BBB) opening, tumor (e.g., pancreatic, brain, and liver metastatic cancer) drug and imaging probe delivery, neurodegenerative disease therapy and cancer ablation6,7,8,9,10,11.
The theranostic arsenal of microbubbles continues to advance in new and exciting directions. Conventional microbubble-FUS delivery applications rely on the co-administration of therapeutic or imaging cargo alongside commercial microbubbles. There is growing interest in enhancing microbubble-FUS delivery capabilities by understanding microbubble shell/biological interactions, exploring custom-made non-commercial microbubble formulations, and generating all-in-one theranostic microbubbles with cargo loaded directly onto the microbubble shell12,13,14. In fact, approximately 40% of lipid microbubble drug delivery studies make use of such shell-loaded microbubbles15. Beyond imaging and drug delivery, microbubble-FUS has also shown promise in enhancing cancer radiotherapy16, and activating antineoplastic effects of otherwise benign shell-loaded agents through sonodynamic therapy17,18.
These conventional and expanded directions in microbubble cancer applications can be more strategically advanced by labeling microbubble shells with radioactive tracers. In the realm of all-in-one cargo-loaded microbubbles, such radiolabeling 1) facilitates gold-standard, quantitative assessment of the on and off-target biodistribution of these loaded microbubble shells, 2) derives pharmacokinetic structure-activity relationships that inform optimal selection of microbubble compositions to maximize on-target delivery, and 3) guides strategic and appropriate image-guided application and treatment planning (e.g., types of tissue targets, dosimetry, drug selection to mitigate off-target safety concerns, utility compared to conventional co-treatment paradigms) of all-in-one cargo-loaded systems15,19. At a preclinical stage, such an understanding of microbubble shell fate can also illuminate broader microbubble-FUS mechanisms of action. For example, lipid transfer from microbubble shells to target cells has been shown to influence FUS-enabled sonoporation12,20. Understanding and optimizing such transfer can thus inform preclinical and clinical microbubble-FUS therapies in which sonoporation is implicated (in vitro transfection, drug delivery, tumor ablation, radiation sensitization, and sonodynamic therapy20,21,22,23,24,25). Dual ultrasound and radioimaging facilities would also enable FUS vessel opening and treatment monitoring (e.g., BBB opening kinetics) from a single agent rather than conventional dual agent designs26. In the same vein, lipid microbubble radiolabeling could serve as an all-in-one single-agent microbubble-FUS/radiotherapy alternative to microbubble-FUS + radiopharmaceutical co-delivery platforms27.
The fragility of microbubbles is an untrivial challenge to such labeling. All existing radiolabeling strategies are limited by purification methodologies known to perturb microbubble stability and size, while some also feature ineffective and unstable radiolabeling28,29,30,31,32. Purification requirements also lead to lengthier protocols. Combined with the use of short-lived radioisotopes (e.g., 18F t1/2 1.8 h,28,29 99mTc t1/2 6 h,32 68Ga t1/2 1 h31), this creates inefficiencies related to radioisotope decay and confines radioimaging and treatment planning timeframes. Collectively, these limitations risk the acquisition of shortened and unrepresentative radioimaging, inaccurate pharmacokinetic data, and inefficient tumor radioisotope delivery.
In this report, these limitations are overcome by leveraging the strong and stable metal chelation capabilities of porphyrin. Porphyrins are organic, heterocyclic macromolecules with a highly conjugated planar ring and a central coordination site that can accommodate a variety of metals. This includes longer-lived radioisotopes such as copper-64 (t1/2 12.7 h), a radiopharmaceutical with positron emission tomography (PET), and γ-counting feasibilities33. When conjugated to a lipid backbone, porphyrins can be readily incorporated into supramolecular structures and subsequently labeled with copper-64 with speed, high chelation efficiency, and serum stability, while maintaining the properties of the parent unlabeled particles33,34. Furthermore, porphyrins are fluorescently active with modular self-quenching in nano and microparticles that is restored upon particle disruption; a complementary readout to PET and γ-counting that facilitates both bulk and microscopic shell fate analysis (Figure 1A)15.
By using porphyrin-lipid as a chelator, these properties were exploited to generate a new one-pot, purification-free microbubble radiolabeling methodology (Figure 1B,C) that overcomes limitations associated with existing microbubble radiolabeling methods. This protocol achieves >95% copper-64 chelation efficiency, does not require post-labeling purification, and preserves microbubble physicochemical properties. It can be integrated easily into the "ground-up" fabrication of lipid microbubbles prior to their activation (Figure 1B). It is versatile and can be applied successfully across custom and commercial microbubble formulations with differing acyl lipid chain length (C16 to C22), charge (neutral and anionic), and porphyrin-lipid compositions (1 mol%, 10 mol%, 30 mol%), generating microbubbles with both radio and fluorescence activity. Its adaptability can also extend beyond porphyrin. The one-pot protocol can be modified to use alternative commercially available chelators (e.g., diethylenetriamine pentaacetate (DTPA)-lipid) and fluorophores (e.g., DiI). It can also be modified to label pre-made microbubble formulations through a "spiking" approach. Accordingly, this method enables the production of tailored, traceable (radio, fluorescent, or dual radio/fluorescent active) microbubbles useful for advancing mechanistic, imaging and therapeutic microbubble-FUS applications. The protocol below outlines the fabrication of lipid microbubbles, application of the one-pot radiolabeling protocol, requisite radiolabeling and physicochemical property characterization, and potential modifications.
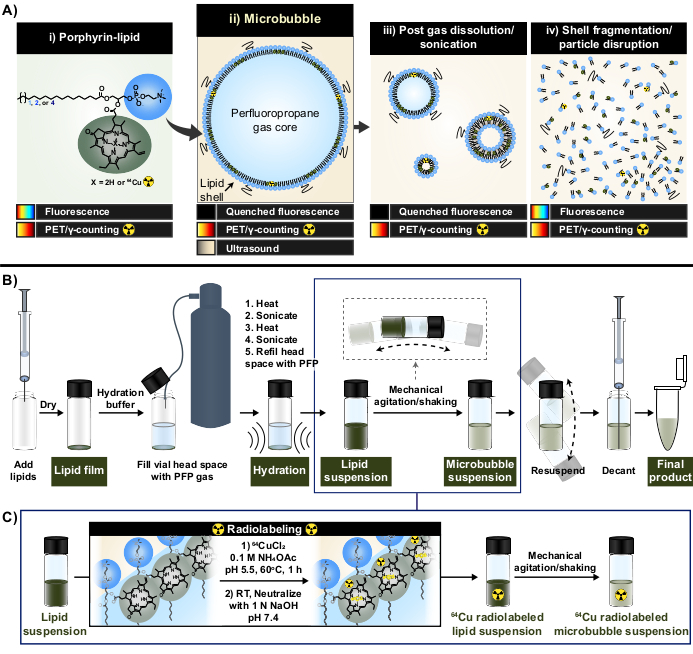
Figure 1: Microbubble fabrication and radiolabeling protocol. (A) Porphyrin-lipid, in the form of pyropheophorbide-a-lipid, serves as a multimodal chelator within this protocol. As a monomer chelated to copper-64 (i), it has PET and imaging capabilities. Its fluorescence is quenched in particle form (microbubbles (ii) and their post-dissolution nanoprogeny (iii)) and unquenched with particle disruption (iv). (B) Lipid film hydration/activation protocol described in this report to generate lipid microbubbles from the ground-up and (C) integration of one-pot radiolabeling between lipid suspension formation and microbubble activation. This figure was adapted with permission from Rajora et al.15. Please click here to view a larger version of this figure.
Protocol
1. Preparations of reagents
- Prepare ammonium acetate buffer (0.1 M, pH 5.5)
- Using an analytical balance, weigh 770.8 mg of ammonium acetate onto a weigh paper. Transfer the weighed amount to a clean 250 mL glass beaker.
- Add 90 mL of double distilled water (ddH2O), measured via a graduated pipette, to the beaker. Add a stir bar and place the beaker on a magnetic stir plate to dissolve the ammonium acetate. Stir at a speed that creates a slight vortex but without solution splashing.
- Calibrate a pH meter according to instrument instructions using standards of pH 4 and 7. Once calibrated, insert the pH probe into the ammonium acetate buffer.
- Add 104 µL of acetic acid to the solution, stir to dissolve, and measure the pH.
NOTE: The pH should be close to 5.5 at this point. - Adjust the pH of the buffer by adding 10 N sodium hydroxide (or hydrochloric acid if the buffer becomes too basic) in 5-10 µL increments using a micropipette. Stir, measure the pH, and repeat as necessary. Make a note of the volume of base/acid added.
- Add enough volume of ddH2O to create a total of 100 mL of buffer.
NOTE: For example, if 45 µL of 10 N sodium hydroxide was used during pH adjustment, 9.851 mL of ddH2O would be added to the beaker (100 mL [target volume]- 90 mL [step 1.1.2] - 0.104 mL [step 1.1.4] - 0.045 mL [step 1.1.5] = 9.851 mL). - Stir the buffer one final time thoroughly before transferring it to a lidded storage container.
- Clean the pH meter as per instrument instructions.
CAUTION: Concentrated aqueous sodium hydroxide and hydrochloric acid can cause skin reactions and should be handled using gloves.
- Prepare hydration buffer (PGG)
- Aspirate phosphate-buffered saline (PBS) into a syringe and equip the end with a polyethersulfone 0.2 µm pore size syringe filter. Filter the PBS into a clean plastic, lidded centrifuge tube.
NOTE: 0.2 µm pore syringe filters of alternative membrane materials (for example, polyvinylidene fluoride) can be used so long as the membrane is compatible with PBS and ammonium acetate. - Combine filtered PBS, propylene glycol, and glycerol via micropipette in an 8:1:1 volumetric ratio to make the hydration buffer (also referred to as PGG). When adding propylene glycol and glycerol, aspirate and wipe any residual droplets of propylene glycol or glycerol from the surface of the pipette tip before slowly pipetting the reagent into the PBS. A clear string-like viscous turbidity will be seen in the PBS.
NOTE: It is recommended to use a p1000 micropipette to first add PBS to a centrifuge tube, followed by propylene glycol and glycerol, as the latter two reagents are viscous. As such, they should be slowly aspirated via the micropipette until fluid movement is no longer seen in the pipette tip and such that no air is uptaken when the pipette tip is removed from the reagent. Micropipette tips with volumetric markings should ideally be used to choose reagent volumes that align with such markings (for example, making 1 mL or 5 mL PGG, and respectively using the 0.1 mL or 0.5 mL marking on the micropipette tip to visualize complete aspiration of propylene glycol and glycerol). When wiping the surface of the micropipette tip, do not wipe at the tip opening, only at the sides. - Pipette up and down with the pipette tip in the solution until the reagents have been homogenously dissolved. Be careful not to introduce any air bubbles into the solution.
- To further ensure a complete mixture of the hydration buffer, cap the centrifuge tube and rotate up and down slowly. Do not vortex.
- Spin the tube at less than 1000 x g for 20-30 s (minimum temperature 4 °C, maximum RT) to remove unobservable air bubbles.
- Aspirate phosphate-buffered saline (PBS) into a syringe and equip the end with a polyethersulfone 0.2 µm pore size syringe filter. Filter the PBS into a clean plastic, lidded centrifuge tube.
- Prepare hydration/radiolabeling buffer (AA-PGG)
- Syringe filter 0.1 M, pH 5.5 ammonium acetate buffer (from step 1.1), and PBS into separate tubes as per step 1.2.1.
- Combine filtered ammonium acetate buffer, filtered PBS, propylene glycol, and glycerol via a p1000 micropipette into a centrifuge tube in a 5:3:1:1 ammonium acetate buffer: PBS: propylene glycol: glycerol volumetric ratio in the listed order. Follow aspirating, mixing, and centrifugation instructions as per steps 1.2.2-1.2.5 to make AA-PGG.
- Instant thin layer chromatography (iTLC) eluent
- Weigh up to 0.1 g of ethylenediaminetetraacetic acid (EDTA) and transfer to a capped vial. Dissolve in ddH2O such that a 2% w/v solution of EDTA is made (for example, for 50 mg of EDTA, add 2.5 mL of ddH2O).
- Combine the 2% w/v EDTA solution with the ammonium acetate buffer from step 1.1 in a 9:1 v/v ratio (90% EDTA solution, 10% ammonium acetate buffer). Cap and store the resulting iTLC eluent.
2. Formation of lipid films
NOTE: This procedure outlines the formation of a lipid film with compositions mimicking the commercial microbubble, Definity®, with porphyrin-lipid substituting the host lipid and constituting 30 mol% of the total lipid. However, the radiolabeling protocol can be applied to diverse lipid formulations (C16, C18, C22 chain lengths, neutral or anionic charge, varying porphyrin-lipid molar compositions). A Supplementary Spreadsheet (Supplementary File 1) is attached that provides calculations, compositions, masses and stock volumes for the described and other formulations. All lipids are commercially available with the exception of the porphyrin-lipid, pyropheophorbide-a-lipid (pyro-lipid), the synthesis of which has been previously described in detail35,36.
- Using Supplementary File 1, determine the total mass needed for each lipid based on the number of films required.
- Weigh an empty 0.5 dram glass vial on an analytical balance.
NOTE: Dust interferes with successful microbubble formation. Thus, blow pressurized air into the vial to remove any dust/particulates if stored uncapped. - Weigh 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) onto weigh paper.
NOTE: The mass weighed should be obtained from step 2.1 plus an additional 0.5-1 mg to account for any loss during sample handling in later steps. - Transfer the DPPC to the weighed glass vial and reweigh it to determine the lipid mass in the vial. This process allows for easier lipid transfer to the glass vial, reduced lipid powder loss/spillage, and more accurate measurement of the lipid mass.
- Repeat steps 2.2 through 2.4 with the other lipids: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000] (DPPE-mPEG), 1,2-dipalmitoyl-sn-glycero-3-phosphate (DPPA), and C16 pyro-lipid.
NOTE: If pyro-lipid is not available in a weighable powder form but rather as a film or aliquot of unknown quantity, it can be dissolved in chloroform to form a stock whose concentration can be calculated through UV-Vis absorbance measurements in methanol using the Beer-Lambert law as previously described35. - Prepare the following organic solvents and solutions in glass test tubes using micropipettes or glass syringes: 1) chloroform, 2) 9:1 v/v chloroform: methanol, and 3) 65:35:8 chloroform:methanol:ddH2O. For the last, pipette the components and mix them in the following order: ddH2O, methanol, then chloroform.
CAUTION: Methanol and chloroform are health hazards, flammable and volatile. Wear eye protection, gloves, and a lab coat, and use a fume hood. - Use the Supplementary File 1 to calculate the volume of organic solvent/solution needed to make lipid stocks and select glass syringes of appropriate volumes.
NOTE: This volume should yield stock concentrations that correspond with 15-100 µL stock aliquot volumes per film that can be readily measured using 25-100 µL glass microliter syringes. - Rinse the glass syringes thrice with chloroform. Pump the plunger back and forth to dry the syringe.
- Measure and add the organic solvent/solutions via the cleaned glass syringe to the individual lipid vials as per the spreadsheet calculations in step 2.7 to form lipid stocks. Dissolve pyro-lipid in chloroform (unless already dissolved as per the step 2.5 note), DPPC and DPPE-mPEG in 9:1 v/v chloroform: methanol and DPPA in 65:35:8 chloroform:methanol:ddH2O. If using the same glass syringe for all additions, rinse and dry between each lipid.
NOTE: If the formulation of choice does not contain DPPA or its C18 chain length variant, then pyro-lipid, host PC lipid, and PEG lipid can all be dissolved in chloroform. - Cap the vials and vortex.
- Add calculated volumes of stock lipid solutions to a new 0.5-dram glass vial (film vial) via a glass microliter syringe. For the first lipid stock, insert the needle tip into the bottom center of the vial and plunge slowly to avoid splashing up the vial walls. For subsequent additions, place the needle tip directly above the liquid level and touch the side of the vial to remove any final drops in a manner that does not expose the needle to the liquid below.
NOTE: Rinse and dry the glass syringe between lipid additions when contamination occurs. If making multiple films, cap both the film and stock vials between additions to minimize solvent evaporation. - Gently swirl the vial manually in an upright position to mix contents. Avoid splashing any solution up the vial walls.
- Uncap (store the cap) and insert a nitrogen line into the headspace of the vial. Adjust the nitrogen flow to cause a slight visible disturbance at the liquid surface but without any funneling or splashing.
- Vortex the vial immediately after inserting the nitrogen line. Start at a low speed sufficient to form a funnel with solvent rising no higher than 1 cm from the bottom of the vial. Avoid solvent splashing. As the solvent evaporates, increase the vortex speed slowly and without pause, maintaining the solvent height until all liquid evaporates. The result will be a thin film coated across the lower third of the vial.
- Place the vial in a vacuum-equipped desiccator and continue to dry the film under vacuum for 8-72 h. Cover the vial (except the opening) or the desiccator with aluminum foil.
NOTE: The protocol can be paused here. The next steps can be performed after film drying, or the films can be stored under argon, sealed with Parafilm, in a -20 °C freezer for up to 1 month, and longer if kept dry.
3. Lipid film hydration
NOTE: If the microbubbles are used in vitro or in vivo, use sterile micropipette tips, tubes, syringes, and needles for steps 3.3 through 5.4 unless otherwise specified.
- Remove the film from the vacuum or, if freezer-stored, allow it to warm to RT.
- Fill a 250 mL beaker with water and heat the water to 70-80 °C.
- Heat a water bath sonicator to 69 °C.
- Micropipette 1 mL of AA-PGG (step 1.3) down the edges of the lipid film vial to avoid bubble creation.
NOTE: When fabricating unchelated control or fluorescent-only microbubbles, use PGG (step 1.2) instead of AA-PGG. - Partially cover the vial opening with a cap, leaving enough space to insert a perfluoropropane (PFP) line. Flow PFP into the vial headspace for 20 s above the liquid, such that the liquid is visibly disturbed but does not splash. Do not flow PFP directly into the suspension. Cap the vial.
NOTE: If the flow is adequate in strength and time, the vial will start to cool to the touch. - Submerge the bottom half of the vial into the 70-80 °C water bath for 1 min. Then, sonicate for at least 30 s in the 69 °C bath sonicator or until the lipid film disperses homogeneously into the AA-PGG. Avoid creating bubbles or prematurely activating microbubble formation (premature activation will appear as milky/cloudy areas in the lipid suspension). Wipe the vial surface when needed to better discern if any unsuspended lipids remain.
NOTE: If the lipid film does not hydrate within 1 min of sonication, re-heat in the 70-80 °C bath, and re-sonicate. - Once the lipid film is homogenously suspended, heat one last time for 1 min and sonicate for an additional 30 s.
- Wipe the vial and allow it to cool passively to RT (~5-10 min).
- Refill the vial headspace with PFP as per step 3.5, cap, and seal the cap edges with Parafilm.
NOTE: The protocol can be paused here and resumed after no later than 8 h.
4. Radiolabeling
NOTE: For unchelated control or fluorescent-only microbubbles, skip to protocol Section 5.
CAUTION: Perform steps 4.4-4.6 of this protocol in a radioactive laboratory unless otherwise specified. 64CuCl2 is a radiological hazard with a risk of multisystem toxicity through skin exposure, inhalation, or ingestion. Whenever possible, handle it in a fume hood indirectly using rubber-tipped forceps. Wear a protective lab coat, a personal ring and badge dosimeter, and double glove when handling. Ensure 64CuCl2 is handled across 2-inch lead shielding. When necessary, transport it in a lead-sheathed container. Shield waste containers and conduct an operational survey for contamination following use.
- Prepare a 60 °C water bath in a glass beaker or large crystallizing dish containing a magnetic stir bar. Use a temperature-controlled hot/stir plate equipped with a thermal probe inserted into the water, set to stir at a rate that produces a weak but visible funnel.
- Transfer a sealed vial containing 64CuCl2 in 0.1 N HCl to a dose calibrator via rubber-tipped forceps.
NOTE: When ordering 64CuCl2, request it be dissolved in 5-20 µL of 0.1 N HCl. A lower volume is critical for preserving microbubble yield. - Note the copper-64 activity measured on the dose calibrator and the time. Remove the vial using forceps and place it in a leaded container.
- Divide the activity noted by the volume reported for the 64CuCl2 to obtain a MBq·mL-1 value.
- Uncap the lipid suspension from step 3.9 and secure it in a vial holder.
- Uncap the 64CuCl2 vial and secure it with forceps.
- Micropipette a volume of the 64CuCl2 solution corresponding to 40-250 MBq of activity and transfer into the lipid suspension. Ensure the pipette tip is submerged into the suspension. Plunge and then pipette up and down to completely transfer the 64CuCl2.
NOTE: The amount of 64CuCl2 added will depend on the intended application for the radiolabeled microbubbles and the sensitivity of the dose calibrator. For longitudinal (up to 48 h post-injection) PET and blood sampling in vivo in mice, a minimum of 220 MBq and 50 MBq, respectively, are recommended. - Cap both lipid suspension and 64CuCl2 vials.
- Using flat rubber-tipped forceps, manually rotate the radioactive lipid suspension up and down at least 5 times to gently mix the 64CuCl2 through the suspension. Avoid shaking or dropping the vial, and avoid bubble formation.
- When right-side-up, gently flick the cap of the vial while keeping the suspension stabilized. This will help any liquid trapped in the cap to gravitate to the bottom of the vial. Carefully partially uncap the vial and insert an 18 G needle-equipped PFP line. Fill the vial headspace with PFP for 20 s as per step 3.5. Cap the vial and seal with Parafilm.
- Measure the vial activity on a dose calibrator and note the time.
NOTE: If adequate activity was not transferred to the vial, repeat Steps 4.5-4.11, adding an appropriate additional volume of 64CuCl2. - Place the vial in a foam vial holder and push through so the bottom half of the vial will be exposed to heat. Place the holder in the stirring 60 °C water bath and heat for 1 h.
- While the chelation reaction ensues, prepare iTLC plates. While wearing fresh gloves, cut glass microfiber chromatography paper into 1 cm x 8 cm strips. Heat the strips in an 80 °C glass-drying oven.
NOTE: This step can be performed in a non-radioactive laboratory. - After 1 h, remove the vial in step 4.12 from the heat and wipe the edges with tissue.
- Rotate the vial up and down manually with rubber-tipped forceps to recondense any condensation on the vial walls into the lipid suspension.
- With the vial in an upright position, flick the cap while stabilizing the tube. Remove the Parafilm and wipe around the cap to remove any trapped bath water.
- Carefully uncap the vial and aspirate 1-2 µL of the lipid suspension. Spot the suspension 1 cm from the bottom center of an iTLC strip and re-cap the vial. Allow the spot to dry.
NOTE: Ideally, a minimum of 2 iTLCs should be spotted per reaction mixture and developed per radiolabeled lipid suspension for certainty. - Micropipette 200 µL of the iTLC eluent (prepared in step 1.4) into the bottom of a 10 mL test tube. House the test tube in a lead container. Add the spotted iTLC to the tube and allow the strip to develop until the eluent is approximately 1 cm from the top edge of the strip.
- Remove the developed iTLC strips using forceps. Hold the strip vertically and cut into thirds over γ-counter and push-cap compatible round-based 5 mL plastic tubes such that each strip third falls directly into an individual third. Insert push caps into the three tubes.
- Measure the strip-containing tubes and an empty/capped control tube on a γ-counter for copper-64 activity and record the associated counts-per-minute (cpm). Subtract the control tube activity from the other readings to correct for background activity.
NOTE: The corrected readings for the bottom third of the strip (piece 1) are associated with copper-64 chelated to lipid suspension particles. The middle section (piece 2) contains a streak of free copper-64 and 64Cu-pyro-lipid chelates in non-supramolecular form. The top section (piece 3) predominantly contains free copper-64. - Calculate radiochemical purity via Equation 1.
 (Equation 1)
(Equation 1)
NOTE: if the cpm for piece 1 seems unreasonably low (for example, lower or equivalent to pieces 2 or 3) or if any readings are above the γ-counter non-linear/saturation threshold, spot a lower volume or a diluted aliquot (1-2 µL) of the radiolabeled suspension for iTLC. - Ensure that the radiochemical purity obtained from both iTLC strips per lipid suspension is ≥ 94% to continue. If not, continue to heat the lipid suspension at 60 °C and monitor the chelation at 30 min intervals via iTLC.
- Uncap the radiolabeled lipid suspension vial and micropipette 8.89 µL of 1 N NaOH into the suspension, pipetting up and down to completely transfer the base and neutralize the suspension. Cap the vial, rotate manually with forceps to invert/revert, and then gently tap the vial cap.
- Fill the headspace with PFP as per step 4.10, cap and seal with Parafilm.
5. Microbubble activation and isolation
- Activate the lipid suspension via a mechanical vial shaker for 45 s at 4530 rpm to generate a milky microbubble suspension. Allow the vial to passively cool to RT for approximately 10 min. The resulting milky suspension will separate into two layers over time.
NOTE: The vial contents should look milky after activation. A clearer suspension post-activation is indicative of unsuccessful activation, the contributors of which will be discussed in later sections. - Once at RT, gently invert/revert the vial to resuspend the microbubble suspension. Set the vial on a flat surface and wait 2 min prior to decanting to obtain the desired microbubble population as follows:
- Equip a 1 mL plastic syringe with an 18 G needle and vent the syringe/needle by aspirating and plunging air in/out. At the 2 min mark, quickly uncap the vial, breaking the Parafilm seal in one motion.
- Draw 400-550 µL from the bottom of the vial (target microbubble population), avoiding aspiration of the top foamy layer of larger undesired microbubble populations.
NOTE: If needed, tilt the vial to one side to collect the end volumes into the syringe to avoid aspirating the foamy/lighter layer.
- Wipe the edges of the needle carefully to remove any foamy contaminants and transfer the isolated microbubble suspension into a microcentrifuge tube. Cap gently (do not abruptly snap the cap open or closed). This is the final working suspension of radiolabeled microbubbles.
- Measure the activity of the final microbubble product on the dose calibrator and note the time. Divide this value by the volume of suspension decanted in step 5.2 to obtain an MBq·mL-1 value to calculate injection volumes depending on the application of interest.
NOTE: The radiolabeled microbubbles are now ready to use. Section 6 can be executed up to 24 h later. For information on how these radiolabeled microbubbles can be injected and tracked in vivo through multimodal (ultrasound, PET, fluorescence) imaging, refer to Rajora et al.15.
6. Validating radiolabeling efficiency
- Resuspend the microbubble suspension through gentle pipetting or vial inversion.
NOTE: Never vortex a microbubble working product. Vortexing destabilizes microbubble suspensions. - Add 10-200 µL of the radiolabeled suspension to a 0.5 mL 30,000 molecular weight cut-off (MWCO) centrifuge filter unit. If using volumes <200 µL, add ddH2O to the filter unit to constitute a total 200 µL volume. House the filter unit in a compatible microcentrifuge tube and cap.
NOTE: Conducting a radiolabeling test prior to and separately from any applied in vitro or in vivo use of radiolabeled microbubbles is recommended to ensure successful protocol completion. In this case, a larger volume (e.g., 200 µL) could be used in this step. When the protocol is subsequently used for a treatment session, prepare treatment volumes/injections first, and then conduct section 6 with the remaining radiolabeled microbubble suspension as early as possible. - Centrifuge for 10 min at 12,000 x g at RT.
NOTE: the microcentrifuge should be surrounded by lead shielding. - Cut the connection between the microcentrifuge tube and its cap with scissors.
- Place the cap in a 20 mL scintillation vial labeled "caps". Transfer the filter unit to a new microcentrifuge tube (tube 2).
- Place the first microcentrifuge tube with infranatant into a 20 mL scintillation vial labeled "tube 1". Add 200 µL ddH2O to the filter unit in tube 2.
- Centrifuge the filter unit in tube 2 at 12,000 x g at RT.
- Repeat steps 6.4 and 6.5. Add the tube 2 cap to the "caps" scintillation vial harboring the tube 1 cap. Place tube 2 in a new 20 mL scintillation vial.
- Cut the cap of the third microcentrifuge tube and place in the "caps" vial as per step 6.4. Transfer the filter unit to a new 20 mL scintillation vial labeled "unit", ensuring that the infranatant remains in tube 3 and is not transferred to the "unit" vial. If drops are seen on the edges of the filter unit, return it to tube 3, cap, and spin down for 10 s. Place tube 3 in a new 20 mL glass scintillation vial.
- Cap the 5 scintillation vials (tube 1, tube 2, tube 3, caps and unit). Prepare one empty and capped 20 mL scintillation vial as a blank control.
- Measure the six scintillation vials on a γ-counter for copper-64 activity. Subtract the blank vial activity from that of other vials. Calculate the radiolabeling/chelation efficiency using Equation 2.
 (Equation 2)
(Equation 2)
NOTE: If the unit cpm is unreasonably low (ex, lower or equivalent to the tubes) or if any readings are above the γ-counter non-linear/saturation threshold, store the scintillation vials in lead containers for up to 4 days to allow the activity to decay until values are below the threshold and remeasure.
7. Microbubble physicochemical characterization
NOTE: Unless a laboratory has designated equipment for radioactive sample processing, microbubble physicochemical characterization must be conducted using non-radioactive, "cold" copper-chelated samples. This "cold" labeling facilitates the assessment of microbubble yield, which is vital for assessing the dose of microbubbles used for one's intended application. Additionally, it allows for comparison with control unchelated microbubbles to ensure the radiolabeling process does not perturb the properties of microbubbles. This "cold" labeling and associated physicochemical characterization should occur prior to radiolabeled microbubble application and can be used as feedback if modifications to radiolabeling are required (see Discussion).
- "Cold" copper microbubble labeling
- Using the volume of 64CuCl2 solution added to the lipid suspension in step 4.7, porphyrin molar% composition within the lipid films and specific activity found on the 64CuCl2 product sheet, calculate the approximate metal:porphyrin molar ratio achieved during radiolabeling. Example calculations can be found in Supplementary File 1.
- Follow sections 1-3 of the current protocol.
- Prepare a 0.1 mg·mL-1 CuCl2 solution in 0.1 N HCl.
- Micropipette the appropriate volume of this CuCl2 solution into the lipid suspension calculated from step 7.1.1 and cap the vial.
- Rotate the vial to mix the CuCl2 into the lipid suspension, fill the headspace with PFP, seal, and heat as per steps 4.9, 4.10, and 4.12. Rubber forceps are not needed for vial handling.
- After 1 h, remove the vial from the heat and wipe the exterior to dry. Allow the vial to cool to RT.
- Neutralize the lipid suspension, mix, fill the headspace with PFP, and seal as per steps 4.23 and 4.24.
- Activate the microbubble suspension and decant to obtain a working product as per steps 5.1-5.3.
- Microbubble sizing
NOTE: Microbubble sizing should be conducted immediately after activation. If assessing the stability of the working suspension, repeat sample preparation and measurements at 30 min intervals. Typically, a 1-2 h window is representative of the timeframe over which the microbubble working suspension would be used/administered post activation. The aim of stability measurements is to ensure microbubble size and yield are maintained across this timeframe so that all treatments administered from the working solution contain similar microbubble populations.- Turn on the Coulter Counter (CC) and set the following parameters using the Edit SOP tool: 30 µm aperture, 0.6-18 µm size range, aperture current 400-600 µA, preamp gain 4-8, 400 bins, flush before and after each run, volumetric analysis, 5 µL sample volume.
- Filter CC electrolyte through a 0.2 µm media vacuum filtration unit. Fill the electrolyte container and a separate container for sample preparation.
- Background measurement: Fill a 10 mL disposable cuvette with 10 mL filtered electrolyte and run a baseline measurement. Ensure the counts are below 400. If not, flush the instrument.
- Sample measurement
- Add 10 mL of filtered electrolyte to a new cuvette. Resuspend microbubble suspension by manually inverting/reverting. Micropipette 5 µL from the bottom center of the vial. Wipe the edges of the pipette tip (except the opening) and plunge the sample directly into the prepared electrolyte.
- Pipette up and down to completely transfer the suspension. Use the pipette tip to gently swirl the electrolyte until the "wisps" of microbubble suspension are dispersed.
- Measure the sample on the CC (two runs per analyte).
NOTE: A 5 µL microbubble sample volume is typically appropriate for samples containing 1-5 x 109 microbubble·mL-1 concentrations. This sample volume may need to be adjusted depending on the specific CC instrument setup and if the sample microbubble yields fall outside the above range.
- Confocal imaging
NOTE: Conduct confocal imaging immediately after microbubble sizing and within the timeframe of retained microbubble stability as per the note in step 7.2.- Resuspend the microbubble suspension and transfer 1-5 µL to the center of a glass microscope slide. Place a cover slip carefully over the microbubble suspension droplet, avoiding any entrapment of air bubbles. The suspension will spread under the cover slip.
- Image the microbubbles at 60x magnification with an oil immersion objective. Obtain images in brightfield and under 633 nm excitation/640-765 nm emission. Overlay the brightfield and fluorescence images.
NOTE: Fluorescence signal should overlap across the shell of all visible particles when the probe is homogenously incorporated within the microbubble shell.
- Spectrofluorometry
NOTE: Spectrofluorometry measurements can be conducted within 24 h of microbubble activation.- Prepare 1% Triton X-100 as previously described35.
- Turn on the spectrofluorometer 15-30 min prior to the first measurement.
- Measure the fluorescence spectra of 1% Triton X-100 using a 410 nm excitation and 600-800 nm emission range in a quartz cuvette. Select the option to normalize the signal by the reference detector signal (often referred to as S1/R1).
NOTE: Triton X-100 easily bubbles when pipetted. As such, when transferring to a cuvette, only plunge to the first micropipette stop. - Rinse the cuvette with methanol and dry with pressurized air in between samples.
- Transfer 6 mL of 1% Triton X-100 to a 15 mL centrifuge tube. Resuspend the microbubble suspension and aspirate 1 µL via micropipette. Wipe the edges of the pipette tip except the opening and transfer the sample to the prepared 1% Triton X-100, pipetting up and down to complete the transfer. Vortex the solution and transfer it to a quartz cuvette.
NOTE: Adjust the ratio of sample: 1% Triton X-100 according to instrument sensitivity and non-linear saturation threshold. - Measure this sample using the parameters in step 7.4.3. This measurement corresponds to "disrupted" particles.
- Repeat steps 7.4.3-7.4.6 using PBS. This measurement corresponds to "intact" particles.
- Baseline-correct the disrupted and intact sample spectra using 1% Triton X-100 and PBS measurements, respectively.
- Calculate quenching efficiency (QE) via Equation 3 using integrated baseline-corrected fluorescence signal of the intact sample in PBS (FPBS) and in 1% Triton X-100 (FTx):
 (Equation 3)
(Equation 3)
- UV-Vis spectroscopy
NOTE: Spectroscopy measurements can be conducted up to 72 h after microbubble activation.- Sonicate an aliquot of the microbubble suspension in a microcentrifuge tube using a bath sonicator at RT until the suspension is transparent. This reduces scattering effects during spectroscopy.
- Turn on the spectrophotometer 10 min prior to the first measurement. Select a scanning interval of 0.25 nm and a 200-800 nm acquisition range. Enable baseline subtraction.
- Use a 1 cm path-length quartz cuvette for measurements. Rinse the cuvette with methanol between measurements and dry with pressurized air.
- Obtain a baseline measurement of methanol.
- Vortex the sonicated, transparent microbubble suspension and transfer 10-50 µL into a microcentrifuge tube containing 200-1000 µL of methanol. Ensure methanol volume is measured and added to the tube via a clean glass microliter syringe. Vortex the solution to obtain a "disrupted" sample.
NOTE: Sample dilution will depend on porphyrin loading efficiency and molar% composition. A 20x dilution is appropriate for a 30 mol% pyro-lipid microbubble composition. - Collect UV-Vis spectrum.
- Repeat steps 7.5.4 through 7.5.6 using PBS instead of methanol.
NOTE: A micropipette can be used to measure PBS volumes instead of a glass microliter syringe.
8. Modifications to protocol
- Alternative chelator
- Prepare lipid films as per section 2, substituting the pyro-lipid for an alternative lipid-conjugated copper chelator (for example, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (ammonium salt), referred henceforth as DTPA-lipid). Use the Supplementary File 1 to calculate the mass and stock volumes required.
NOTE: Testing various molar compositions of an alternative chelator is likely needed to determine the upper limit beyond which stable microbubbles with high yields cannot be generated. - Follow sections 3 through 6 to generate and characterize radiolabeled microbubbles.
- Follow steps 7.1 through 7.3 to characterize "cold" copper-chelated microbubbles. Only brightfield confocal microscopy image acquisition is required to assess particle morphology. If the alternative chelator is fluorescent, conduct confocal microscopy with associated excitation and emission wavelengths in addition to steps 7.4 and 7.5.
- Prepare lipid films as per section 2, substituting the pyro-lipid for an alternative lipid-conjugated copper chelator (for example, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (ammonium salt), referred henceforth as DTPA-lipid). Use the Supplementary File 1 to calculate the mass and stock volumes required.
- Alternative fluorophore
- Prepare lipid films as per section 2, substituting the pyro-lipid for an alternative lipid-conjugated or intercalating fluorophore (e.g., DiI). Use Supplementary File 1 to calculate the mass and stock volumes required.
NOTE: Testing various molar compositions of an alternative fluorophore is likely needed to determine the upper limit beyond which stable microbubbles with high yields cannot be generated. - Follow section 3 using PGG instead of AA-PGG.
- Complete steps 5.1 through 5.3 and steps 7.2 through 7.5.
- Prepare lipid films as per section 2, substituting the pyro-lipid for an alternative lipid-conjugated or intercalating fluorophore (e.g., DiI). Use Supplementary File 1 to calculate the mass and stock volumes required.
- "Spiking" approach: Labeling pre-formed microbubble lipid suspensions
- Generate a porphyrin-lipid film as per section 2, using only pyro-lipid with no other lipid constituents. Refer to the Supplementary File 1 for pyro-lipid quantities.
- Hydrate the pyro-lipid film as per section 3 using 100-200 µL of AA-PGG (or PGG if radiolabeling is not required) instead of 1 mL of hydration buffer.
- Transfer the entire pyro-lipid suspension to a pre-made lipid microbubble suspension.
- Fill the headspace with PFP, heat, and sonicate the suspension and seal under PFP as per steps 3.4 through 3.9. Monitor for dispersion of the pyro-lipid suspension into the pre-made lipid microbubble suspension and conduct heat/sonication cycles until completely dispersed.
NOTE: If using a septum-sealed commercial microbubble vial, the pyro-lipid suspension can be introduced to the vial through a syringe/needle without refilling the vial headspace with PFP. - Conduct radiolabeling (see step 8.3.5.1 below), activation, isolation (see NOTE below), and associated characterization as per sections 4 through 7.
- An alternative approach is to first radiolabel the hydrated pyro-lipid suspension, monitor for >94% radiochemical purity, neutralize with 1 N NaOH (modify the volume according to AA-PGG volume used to hydrate the pyro-lipid film), and then introduce the radiolabeled pyro-lipid suspension into the pre-made microbubble lipid suspension as per step 8.3.3.
NOTE: This modified approach should only be used to generate fluorescent or multimodal radiolabeled microbubbles if followed by an isolation process that removes submicron multilamellar vesicles created during lipid hydration but not incorporated into microbubbles. See Discussion for further details.
- An alternative approach is to first radiolabel the hydrated pyro-lipid suspension, monitor for >94% radiochemical purity, neutralize with 1 N NaOH (modify the volume according to AA-PGG volume used to hydrate the pyro-lipid film), and then introduce the radiolabeled pyro-lipid suspension into the pre-made microbubble lipid suspension as per step 8.3.3.
Results
The key quantifiable results when fabricating radiolabeled microbubbles are radiochemical purity and radiolabeling efficiency. This protocol uses iTLC and a validated centrifugal procedure, respectively, to characterize each. Figure 2A shows that average radiochemical purities and efficiencies of ≥95% were achieved across commercial microbubble mimicking formulations in which the host lipid was substituted for pyro-lipid at compositions of 1 mol%, 10 mol%, or 30 mol% of the total lipid. The 1 mol% and 10 mol% pyro-lipid formulations required a more concentrated lipid suspension (0.15 mL vs 1 mL) to achieve these values. The high radiochemical purities and chelation efficiencies achieved in the current protocol negated any need for post-labeling purification.
This was also true for alternative formulations composed of C16, C18, and C22 chain length lipids with an overall neutral or anionic surface charge (Figure 2B). These variants were loaded with a 30 mol% pyro-lipid composition, based on an upper limit previously determined to maintain average microbubble size, acceptable microbubble yields (>1 x 109 microbubbles·mL-1), and limit >8 µm microbubble population generation while maximizing pyro-lipid and its activatable fluorescence and metal chelation capabilities15. To this end, the 30 mol% formulations demonstrated the highest (as high as 99%) radiochemical purities and chelation efficiencies. The foundational lipid compositions of all formulations explored in Figure 2 were based on the most predominantly clinically explored commercial microbubble formulation, while the chain lengths and charge variants represent the most widely preclinically explored compositions15. This lends confidence that the presented radiolabeling protocol can be applied across most microbubble compositions of interest to the therapeutic ultrasound field.
Quantitative microbubble physicochemical characterization is typically unfeasible for radioactive microbubbles. By the time the radioactivity decays (over days and even weeks, depending on the isotope and quantity of activity used), microbubble gas cores will have largely dissolved, generating microbubble fragments. Thus, the particle suspension will not accurately capture the theranostically active species that were first activated or administered. As such, visual observation during radioactive microbubble preparation and a paired "cold" chelation analog protocol are vital. Specifically, key visual observations are needed at the lipid film hydration and activation steps (Figure 3). In a successful, complete hydration, lipid films will completely dissociate from the vial walls and self-assemble into vesicles that distribute homogenously in the hydration buffer. The end result is a transparent solution (Figure 3A). Incomplete hydrations will feature a lipid film that continues to adhere to the vial wall or aggregate in the suspension (Figure 3B). The former may not be easily noticed, and thus wiping any water off the surface of the vial and careful inspection are recommended during sonication. Premature activation of the microbubbles should be avoided during hydration and can be easily observed as the creation of persistent bubbles at the suspension surface and milky/cloudy whisps within the suspension (Figure 3C). This inappropriate activation can reduce microbubble yields and size distribution reproducibility. It can be avoided by using a bath water temperature above the transition temperature of the host lipid constituent, filling the bath sonicator to the appropriate water level that facilitates visible sonication/liquid disturbance but without "jumping" off the hydration buffer or bath water and careful avoidance of vial shaking or abrupt agitation. Once the microbubble suspension is purposefully activated through controlled, high-speed mechanical agitation, a milky, opaque suspension will form that separates into a lighter/whiter and foamier upper layer containing inappropriately large particles (>8 µm diameter, for example), and the target bottom layer containing microbubbles of interest (Figure 3D), which is isolated through syringe/needle aspiration. Suboptimal activation, resulting in lower yields of target microbubble populations, yields a less milky/opaque and often translucent lower layer that looks like a cloudy version of a hydrated lipid suspension (Figure 3E).
Objective radiolabeled microbubble physicochemical characterization is conducted using analogous "cold" copper-chelated microbubbles. Key endpoints for this characterization include: 1) quantification of microbubble average size and yield, 2) evaluating microbubble storage/working timeframe stability, 3) validating successful pyro-lipid loading across the microbubble shell, and 4) ensuring these physicochemical properties are unchanged by the radiolabeling process integrated within the microbubble fabrication procedure. Figures 4 and Figure 5 illustrate such characterization for a copper-chelated C16 anionic microbubble formulation with a pyro-lipid composition of 30 mol% of the total lipid. Characterization is presented in contrast to that of control, unchelated microbubbles.
Figure 4 illustrates representative sizing data for chelated and unchelated microbubbles. The number distributions (Figure 4A) demonstrate a larger presence of small bubbles and a monotonic decay in microbubble number with size. The microbubble volume distributions (Figure 4B) exhibit unimodal peaks corresponding to an average size of 6 µm. As larger microbubbles comprise larger gas volumes, volume distributions are expected to skew towards higher microbubble average sizes. When averaged by number-weighted mean, sizes of 1.5-2 µm and microbubble yields of 3 x 109 microbubbles·mL-1 are achieved (Figure 4C,D), which remain stable 1 h post microbubble activation and isolation. These results are typical for stable lipid microbubbles generated through a lipid hydration protocol without any further centrifugal size isolation. Importantly, the size distributions, average sizes, concentrations, and stability of the microbubbles are unchanged with the integration of the chelation conditions within microbubble ground-up fabrication. These values are summarized in Table 1, alongside those of other microbubble formulations, which also showcase the retention of physicochemical properties with copper chelation.
The morphological and optical properties (Figure 5) of the microbubbles are also maintained with "cold" copper chelation. Porphyrin fluorescence can be observed homogeneously outlining the shells of chelated and unchelated microbubbles, demonstrating successful pyro-lipid chelator incorporation within the microbubble shells (Figure 5A). Successful loading can also be discerned through UV-Vis spectroscopy and spectrofluorometry. Disrupted microbubbles diassemble into monomeric lipid species. As such, the UV-Vis spectra (Figure 5B) of disrupted microbubbles match that of free pyro-lipid. It contains two prominent peaks: a Soret band in the blue region and a Q-band in the red region. To normalize for concentration and facilitate more objective comparison amongst microbubbles, absorbance spectra are presented as the molar emissivity across wavelengths. Raw UV-Vis absorbance spectra of disrupted microbubbles can be used to quantify pyro-lipid encapsulation and concentration in microbubble suspensions by applying the Beer-Lambert law (path length of 1 cm, extinction coefficients of 97,000 M-1·cm-1 or 45,000 M-1·cm-1 at 410 nm and 667 nm respectively). The microbubbles characterized in Figure 5 exhibit 85%-90% pyro-lipid encapsulation efficiency and porphyrin concentrations of ~0.17 mM. Characterizing both microbubble and porphyrin concentrations allows one to estimate administered doses of microbubble and porphyrin. The high encapsulation efficiency demonstrates effective pyro-lipid loading in microbubbles. This is supported by UV-Vis spectra of intact microbubbles, depicting a signature red shift of the Qy band to 674 nm and 702 nm. The latter is seen specifically with high loading and ordered aggregation of porphyrin into microbubble shells versus vesicular structures, which only depict a single red-shifted peak between 670-680 nm15,37. Effective loading of porphyrin into microbubbles at compositions greater than 5 mol% can be observed through high (>90%) quenching of porphyrin fluorescence in intact microbubbles15, which is restored when particles are disrupted (Figure 5C). Similar to sizing data, these optical properties are preserved with the radiolabeling conditions associated with the current protocol (summarized in Table 1). Collectively, these results demonstrate the achievement of all endpoints for successful microbubble fabrication, porphyrin chelator incorporation, and property retention of chelated microbubbles.
The current microbubble radiolabeling protocol was established by exploiting the copper chelation capabilities and multimodality of pyro-lipid. However, pyro-lipid is currently commercially unavailable. Research collaborations are encouraged to obtain pyro-lipid if resources are unavailable for its synthesis in-house or externally. If neither option is available, it is possible to modify the current protocol to generate unimodal fluorescent or radioactive microbubbles using commercially available fluorophores and chelators, respectively. These alternative chelators/tracers must be incorporable within a microbubble lipid shell. Figure 6 demonstrates representative microbubbles constructed with two such moieties: DiI and DTPA-lipid.
DTPA-lipid incorporation into a C16 anionic microbubble formulation yields 1.1 µm microbubbles with an 11 x 109 microbubbles·mL-1 yield and similar morphology as chelated C16 anionic pyro-lipid microbubbles (Figure 6B,C). A preliminary assessment of the DTPA microbubble copper chelation abilities was conducted using "cold" CuCl2. When copper chelation was integrated into the DTPA microbubble fabrication process, size and yield remained unchanged. To confirm that DTPA-lipid within the microbubbles was available for copper chelation, ICP-MS was conducted on centrifuge-filtered microbubbles. A definitive copper signal was detected, which corresponded to 90%-100% chelation efficiency when compared to the signal achieved from equivalent copper-spiked unlabeled controls. In-house experience is that ICP-MS yields more variable copper chelation efficiencies than γ-counting, and thus, the latter is recommended to quantify chelation/radiolabeling efficiency in the current protocol. These results highlight the importance of this recommendation but also provide proof-of-concept demonstration that the copper chelation protocol presented in this report is translatable to chelators beyond pyro-lipid.
As can be seen in Figure 6D, the current protocol can also be used to successfully incorporate DiI into a C16 anionic microbubble formulation at a 5 mol% composition. This leads to strong homogenous fluorescent labeling of the microbubble shell (similar to when pyro-lipid is used as illustrated in Figure 6B) and generates microbubbles with an average size of 1.7 µm and yield of 1.5 x 109 microbubbles·mL-1. Overall, the results presented in Figure 6 demonstrate that the current microbubble fabrication and labeling protocol can be implemented to incorporate alternative probes and chelators into microbubble formulations if pyro-lipid is inaccessible.
This protocol focuses on the radiolabeling of lipids MBs. Its natural extension is the in vivo application of these radiolabeled microbubbles, which was essayed in a recent report that characterized the fragmentation, circulation kinetics, and kinetic biodistribution of a series of radiolabeled analogs of commercial lipid microbubbles15. The results of this extended work will be discussed in the subsequent section in the context of the applications and future utility of radiolabeled microbubbles.

Figure 2: Radiolabeling purities and efficiencies. Radiolabeling purities and efficiencies obtained following the application of the current microbubble radiolabeling protocol during ground-up synthesis of microbubbles with (A) varying pyro-lipid chelator compositions and (B) lipid chain lengths and microbubble charge. Anionic microbubbles are specified with (-), while zwitterionic (i.e. neutral) are specified with "n". Data are presented as averages ± standard deviation. This figure was adapted with permission from Rajora et al.15. Please click here to view a larger version of this figure.
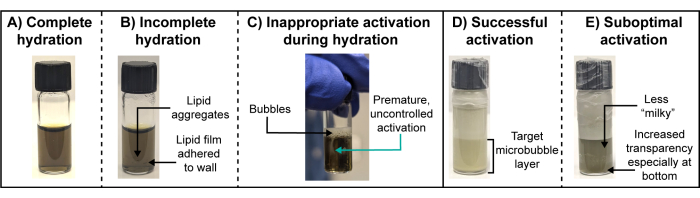
Figure 3: Optimal and suboptimal lipid film hydrations and microbubble activations. (A-E) Representative photographs of (A,D) optimal and (B,C,E) suboptimal lipid film hydrations and microbubble activations. Please click here to view a larger version of this figure.

Figure 4: Representative sizing, yields and stability of unchelated and copper-chelated porphyrin microbubbles. (A) number size distribution, (B) volume size distribution, (C) microbubble yield, and (D) number averaged sizes of unchelated (in black) and copper-chelated (in pink) porphyrin microbubbles with a 30 mol% pyro-lipid composition. The yield and size of the microbubbles (C and D) were measured at 30 min intervals to ensure working suspension stability. Data are presented as an average standard deviation for (C) and (D) of n = 4-7 replicates. This figure was adapted with permission from Rajora et al.15. Please click here to view a larger version of this figure.
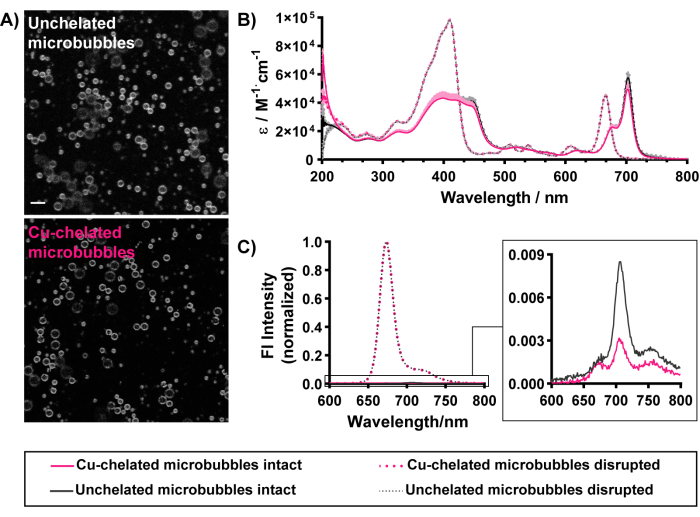
Figure 5: Morphological and optical characterization of unchelated (in black) and copper-chelated (in pink) porphyrin microbubbles with a 30 mol% pyro-lipid composition. (A) Gray scale confocal imaging (pyro signal depicted) demonstrates the homogenous incorporation of pyro-lipid within chelated and unchelated microbubble shells (scale bar = 5 µm). (B) UV-Vis spectra obtained for intact microbubbles (in PBS, solid lines) and methanol-disrupted microbubbles (dashed lines). (C) Fluorescence spectra of intact microbubbles in PBS (solid lines, magnified inset) and 1% Triton X-100 disrupted microbubbles (dashed lines). This figure was adapted with permission from Rajora et al.15. Please click here to view a larger version of this figure.
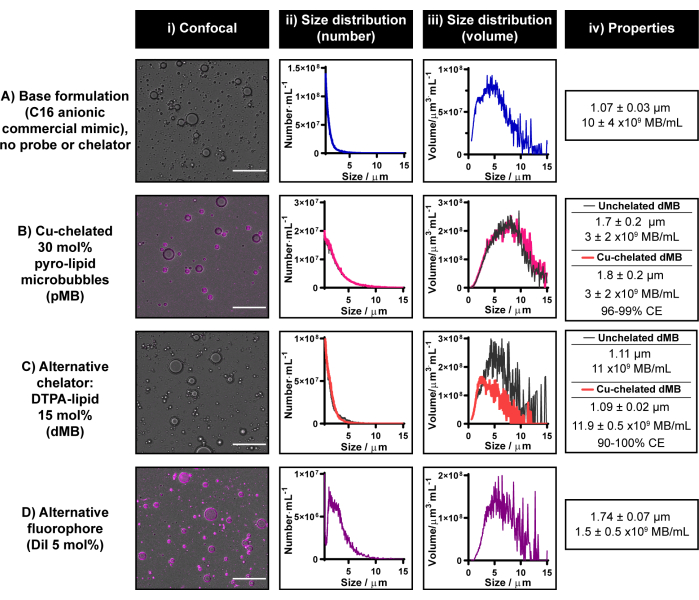
Figure 6: Representative microbubbles constructed with commercially available fluorophores and chelators. (A-D) Example usage of (C) alternative chelator (DTPA-lipid) and (D) fluorophore (DiI) to (B) pyro-lipid incorporated separately into a (A) C16 anionic commercial lipid microbubble formulation. Microbubbles were characterized via confocal microscopy (scale bar = 20 µm) (i) and electrical impedance sizing generating number-weighted (ii) and volume-weighted (iii) size distributions. The average size and yield of the microbubbles are summarized in (iv). Data are presented as an average of n = 2-7 replicates in ii-iv. Please click here to view a larger version of this figure.
| Microbubble variant | Yield | Gas volume | Mean size by number distribution [µm] | Mean size by volume distribution [µm] | Q702nm:Q674nm | Fl quenching efficiency [%] |
| [x109 MB▪mL-1] | [x1010 µm3 ▪mL-1] | |||||
| In-house Definity | 10 ± 4 | 1.8 ± 0.2 | 1.07 ± 0.03 | 3.64 ± 0.09 | N/A | N/A |
| C16 (-) | 3 ± 2 | 6 ± 3 | 1.7 ± 0.2 | 6.0 ± 0.8 | 2.5 ± 0.1 | 97.5 ± 0.8 |
| Cu-C16 (-) | 3 ± 2 | 4 ± 2 | 1.8 ± 0.2 | 6.6 ± 0.8 | 2.3 ± 0.2 | 98 ± 2 |
| C16 n | 8.4 ± 0.4 | 2.3 ± 0.8 | 1.1 ± 0.2 | 4.6 ± 0.1 | 2.1 ± 0.5 | 98.2 ± 0.3 |
| Cu-C16 n | 6.2 ± 0.5 | 2.9 ± 0.9 | 1.3 ± 0.2 | 5.1 ± 0.3 | 1.8 ± 0.6 | 98.7 ± 0.2 |
| C18 (-) | 8 ± 1 | 3.9 ± 0.9 | 1.5 ± 0.1 | 4.0 ± 0.5 | 2.5 ± 0.3 | 97 ± 1 |
| Cu-C18 (-) | 7 ± 2 | 4 ± 1 | 1.5 ± 0.2 | 4.9 ± 0.9 | 2.2 ± 0.5 | 98.5 ± 0.6 |
| C18 n | 8 ± 1 | 3 ± 1 | 1.5 ± 0.1 | 4.0 ± 0.8 | 1.8 ± 0.7 | 98.6 ± 0.5 |
| Cu-C18 | 7.8 ± 0.2 | 3.9 ± 0.2 | 1.42 ± 0.04 | 4.8 ± 0.1 | 1.8 ± 0.7 | 98.7 ± 0.3 |
Table 1: Physicochemical properties of unchelated and Cu-chelated pDefs. Anionic microbubbles are specified with (-), while zwitterionic (i.e. neutral) are specified with "n". All measurements represent an average ± standard deviation (n = 3-7). This figure was adapted with permission from Rajora et al.15.
Supplementary Figure 1: Comparison of "ground-up" (grey) and "spiking" (pink) approaches for pyro-lipid introduction into lipid microbubble shells. (A) Confocal imaging of microbubbles "spiked" with pyro-lipid at at quantities corresponding with total pyro-lipid compositions of 1 mol%, 10 mol%, and 30 mol% of total lipid (scale bar = 20 µm). (B) UV-Vis spectra (i-iii) of pyro-lipid microbubbles generated through the "spiked" (magenta) or "ground-up" approach (black). (Biv) Percentage of pyro-lipid in microbubble cakes versus infranatants following centrifugal removal of submicron species. (C) Number and volume distributions (i-iii) of pyro-lipid microbubbles made through "ground-up" (black) and "spiking" approaches, and corresponding average microbubble sizes (iv) and yields (v). Please click here to download this File.
Supplementary Figure 2: Radio-UPLC characterization of microbubble lipid suspensions composed of 1 mol%, 10 mol%, and 30 mol% pyro-lipid and radiolabeled with copper-64. The left-hand spectra are from a radiation detector, while the right is from a 400 nm absorbance channel. Unfortunately, due to potential contaminants, this data could not be used to quantify radiochemical purity. However, it does demonstrate an overlap in elution times of peaks associated with [64Cu]Cu2+ signal and pyro-lipid, indicating successful radiolabeling. This figure was adapted with permission from Rajora et al.15. Please click here to download this File.
Supplementary Figure 3: Effect of increasing pyro-lipid molar composition. Effect on (A) associated number distributions, (B) volume distributions, (C) number averaged size, (D) volume averaged size, (E) yield, (F) fluorescence quenching efficiency and integrated fluorescence signal (410 nm excitation, 600-800 nm excitation) associated with intact (PBS) and disrupted (in 1% Triton X-100). Data is presented as an average of n = 3 replicates ± standard deviation. This figure was adapted with permission from Rajora et al.15. Please click here to download this File.
Supplementary File 1. Please click here to download this File.
Discussion
The current lipid microbubble radiolabeling protocol achieves >95% radiochemical purity, >95% chelation efficiency, and retention of microbubble physicochemical properties without necessitating any post-labeling purification. These accomplishments represent advancements previously unattained for existing labeling protocols. Lack of purification steps allows quicker use of radioisotopes (in this case, copper-64), and thus, reduction of inefficient activity loss from radioactive decay. The resulting retention of microbubble properties, combined with the known stability of copper-porphyrin chelation33, better ensures that any subsequent radioimaging or therapy is representative of the microbubble of interest vs that associated with free radioisotope or purification-modified microbubble populations.
The current protocol is also the first to integrate parallel "cold" labeling and key characterization techniques to ensure such retention of microbubble physicochemical properties. In fact, this is the first microbubble radiolabeling protocol in which microbubble size, yield, and stability are robustly characterized. Understanding these properties is crucial for the application of radiolabeled microbubbles. Knowledge of microbubble size, gas volumes, and yield are required to determine microbubble doses, which in turn affect the safety and efficacy of the microbubble-FUS treatment. For example, microbubble-FUS BBB opening is associated with an upper limit for microbubble dose/gas volume, beyond which post-FUS hemorrhage and inflammation pose safety concerns38. For all-in-one shell-loaded microbubbles, this dose is also tied to the dose of the loaded drug. Size and yield also affect imaging applications, generating shielding artifacts with high concentrations of microbubble gas volumes39. Large, >8 µm microbubbles present the additional safety risk of lodging in capillaries and forming gas emboli40. Intuitively, this would also impact the pharmacokinetics and biodistribution of microbubble shells. For example, lungs were previously reported as a primary site of radiolabeled microbubble shell accumulation28,32. In the absence of microbubble characterization, it remains unclear if lung entrapment of larger-sized microbubbles contributed to this biodistribution profile. Specific to the use of porphyrin, characterizing microbubble optical properties ensures efficacious porphyrin shell loading, which can influence metal:porphyrin ratios and in turn chelation efficiency, as will be discussed below. Porphyrin is also a theranostic agent of interest for microbubble multimodal imaging37, synergistic photodynamic therapy41, and sonodynamic therapy42. Characterizing its loading and fluorescence within microbubbles guides therapeutic porphyrin microbubble studies and ensures these properties are unchanged with radiolabeling to facilitate true image-guided therapy planning. Thus, it is strongly implored that "cold" labeling and fabrication of unlabeled microbubbles be conducted in parallel to radiolabeling.
This protocol leverages the strong copper chelation capabilities of porphyrins and the known self-assembly of porphyrin-lipid within microbubble shells37. Though focused on copper-64, this protocol opens the door to alternative one-pot metallochelated microbubble preparations as the broader classes of porphyrins can bind Zn, Ni, Mn, Pd, In, Lu, Cd, Sn, Ga, Co, and beyond43,44,45,46. Such porphyrin-metal chelation typically occurs at one of two steps during porphyrin supramolecular synthesis: 1) pre-insertion, where porphyrin building blocks are chelated prior to their introduction into particles, or 2) post-insertion, in which porphyrin is already assembled into a particle before conducting metal chelation33,43. When using radioisotopes, the first is associated with inefficient radioactive decay during lipid film drying, which in turn increases radiation safety risks. The latter, when applied to microbubbles, would risk particle destabilization. As such, a hybrid approach was generated in the current protocol in which the chelator was introduced during "ground-up" lipid film formation, but radiolabeling was conducted after lipid film hydration and prior to microbubble activation. This strategy allowed radiolabeling to be easily incorporated into a typical microbubble fabrication process, making it amenable to various microbubble formulations. Furthermore, this integration enabled the use of simple instrumentation and minimized the number of specialized radioactively "hot" equipment.
This hybrid approach is also distinct from existing microbubble radiolabeling protocols, which are largely divided into two predominant approaches: 1) Synthesis and radiolabeling of a chelator, which is then "spiked" into pre-formed microbubble lipid suspensions28,47, and 2) radiolabeling of a biotinylated moiety that is subsequently incubated with commercial streptavidin functionalized commercial microbubbles32,29. This latter approach bears the advantage of conserving radioisotope use. In the presented "ground-up" and reported "spiked" protocols, the radioisotope-chelator is incorporated prior to microbubble activation and thus, both target decanted microbubbles and unwanted large, foamy particle populations are labeled. This limitation means that a higher amount of radioisotope is required for "ground-up" and "spiked" approaches, half of which goes to waste. However, as described above, post-activation radiolabeling risks microbubble destabilization and, alongside previous "spiking" protocols, requires the removal of unintegrated radiolabeled chelator.
However, the "spiking" approach does open avenues to radiolabeling pre-formed microbubble lipid suspensions (for example commercial microbubbles) when lipid film generation is not feasible or desired. The current "ground-up" radiolabeling protocol can be adapted to mimic such a "spiking" approach (step 8.3). Here, a pyro-lipid film is generated and then hydrated to form a pyro-lipid suspension of vesicles. This suspension is then "spiked" into a pre-formed microbubble lipid suspension and heated/sonicated to integrate the pyro-lipid. Radiolabeling can occur either before or directly after "spiking" (but prior to activation). As can be seen in Supplementary Figure 1A, this adapted protocol labels in-house commercial microbubble formulations with "spiked" pyro-lipid chelator at compositions of 1 mol%, 10 mol%, or 30 mol% of the total lipid.
This "spiking" approach features a key limitation compared to the unmodified "ground-up" approach at focus in the current protocol: incomplete pyro-lipid integration. As can be seen on confocal imaging (Supplementary Figure 1A), pyro-lipid signal does not localize strictly in a ring-like, homogenous fashion around the microbubble shell as it is during "ground-up" incorporation. Instead, the "spiking" approach is associated with patchy areas of fluorescence visible across the microbubbles, some of which have fuzzy versus crisp fluorescent outlines. Higher non-uniform background fluorescence is also observable. Centrifugation of these "spiked" microbubbles to remove submicron species eliminates this background fluorescence and much of the patchy signal. This suggests that pyro-lipid vesicles may have been absorbed but not completely integrated into the microbubble shell, a conjecture supported by UV-Vis characterization of the "spiked" versus "ground-up" incorporation of pyro-lipid (Supplementary Figure 1B). The 704 nm Q-band associated specifically with pyro-lipid inclusion in microbubbles (at >5 mol% composition) is reduced with "spiking". Instead, a more prominent peak is observed at 674 nm. Quantification of pyro-lipid distributed to microbubble cakes versus infranatant sub-micron species demonstrates that only a quarter of the pyro-lipid is distributed to the microbubbles with "spiking". Contrarily, higher pyro-lipid amounts are incorporated into the microbubbles for "ground-up" chelator introduction. The lower pyro-lipid incorporation within "spiked" microbubbles is likely the contributing factor to their size distributions resembling microbubbles without probe vs associated "ground-up" microbubbles (Supplementary Figure 1C). The presence of unincorporated radiolabeled pyro-lipid structures can confound radioimaging or therapy results. As such, similarly to existing protocols, a "spiking" approach must be followed by a centrifugation or decant step that removes unincorporated pyro-lipid species. Taking all of the above into account, the "ground-up" radiolabeling strategy presented in the current protocol is more strongly recommended. It allows for higher chelator integration compared to "spiking" and enables customizability of the microbubble formulation, unlike pre-formed commercial microbubbles.
The "ground-up" copper chelation protocol was established through a systematic exploration of reaction parameters as previously described48. This optimization process provided insight into critical steps for producing quality microbubbles while simultaneously enabling efficient and timely copper chelation: 1) maintaining a 10% glycerol and 10% propylene glycol excipient content prior to microbubble activation, 2) ensuring the chelation temperature remains between 60-80 °C, 3) hydrating films with AA-PGG on the same day as radiolabeling (prolonged exposure of lipid suspension to acidic conditions should be refrained), 4) neutralizing lipid suspension prior to activation and 5) ensuring "cool down" steps following hydration, chelation, and activation. The porphyrin:copper ratio should be kept above 10:1. An excess of porphyrin with respect to copper is needed to maintain microbubble size, yield, stability, and chelation efficiency, as well as dual radio and fluorescence imaging capabilities since copper chelation quenches porphyrin fluorescence. The 1 mL reaction volume and 1 h chelation time yielded efficient and pure radiolabeling for 30 mol% pyro-lipid suspension compositions, corresponding to <1% of the pyro-lipid moieties in the microbubbles being labeled. Lowering the pyro-lipid compositions in lipid films to 1 mol% and 10 mol% reduced the pyro-lipid excess for the same copper-64 addition and required modification to the radiolabeling reaction. A more concentrated reaction was required, and thus, the associated microbubble films were hydrated with 0.15 mL of AA-PGG. After neutralization, PGG was added to yield a 1 mL radiolabeled lipid suspension volume prior to microbubble activation. The 1 mol% composition required longer heating (1.5-2 h) to yield ≥95% radiolabeling efficiency and purity. Thus, if radiolabeling challenges are encountered (i.e., ≤90% radiochemical purity and efficiency), a longer reaction time and lower reaction volume (i.e., more concentrated lipid suspension) can be trialed.
This protocol used iTLC and a validated centrifugal filtration technique15 to quantify radiochemical purity and radiolabeling efficiency, respectively. An in vivo readout for successful and stable copper-64 chelation is an absence of bladder signal (free copper-64 undergoes renal excretion while lipid supramolecular structures, like microbubble shells, undergo hepatobiliary/fecal excretion)15. Chelation efficacy was validated by radio-high-performance liquid chromatography (Supplementary Figure 2), which, if available, is another means of evaluating radiochemical purity and labeling efficiency. This protocol presented iTLC and centrifugal filtration as quicker and simpler methods that do not require specialty skillsets or instruments and rather use equipment that research laboratory nuclear facilities would have higher likelihood of possessing. To this end the centrifugal filtration protocol was validated for separation of free and chelated copper using 30,000 MWCO units (100,000 MWCO units can also be used). If using an alternative radioisotope/metal, the centrifuge speed, time and number of washes may need to be modified. If an alternative metal is used for which the copper-metal chelation stability is unknown or precarious, an appropriate serum stability test should also be conducted.
Beyond radiolabeling challenges, microbubble fabrication challenges may also arise. As previously introduced, microbubbles are fragile, and steps towards their fabrication require precision and careful handling, without which issues in hydration, activation, reproducibility, stability, and yield can be encountered, as depicted in Figure 3. Other factors that promote successful microbubble fabrication include 1) use of fresh, dry lipids (store dry lipids in a desiccator and protect from ice), 2) avoiding the use of lipid aliquots that have been subjected to heat (for example, through speed vacuuming), and 3) preparing fresh hydration buffer devoid of air bubbles. In circumstances where the fabrication of control microbubbles yields suitable products but not those generated following radiolabeling, additional considerations may be at play. The temperature and length of the chelation reaction may be outside of the 60-80 °C and 0-2 h timeframes for which the current protocol has been validated. Additionally, it may be feasible that the specific activity of the copper-64 or other radioisotopes may be significantly lower than that associated with the current study. This would result in a greater ratio of porphyrin species being labeled for the same level of activity (ex lower than the 10:1 porphyrin:copper excess for which this protocol was validated), which could disrupt microbubble activation.
Many of the above challenges in successful microbubble fabrication can be mitigated by first trialing the described microbubble fabrication in the absence of radiolabeling, especially if users are new to microbubble synthesis. It is recommended to first generate control unchelated microbubbles, and subsequently essay the labeling protocol with "cold" non-radioactive copper. As described above, this "cold" chelation process is vital for obtaining representative physicochemical characterization of radiolabeled microbubbles. "Cold" chelation also serves as an important first step in ensuring that any modifications made to the described protocol (for example, altered chelation temperature, volume, reaction time, porphyrin:copper ratio, radioisotope, alternative chelator) retain the desired microbubble physicochemical properties.
One such change may be the use of alternative chelators and probes. A limitation of the current protocol is its foundational use of a commercially unavailable chelator. As such, modifications are presented (section 8 of the protocol) that enable the use of commercially available chelators and fluorescent probes in lieu of pyro-lipid. Test data associated with the incorporation of DiI or DTPA-lipid as alternatives to pyro-lipid were obtained (Figure 6). DiI is a commercially available probe that has been incorporated into microbubble shells to study microbubble-FUS mechanisms and model drug agents49,50,51. To the best of our knowledge, this is the first report of a DTPA-lipid microbubble. The successful substitution of pyro-lipid with these alternative moieties speaks to the versatility of the current microbubble fabrication and labeling protocol. It can likely be applied to a broader array of probes, particularly those tethered to or which can integrate amongst phospholipids.
Though substitutable in this protocol, pyro-lipid provides the unique advantage of harboring complementary radioimaging and fluorescence tracking abilities within a single organic molecule. This multimodality can be advantageous for monitoring microbubble shell delivery and biodistribution. This is especially true when using pyro-lipid compositions ≥10 mol%. At increasing compositions, pyro-lipid within microbubble shells (and supramolecular structures in general) becomes increasingly fluorescently quenched15. Upon particle disruption, higher porphyrin compositions yield substantially stronger fluorescence signals (Supplementary Figure 3). This fluorescence unquenching can serve as an additional readout when imaging microbubble shell fate. For example, while PET facilitates quantification of absolute shell delivery/biodistribution, fluorescence imaging can capture whether such accumulation is associated with particle disruption.
However, one of the limitations of higher pyro-lipid molar compositions is the effects it has on microbubble size and yield. Replacing host lipids with increasing pyro-lipid compositions within a commercial lipid microbubble formulation was found to produce larger bubbles, lower microbubble yields, and more unstable microbubbles15. For ease of reference, these findings are summarized in Supplementary Figure 3 for the 1 mol%, 10 mol%, and 30 mol% pyro-lipid compositions for which the current radiolabeling protocol has been validated. This alteration in microbubble size with probe addition is not exclusive to pyro-lipid. For example, at a 5 mol% composition, DiI inclusion into a commercial lipid microbubble formulation reduced microbubble yield 10-fold and increased microbubble size by over 60% (Figure 6). In comparison, a 5 mol% pyro-lipid composition did not exhibit significantly different yield, volume average size, or peak volume but did increase microbubble number-averaged size by 25%15. Furthermore, increasing pyro-lipid composition from 1 mol% to 30 mol% does not significantly impact shell circulation and clearance pathways15. However, if retention of size distributions associated with probe-less or commercial formulations is desired, the current microbubble fabrication and radiolabeling protocol can be applied using a 1 mol% pyro-lipid composition. This low quantity of pyro-lipid inclusion does not significantly change any sizing or yield parameters associated with baseline probe-less formulations (Supplementary Figure 3). It does, however, come at the expense of losing strong activatable fluorescence capabilities associated with higher pyro-lipid compositions. A compromising solution could be the selection of a 10 mol% pyro-lipid composition. Overall, given that pyro-lipid compositions as low as 1 mol% were amenable to the current radiolabeling protocol, a large degree of modularity exists from which microbubble sizing, yield, and fluorescence capabilities can be tailored.
Overall, the overarching adaptability of the current radiolabeling protocol can enable the multitude of radiolabeled microbubble applications overviewed in the Introduction. Perhaps the most relevant to the current landscape of the microbubble-FUS field is the radiotracing of microbubble shell fate for image-guided drug delivery platform design. This application of radiolabeled microbubbles was explored in a recent study15. The current radiolabeling protocol was applied to generate a series of multimodal copper-64 labeled porphyrin microbubbles with varying acyl chain length and charge, representing the most widely studied lipid microbubble compositions in clinical and preclinical literature. The in vivo gas dissolution, shell clearance, biodistribution, and disassembly kinetics were monitored in healthy and tumor-bearing mice via ultrasound, PET, and fluorescence imaging, yielding a first-of-its-kind longitudinal and systematic lipid microbubble pharmacokinetic study. Key findings included: 1) Microbubble cores dissolve over minutes where increasing microbubble lipid chain length led to slower dissolution of neutral microbubbles and faster dissolution of anionic microbubbles (most similar to commercial formulations); 2) The remnant shells circulated for over 24 h in the blood and were cleared through a hepatobiliary/splenic/fecal pathway; 3) Such clearance was dependent on microbubble composition such that shorter chain length shells underwent higher hepatic processing while longer chain length shells exhibited higher splenic uptake; 4) Microbubble shells underwent preferential accumulation in tumors compared to surrounding tissue as early as the first PET time-point following injection (1 h), with C18 neutral shelled microbubbles exhibiting the highest passive and FUS-enhanced uptake (maximum enhancement at 3.5 h post-treatment) despite facilitating similar levels of FUS-mediated vessel opening (as determined by co-administration of Evans blue); 5) In general, FUS enhancements in tumor shell delivery were modest and not equal amongst all microbubble formulations, demonstrating that FUS-enhanced cargo-loaded shell delivery cannot be achieved ubiquitously across diverse microbubbles and may require higher FUS pressures; 6) microbubble shells within the tumor, liver and spleen were predominantly found in the extravascular space; and 7) shorter chain length shells underwent faster disassembly, wherein the tumor featured the highest rates of shell fragment disassembly. These findings clarified conventional wisdom within the microbubble-FUS field and overturned certain assumptions surrounding optimal microbubble designs for shell-based tumor delivery. Key initial guidance included the use of C18 lipids to formulate drug-loaded microbubbles for splenic targets, C16 lipids for hepatic targets, neutral lengthier chains for ultrasound contrast imaging and longer blood circulation, neutral C18 lipids for tumor targets, contraindicated use of drugs with hepatosplenic toxicity within all-in-one shell-loaded lipid microbubbles and application of such systems for tumors with low baseline vascular permeability. This study thus initiated structure-activity relationships that could inform more optimal microbubble design and provided a blueprint for further explorations, all of which were enabled by the current radiolabeling protocol. This capacity was furthered by using the obtained microbubble pharmacokinetic dataset to build an adapted deep learning tool for automatic PET/CT organ segmentation, allowing for more efficient pharmacokinetic data analysis.52
The focus of the current protocol was placed on the radiolabeling of lipid microbubbles. However, in the context of cargo-loaded ultrasound agent designs, one would be remiss to overlook nanodroplets and polymeric microbubbles. Nanodroplets are phase-changing systems that consist of a liquid perfluorocarbon core encapsulated by lipid, protein, or polymer shells. Under higher-intensity FUS, these nanodroplets are converted into microbubbles through acoustic vaporization of the liquid core. This mechanism of FUS activity and smaller size of nanodroplets bear potential advantages for shell-loaded delivery: 1) longer stability in vivo, 2) greater permeability in tissue and higher tumor delivery, 3) facility for both vascular and extravascular activity, and 4) rapid drug release following acoustic vaporization50,53,54. Thus, radiolabeling of cargo-loaded nanodroplets would also be beneficial for radiotracking and image-guided treatment planning in future work. The current radiolabeling protocol could be incorporated readily into nanodroplets fabricated via the condensation method, which has previously been shown to allow porphyrin-lipid loading within nanodroplet shells35.
Polymeric microbubbles are also thought to exhibit shell-loaded drug delivery advantages over lipid microbubbles due to their stiff and modular shells yielding higher putative stability to drug loading, cargo encapsulation, and drug loading tunability through polymer shell thickness and material composition modulation55,56,57. Due to their stability, polymeric microbubbles may not encounter the same radiolabeling limitations as lipid microbubbles, which the current protocol was designed to overcome. However, the current protocol can still be used to inform polymeric microbubble radiolabeling on two accounts: 1) microbubble characterization and 2) chelation efficiency. Polymeric microbubble radiolabeling is limited in the study but typically involves functionalizing the surface of polymeric shells with chelators (for example, DOTA and NOTA), followed by isotope addition, heating, and washing to remove free isotope27,58. Similarly to existing lipid microbubble radiolabeling protocols, these reports do not characterize microbubble physicochemical properties post-labeling. Thus, the current protocol can be used as a blueprint to standardize the use of "cold" labeling and characterization when radiolabeling any type of microbubble shell. Furthermore, there is room for improvement in polymeric microbubble radiolabeling yields (reported range of 42%-85% for chelator-grafted microbubbles58). The use of porphyrin as a strong and efficient radioisotope chelator in this study may be translated in future work by adapting existing porphyrin-polymer shell conjugation techniques59 prior to copper chelation. Overall, polymeric microbubbles are not as popular as lipid microbubbles. Lipid microbubbles are the only shell variant currently clinically approved for human use, making them the material of choice for more rapid translation of therapeutic microbubble-FUS platforms. Furthermore, the few head-to-head polymer versus lipid-shelled therapeutic FUS comparisons suggest lipid shells facilitate greater vessel permeabilization, more rapid FUS-triggered drug release, stronger tissue ablation, and faster temperature elevations in high-intensity FUS paradigms60,61,62. Collectively, lipid microbubbles are thus more widely studied for therapeutic-FUS applications than other shell variants. Accordingly, the focus of the current radiolabeling protocol on lipid microbubbles aligns with that of the broader microbubble-FUS field.
Beyond informing microbubble-FUS mechanisms and cargo-loaded microbubble designs, radiolabeled microbubbles also have proposed utility for radiotherapy27. Interest in combining FUS and radiotheranostic activity within a single agent is built on evidence that microbubble-FUS acts synergistically with radiotherapy to strengthen radiotherapy antineoplastic response16. The current radiolabeling protocol could be adapted to employ copper-67 (more suitable for radiotherapy63) instead of copper-64 to generate such a radiotheranostic microbubble. However, the pharmacokinetic study completed on the foundation of the current lipid microbubble radiolabeling strategy demonstrated high hepatosplenic accumulation of the microbubble shells15. This off-target accumulation is an important consideration if radiolabeled microbubbles were applied as dual FUS/radiotherapy agents.
In this context, hepatosplenic toxicity mitigation strategies would be needed. For example, as thoroughly reviewed by Navarro-Becerra and Borden64, numerous authors have functionalized lipid microbubbles with tumor-targeting ligands (for example, VEGFR2 mAb, RGD, folate) through biotin/avidin coupling, and electrostatic absorption. This biofunctionalization increases shell-loaded cargo delivery to tumors, decreases off-target tissue accumulation, and enhances tumor therapy32,65,66. As functionalization is typically electrostatic in nature, the current radiolabeling and microbubble fabrication protocol could be used as-is prior to ligand absorption. Adjustments to the radiolabeling protocol may be needed to accommodate any heat-sensitive peptide and protein ligands that must be incorporated covalently (for example, PEG-lipid conjugated peptides). In these cases, the chelation reaction time can be extended while lowering the reaction temperature to 37 °C, an approach that has yielded efficient porphyrin-copper chelation within lipoprotein nanoparticles while preserving targeting capabilities34. However, it is unlikely that the addition of targeting ligands to the surface of radiolabeled microbubbles would completely alleviate hepatosplenic toxicity concerns associated with higher-dose copper-67 microbubble radiotherapy. For example, functionalizing microbubbles with p-selectin antibodies doubled their tumor delivery in mice and decreased liver accumulation by 4x, yet liver accumulation was still at a substantial 9% ID/g (twice as high as tumor accumulation) 1 h post-injection32. A more promising alternative to targeting ligands may be locoregional delivery of radiotherapeutic microbubbles through intratumoral injection. Though an atypical means of administering microbubbles, intratumoral injection of microbubbles has been shown by Bismuth et al.39 to achieve strong tumor ablation (50% tissue perforation) with a single 60 s (MI 0.9) FUS treatment. One would anticipate even stronger tumor ablation if said microbubbles had additional radiotherapeutic capabilities. To this end, future work may also benefit from using oxygen cores in radiolabeled microbubbles to further enhance microbubble-FUS/radiotherapy synergy by alleviating tumor hypoxia67,68. Prior to their application as dual FUS/radiotherapeutic agents, intratumorally delivered radiolabeled microbubbles should be pharmacokinetically assessed to ensure that locoregional delivery does not cause systemic leakage. This highlights the intrinsic importance of radiotracing across all proposed applications of radiolabeled microbubbles made feasible by the current study.
In summary, the current protocol delivers advances in microbubble radiolabeling. Its collective advantages include enhanced "ground-up" chelator incorporation, high chelation efficiency, absence of post-labeling free radioisotope or chelator purification, preservation of microbubble physicochemical properties, versatile application across diverse microbubble formulations, adaptability to alternative chelators and fluorophores and customization of chelator composition and associated multimodality and particle size. Ultimately, it yields tailored radio and/or fluorescently active microbubbles that can advance mechanistic insights and theranostic capabilities of microbubble-FUS. These applications can include the acquisition of representative, quantitative pharmacokinetic data, broadening microbubble multimodal imaging capabilities, facilitating image-guided therapy optimization, and enabling synergistic microbubble-FUS radio (and/or porphyrin) therapies.
Disclosures
The authors report no conflicts of interest.
Acknowledgements
We thank Deborah Scollard and Teesha Komal (University Health Network Spatio-Temporal Targeting and Amplification of Radiation Response (STTARR) program, Toronto, Ontario) for their technical services and guidance. We also thank Mark Zheng and Dr. Alex Dhaliwal for their technical assistance during confocal microscopy and the Advanced Optical Microscopy Facility (Toronto, Ontario) for providing associated equipment. We acknowledge our funding sources: the Canadian Institutes of Health Research, the Terry Fox Research Institute, the Natural Sciences and Engineering Research Council of Canada, the Canada Foundation for Innovation, the Princess Margaret Cancer Foundation, Canada Research Chairs Program, the McLaughlin Centre, the Vanier Scholarship Program, the Ontario Graduate Student Scholarship Program, Prostate Cancer Canada, and the Peterborough K. M. Hunter Charitable Foundation.
Materials
| Name | Company | Catalog Number | Comments |
| 64CuCl2 | Washington University School of Medicine, Mallinckrodt Institute of Radiology | N/A | Order in small volume (<10 µL) dissolved in 0.1 N HCl |
| Acetic acid | Any company | ≥ 95% purity | |
| Aluminum foil | Any company | ||
| Ammonium acetate | Any company | Purity: ≥ 98% | |
| Balance - analytical | Any company | Able to measure down to 0.1 mg | |
| Bath sonicator | Any company | Can be heated to 69 oC | |
| CC aperture - 30 micron | Beckman Coulter | A36391 | Particle diameter range: 0.6-18 um |
| CC electrolyte | Beckman Coulter | 8546719 | Isoton II diluent |
| CC Software | Beckman Coulter | Multisizer 4e | |
| Centrifuge filter units (0.5 mL 30,000 MWCO) with compatible microcentrifuge tubes | MilliporeSigma | UFC503096 | Amicon Ultra - 0.5 mL |
| Centrifuge tubes - 15 mL with caps | Any company | ||
| Chloroform | Any company | Purity: ≥ 99.8% | |
| Coulter counter | Beckman Coulter | B43905 | Multisizer 4e Coulter Counter |
| Cover slips | VWR | 48393081 | VWR micro cover glass |
| CuCl2 | Any company | Ensure not oxidized | |
| CuCl2 | |||
| Cuvette- quarts, 1 cm path length | Any company | ||
| Cuvettes - 10 mL plastic for CC measurements | Beckman Coulter | A35471 | Coulter Counter Accuvette ST |
| ddH2O | Any company | Can be obtained through an ultrapure water purification system | |
| DiI (1,1'-Dioctadecyl-3,3,3',3'-Tetramethylindocarbocyanine Perchlorate) | Any company | Powder form | |
| Dose calibrator | Any company | Able to read copper-64 | |
| DPPA (1,2-dipalmitoyl-sn-glycero-3-phosphate (sodium salt)) | Avanti Polar Lipids | 830855P | Powder form |
| DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine) | Avanti Polar Lipids | 850355P | Powder form |
| DPPE-MPEG (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-5000] (ammonium salt)) | Avanti Polar Lipids | 880200P | Powder form |
| DTPA-lipid (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (ammonium salt)) | Avanti Polar Lipids | 790106P | Powder form |
| EDTA (Ethylenediaminetetraacetic acid) | Any company | ||
| Gamma counter | Any company | Able to read copper-64 | |
| Gamma counting tube push caps | Globe Scientific | 22-171-665 | Flanged plug caps for 12 mm tubes |
| Gamma counting tubes | Sarstedt | 55.1579 | 5 mL, 75 x 12 mm, PS |
| Glass beaker - 250 mL | Any company | Able to withstand temperatures up to 100 oC | |
| Glass drying oven | Any company | Can be heated to 80 oC | |
| Glass microliter syringes - 25, 50, 100, 1000 µL | Any company | Compatible with organic solvents | |
| Glass scintillation vials - 20 mL | VWR | 66022-081 | VWR® Scintillation Vials, Borosilicate Glass, with Screw Caps, With pulp foil liner |
| Glass vials - 0.5 dram | VWR | 66011-020 | VWR Vial 1/2 dram, with black phenolic screw cap and polyvinyl-faced pulp liner |
| Glycerol | Sigma Aldrich | G7757-1L | Purity: ≥ 99.0% |
| Graduated pipette/gun | Any company | ||
| Hot/stir plate | Equipped with temperature prob for automatic tempearture control | ||
| Hydrochloric acid - 0.1 N | Any company | ||
| iTLC plates | Agilent | A120B12 | iTLC-SA chromatography paper |
| Laboratory tissues | Any company | ||
| Media vaccuum filtration unit | Any company | 0.22 micron pore size, PES membrane, 500 mL funnel capacity | |
| Methanol | Any company | Purity: ≥ 99.8%, HPLC grade, meets ACS specifications | |
| Microcentrifuge tubes non sterile - 1.5 mL | Any company | ||
| Microcentrifuge tubes sterile - 1.5 mL | Any company | ||
| Micropipetes - p1000, p200, p20, p10 | Any company | Ensure are calibrated | |
| Microscope slides | Fisher Scientific | 12-550-15 | Superfrost Plus Microscope Slides Precleaned |
| Needles - 18 G | Sterile | ||
| Parafilm | Any company | ||
| PBS | Sigma Aldrich | D8537-500ML | DPBS, modified, without calcium chloride and magnesium chloride, liquid, sterile-filtered, suitable for cell culture |
| PFP | FluoroMed | APF-N40HP | Purity: ≥ 99.8% |
| PFP line | Any company | 1/4 inch diameter plastic hose cut about 50 cm in length | |
| PFP regulator | Swagelok | SS-1RF4 and SS-4HC-1-4 | |
| pH meter | Any company | ||
| pH standards 4 and 7 | Any company | ||
| Pipette tips for p1000, p200, p10 - non sterile | Any company | ||
| Pipette tips for p1000, p200, p10 - sterile | Any company | ||
| Plastic syringe - 1 mL | Any company | Sterile | |
| Propylene glycol | BioShop | PRO888.500 | Purity: ≥ 99.5% |
| Pyro-lipid | N/A | Made in-house | |
| Rubber tipped forceps | Any company | Mix of fine-tipped and flat/square edges recommended | |
| Scissors | Any company | ||
| Sodium hydroxide - 1 N | Any company | ||
| Sodium hydroxide - 10 N | Any company | ||
| Spectrofluorometer | Any company | Capable of 410 nm excitation and 600-850 nm emission | |
| Spectrofluorometry software | Horiba | FluorEssence | |
| Spectrometer | Any company | ||
| Syringe - 1 mL | Any company | Disposible, plastic, sterile | |
| Syringe filters - 0.2 micron pore size | Any company | Membrane material: PES or other compatible with ammonium acetate/acetic acid and PBS | |
| Test tube - 10 mL | |||
| Triton X-100 | Any company | ||
| Vacuum desicator/vacuum | Any company | ||
| Vialmix | Lantheus Medical Imaging | 515030-0508 | Referred to in protocol as a mechanical vial shaker |
| Weigh paper | Any company | To avoid losing product, cutting weigh paper into 3x3 cm squares is recommended |
References
- Itani, M., Mattrey, R. F. The effect of inhaled gases on ultrasound contrast agent longevity in vivo. Mol Imaging Biol. 14 (1), 40-46 (2012).
- Kong, W. T., Wang, W. P., Huang, B. J., Ding, H., Mao, F. Value of wash-in and wash-out time in the diagnosis between hepatocellular carcinoma and other hepatic nodules with similar vascular pattern on contrast-enhanced ultrasound. J Gastroenterol Hepatol. 29 (3), 576-580 (2014).
- Wilson, S. R., Burns, P. N. Microbubble-enhanced us in body imaging: What role. Radiology. 257 (1), 24-39 (2010).
- Hynynen, K., Mcdannold, N., Vykhodtseva, N., Jolesz, F. Noninvasive mr imaging-guided focal opening of the blood-brain barrier bbb in rabbits. Radiology. 220 (3), 640-646 (2001).
- He, W., et al. Enhanced ablation of high intensity focused ultrasound with microbubbles: An experimental study on rabbit hepatic vx2 tumors. Cardiovasc Intervent Radiol. 34 (5), 1050-1057 (2011).
- Lipsman, N., et al. Blood-brain barrier opening in alzheimer's disease using mr-guided focused ultrasound. Nat Commun. 9 (1), 2336 (2018).
- Burke, C. W., Klibanov, A. L., Sheehan, J. P., Price, R. J. Inhibition of glioma growth by microbubble activation in a subcutaneous model using low duty cycle ultrasound without significant heating. J Neurosurg. 114 (6), 1654-1661 (2011).
- Dimcevski, G., et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J Control Release. 243, 172-181 (2016).
- Haram, M., Hansen, R., Bouget, D., Myhre, O. F., Davies, C. L., Hofsli, E. Treatment of liver metastases with focused ultrasound and microbubbles in patients with colorectal cancer receiving chemotherapy. Ultrasound Med Biol. 49 (9), 2081-2088 (2023).
- Mainprize, T., et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: A clinical safety and feasibility study. Sci Rep. 9 (1), 321 (2019).
- Karakatsani, M. E., et al. Focused ultrasound mitigates pathology and improves spatial memory in alzheimer's mice and patients. Theranostics. 13 (12), 4102-4120 (2023).
- Aron, M., Vince, O., Gray, M., Mannaris, C., Stride, E. Investigating the role of lipid transfer in microbubble-mediated drug delivery. Langmuir. 35 (40), 13205-13215 (2019).
- Wang, S., Samiotaki, G., Olumolade, O., Feshitan, J. A., Konofagou, E. E. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol. 40 (1), 130-137 (2014).
- Huynh, E., Rajora, M. A., Zheng, G. Multimodal micro, nano, and size conversion ultrasound agents for imaging and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 8 (6), 796-813 (2016).
- Rajora, M. A., et al. Quantitative pharmacokinetics reveal impact of lipid composition on microbubble and nanoprogeny shell fate. Adv Sci. 11 (4), 2304453 (2024).
- Leong, K. X., Sharma, D., Czarnota, G. J. Focused ultrasound and ultrasound stimulated microbubbles in radiotherapy enhancement for cancer treatment. Technol Cancer Res Treat. 22, 1-9 (2023).
- Li, Y., et al. Ultrasound-triggered release of sinoporphyrin sodium from liposome-microbubble complexes and its enhanced sonodynamic toxicity in breast cancer. Nano Research. 11, 1038-1056 (2017).
- Nomikou, N., Fowley, C., Byrne, N. M., Mccaughan, B., Mchale, A. P., Callan, J. F. Microbubble-sonosensitiser conjugates as therapeutics in sonodynamic therapy. Chem Commun. 48 (67), 8332-8334 (2012).
- Chakravarty, R., Hong, H., Cai, W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Mol Pharm. 11 (11), 3777-3797 (2014).
- Delalande, A., Leduc, C., Midoux, P., Postema, M., Pichon, C. Efficient gene delivery by sonoporation is associated with microbubble entry into cells and the clathrin-dependent endocytosis pathway. Ultrasound Med Biol. 41 (7), 1913-1926 (2015).
- Sheikov, N., Mcdannold, N., Vykhodtseva, N., Jolesz, F., Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 30 (7), 979-989 (2004).
- Conway, G. E., Paranjape, A. N., Chen, X., Villanueva, F. S. Development of an in vitro model to study mechanisms of ultrasound-targeted microbubble cavitation-mediated blood-brain barrier opening. Ultrasound Med Biol. 50 (3), 425-433 (2024).
- Hu, S., Zhang, X., Unger, M., Patties, I., Melzer, A., Landgraf, L. Focused ultrasound-induced cavitation sensitizes cancer cells to radiation therapy and hyperthermia. Cells. 9 (12), 2595 (2020).
- Zhao, C., et al. Synergistically augmenting cancer immunotherapy by physical manipulation of pyroptosis induction. Phenomics. , (2024).
- Tachibana, K., Uchida, T., Ogawa, K., Yamashita, N., Tamura, K. Induction of cell-membrane porosity by ultrasound. Lancet. 353 (9162), 1409 (1999).
- Arif, W. M., et al. Focused ultrasound for opening blood-brain barrier and drug delivery monitored with positron emission tomography. J Control Release. 324, 303-316 (2020).
- Da Ros, V., et al. PVA-microbubbles as a radioembolization platform: Formulation and the in vitro proof of concept. Pharmaceutics. 15 (1), 217 (2023).
- Tartis, M. S., et al. Dynamic micropet imaging of ultrasound contrast agents and lipid delivery. J Control Release. 131 (3), 160-166 (2008).
- Willmann, J. K., et al. Targeted microbubbles for imaging tumor angiogenesis: Assessment of whole-body biodistribution with dynamic micro-pet in mice. Radiology. 249 (1), 212-219 (2008).
- Ingram, N., et al. Ultrasound-triggered therapeutic microbubbles enhance the efficacy of cytotoxic drugs by increasing circulation and tumor drug accumulation and limiting bioavailability and toxicity in normal tissues. Theranostics. 10 (24), 10973-10992 (2020).
- Hernández-Gil, J., et al. Development of 68ga-labelled ultrasound microbubbles for whole-body pet imaging. Chem Sci. 10 (215), 5603-5615 (2019).
- Warram, J. M., et al. Biodistribution of p-selectin targeted microbubbles. J Drug Target. 22 (5), 387-394 (2014).
- Liu, T. W., Macdonald, T. D., Shi, J., Wilson, B. C., Zheng, G. Intrinsically copper-64-labeled organic nanoparticles as radiotracers. Angew Chem Int Ed Engl. 51 (52), 13128-13131 (2012).
- Rajora, M. A., et al. Tailored theranostic apolipoprotein e3 porphyrin-lipid nanoparticles target glioblastoma. Chem Sci. 8 (8), 5371-5384 (2017).
- Yoo, K., Dhaliwal, A., Chen, J., Sheeran, P. S., Zheng, G. Synthesis and characterization of multi-modal phase-change porphyrin droplets. J Vis Exp. 176, e62665 (2021).
- Lovell, J. F., et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater. 10 (4), 324-332 (2011).
- Huynh, E., Jin, C. S., Wilson, B. C., Zheng, G. Aggregate enhanced trimodal porphyrin shell microbubbles for ultrasound, photoacoustic, and fluorescence imaging. Bioconjug Chem. 25 (4), 796-801 (2014).
- Mcmahon, D., Hynynen, K. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics. 7 (16), 3989-4000 (2017).
- Bismuth, M., Katz, S., Rosenblatt, H., Twito, M., Aronovich, R., Ilovitsh, T. Acoustically detonated microbubbles coupled with low frequency insonation: Multiparameter evaluation of low energy mechanical ablation. Bioconjug Chem. 33 (6), 1069-1079 (2022).
- Kutscher, H. L., et al. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release. 143 (1), 31-37 (2010).
- You, Y., et al. Porphyrin-grafted lipid microbubbles for the enhanced efficacy of photodynamic therapy in prostate cancer through ultrasound-controlled in situ accumulation. Theranostics. 8 (6), 1665-1677 (2018).
- Chen, X., Qin, B., Whitehurst, D., Helfield, B., Lavery, L., Villanueva, F. S. Sonodynamic therapy using protoporphyrin ix encapsulated microbubbles inhibits tumor growth. 2017 IEEE International Ultrasonics Symposium. , 1-4 (2017).
- Cheng, M. H. Y., et al. Targeted theranostic 111in/lu-nanotexaphyrin for spect imaging and photodynamic therapy. Mol Pharm. 19 (6), 1803-1813 (2022).
- Cheng, M. H. Y., Cevallos, A., Rajora, M. A., Zheng, G. Fast, facile, base-free microwave-assisted metallation of bacteriochlorophylls and corresponding high yield synthesis of tookad. J Porphyrins Phthalocyanines. 25 (07n08), 703-713 (2021).
- Macdonald, T. D., Liu, T. W., Zheng, G. An MRI-sensitive, non-photobleachable porphysome photothermal agent. Angew Chem Int Ed Engl. 53 (27), 6956-6959 (2014).
- Shao, S., et al. Functionalization of cobalt porphyrin-phospholipid bilayers with his-tagged ligands and antigens. Nat Chem. 7 (5), 438-446 (2015).
- Teh, J. H., et al. A kit-based aluminium-[(18)f]fluoride approach to radiolabelled microbubbles. Chem Commun (Camb). 57 (88), 11677-11680 (2021).
- Rajora, M. A. . Supramolecular Structure-Enabled Delivery of Porphyrin to Glioblastomas Beyond the Blood-Brain Barrier [Doctor of Philosophy thesis]. , (2024).
- Carugo, D., et al. Modulation of the molecular arrangement in artificial and biological membranes by phospholipid-shelled microbubbles. Biomaterials. 113, 105-117 (2017).
- Dhaliwal, A. . Utilizing Porphyrin to Improve Ultrasound-Activated Supramolecular Agent Design for Solid Tumor Delivery [Doctor of Philosophy thesis]. , (2023).
- Kilroy, J. P., Klibanov, A. L., Wamhoff, B. R., Bowles, D. K., Hossack, J. A. Localized in vivo model drug delivery with intravascular ultrasound and microbubbles. Ultrasound Med Biol. 40 (10), 2458-2467 (2014).
- Dhaliwal, A., et al. Deep learning for automatic organ and tumor segmentation in nanomedicine pharmacokinetics. Theranostics. 14 (3), 973-987 (2024).
- Shakya, G., Cattaneo, M., Guerriero, G., Prasanna, A., Fiorini, S., Supponen, O. Ultrasound-responsive microbubbles and nanodroplets: A pathway to targeted drug delivery. Adv Drug Deliv Rev. 206, 115178 (2024).
- Honari, A., Merillat, D. A., Bellary, A., Ghaderi, M., Sirsi, S. R. Improving release of liposome-encapsulated drugs with focused ultrasound and vaporizable droplet-liposome nanoclusters. Pharmaceutics. 13 (5), 609 (2021).
- Barmin, R. A., et al. Polymeric materials for ultrasound imaging and therapy. Chem Sci. 14 (43), 11941-11954 (2023).
- Chen, Y., Liang, Y., Jiang, P., Li, F., Yu, B., Yan, F. Lipid/PLGA hybrid microbubbles as a versatile platform for noninvasive image-guided targeted drug delivery. ACS Appl Mater Interfaces. 11 (45), 41842-41852 (2019).
- Barmin, R. A., et al. Engineering the acoustic response and drug loading capacity of pbca-based polymeric microbubbles with surfactants. Mol Pharm. 19 (9), 3256-3266 (2022).
- Barrefelt, A. A., et al. Multimodality imaging using SPECT/CT and MRI and ligand functionalized 99mTc-labeled magnetic microbubbles. EJNMMI Res. 3 (1), 12 (2013).
- Song, R., Hu, D., Chung, H. Y., Sheng, Z., Yao, S. Lipid-polymer bilaminar oxygen nanobubbles for enhanced photodynamic therapy of cancer. ACS Appl Mater Interfaces. 10 (43), 36805-36813 (2018).
- Böhmer, M. R., Chlon, C. H. T., Raju, B. I., Chin, C. T., Shevchenko, T., Klibanov, A. L. Focused ultrasound and microbubbles for enhanced extravasation. J Control Release. 148 (1), 18-24 (2010).
- Zhang, S., et al. Compare ultrasound-mediated heating and cavitation between flowing polymer- and lipid-shelled microbubbles during focused ultrasound exposures. J Acoust Soc Am. 131 (6), 4845-4855 (2012).
- Cao, Y., et al. Drug release from phase-changeable nanodroplets triggered by low-intensity focused ultrasound. Theranostics. 8 (5), 1327-1339 (2018).
- Krasnovskaya, O. O., et al. Recent advances in (64)cu/(67)cu-based radiopharmaceuticals. Int J Mol Sci. 24 (11), 9154 (2023).
- Navarro-Becerra, J. A., Borden, M. A. Targeted microbubbles for drug, gene, and cell delivery in therapy and immunotherapy. Pharmaceutics. 15 (6), 1625 (2023).
- Fan, C. -. H., et al. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 34 (8), 2142-2155 (2013).
- Luo, T., et al. Ultrasound-mediated destruction of oxygen and paclitaxel loaded dual-targeting microbubbles for intraperitoneal treatment of ovarian cancer xenografts. Cancer Lett. 391, 1-11 (2017).
- Fix, S. M., et al. Oxygen microbubbles improve radiotherapy tumor control in a rat fibrosarcoma model - a preliminary study. PLoS One. 13 (4), e0195667 (2018).
- Eisenbrey, J. R., et al. Sensitization of hypoxic tumors to radiation therapy using ultrasound-sensitive oxygen microbubbles. Int J Radiat Oncol Biol Phys. 101 (1), 88-96 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved