Assembly of a Reflux System for Heated Chemical Reactions
Обзор
Source: Laboratory of Dr. Philip Miller — Imperial College London
Many chemical experiments require elevated temperatures before any reaction is observed, however heating solutions of reactants can lead to loss of reactants and/or solvent via evaporation if their boiling points are sufficiently low. In order to ensure no loss of reactants or solvent, a reflux system is used in order to condense any vapors produced on heating and return these condensates to the reaction vessel.
Принципы
A reflux system is normally operated using a vertical connection of a water-cooled-glass column (reflux condenser) to the outlet of the reaction vessel. This piece of glassware consists of a jacketed column with water inlet and outlet ports that allow cold tap water to flow through the outer jacket, while reaction vapors are forced through the inner column. The flowing cold water ensures these vapors are condensed on the walls of the inner column and gravity will return these condensates to the reaction vessel. Once a steady reflux has been reached a constant drip of fluid back to the reaction solution should be established. In this fashion, reactions can be left indefinitely without ever needing more solvent to be added. This video will explain the process of connecting the glassware and establishing a steady reflux.
Процедура
1. Preparation of Glassware
- Ensure the glassware is free of chemical contaminants and if necessary of moisture by allowing to heat in an oven at 100 °C for approximately 30 min.
- Once cooled, ensure all ground glass joints are contaminant free using either a dry or acetone soaked cleaning tissue specially made for laboratory applications.
- After dissolving regents in a suitable solvent in an appropriate reaction vessel (typically a round-bottomed flask) and adding a magnetic stirrer bar, connect the reflux condenser and reaction vessel via the ground glass joint. Attach a Keck clip to the joint.
- Attach tubing from a cold-water tap to the bottom-most inlet of the reflux condenser and another piece of tubing from the top-most outlet to a sink. Start flowing cold water through the outer jacket of the condenser at such a rate that the sink does not overfill.
2. Heating of Reactants
- Using a hotplate stirrer, submerge the reaction vessel into a heating bath (oil or water) until the level of the bath is just above the level of the solution within the vessel and hold in place using a ring stand, clamp, and boss.
- Start stirring the reaction. Heat the bath to about 15 °C over the boiling point of the solvent. Once equilibrium between evaporation and condensation has been reached a steady drip of condensed solvent will start falling back into the reaction vessel from the condenser column.
3. Dismantling the Apparatus
- Once the desired time has passed remove the reaction vessel/reflux apparatus from the bath by clamping it higher up the ring stand.
- Allow the water to continue flowing through the reflux condenser until the solution has reached room temperature.
- Turn off the flow of water and disconnect the condenser from the reaction vessel.
- Empty any remaining water in the condenser into the sink and remove tubing from inlet and outlet.
Результаты
The outcome can be observed after spectroscopic characterization of the resultant solution, as the two reagents should now have reacted to form a new product. Typically, various purification strategies will be required to separate the desired product from undesired side reactions.
In this example, a transesterification reaction between dimethyl terephthalate (DMT) and ethylene glycol has occurred to afford bis(2-hydroxyethyl) terephthalate and methanol (Scheme 1). The refluxing solvent will be the methanol that is being produced (b.p. 65 °C). After heating the starting material (Figure 1) under reflux for 45 min, nuclear magnetic resonance (NMR) spectroscopy can be used to ensure product formation, as shown in Figure 2.

Scheme 1. Transesterification reaction between dimethyl terephthalate and ethylene glycol.
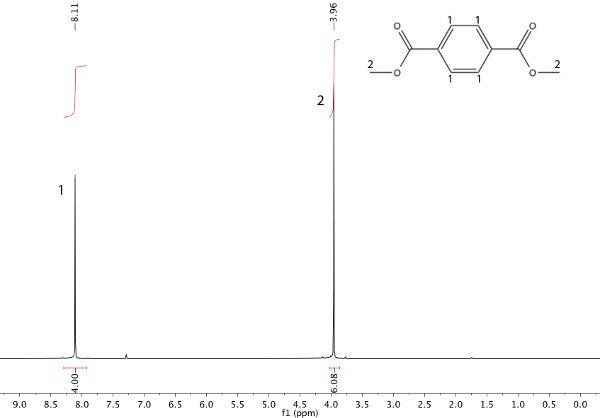
Figure 1. 1H NMR spectrum of starting material: dimethyl terephthalate (DMT).
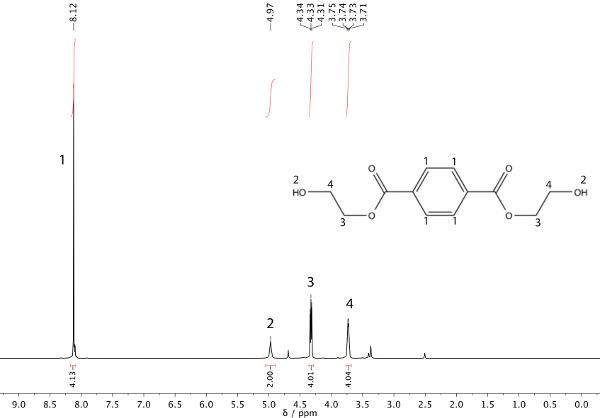
Figure 2. 1H NMR spectrum of product: bis(2-hydroxyethyl) terephthalate.
Заявка и Краткое содержание
Performing reactions under reflux is an important technique to understand. Apart from providing a system whereby solvent and volatile reagents are recycled, it also allows fine control of reaction temperature, as this will be held constant at the boiling point of the chosen solvent. By careful choice of solvent, one can control the temperature within a very narrow range.
More advanced techniques can utilize refluxing solvents to perform sophisticated purification techniques such as Soxhlet extractions or fractional distillation. The later of which is used industrially on a huge scale, for instance in petroleum refineries in order to separate crude oil into various petrol fractions of different boiling points.
Теги
Перейти к...
Видео из этой коллекции:

Now Playing
Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
168.6K Просмотры

Введение в катализ
Organic Chemistry
34.7K Просмотры

Conducting Reactions Below Room Temperature
Organic Chemistry
70.8K Просмотры

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.7K Просмотры

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
56.4K Просмотры

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.5K Просмотры

Purifying Compounds by Recrystallization
Organic Chemistry
710.9K Просмотры

Separation of Mixtures via Precipitation
Organic Chemistry
158.2K Просмотры

Solid-Liquid Extraction
Organic Chemistry
238.4K Просмотры

Rotary Evaporation to Remove Solvent
Organic Chemistry
213.0K Просмотры

Fractional Distillation
Organic Chemistry
335.1K Просмотры

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.9K Просмотры

Performing 1D Thin Layer Chromatography
Organic Chemistry
290.3K Просмотры

Column Chromatography
Organic Chemistry
361.5K Просмотры

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
249.2K Просмотры
Авторские права © 2025 MyJoVE Corporation. Все права защищены