Determining the Solubility Rules of Ionic Compounds
Обзор
Source: Laboratory of Dr. Neal Abrams — SUNY College of Environmental Science and Forestry
An ionic compound's solubility can be determined via qualitative analysis. Qualitative analysis is a branch of analytical chemistry that uses chemical properties and reactions to identify the cation or anion present in a chemical compound. While the chemical reactions rely on known solubility rules, those same rules can be determined by identifying the products that form. Qualitative analysis is not typically done in modern industrial chemistry labs, but it can be used easily in the field without the need of sophisticated instrumentation. Qualitative analysis also focuses on understanding ionic and net ionic reactions as well as organizing data into a flow chart to explain observations and make definitive conclusions.
Many cations have similar chemical properties, as do the anion counterparts. Correct identification requires careful separation and analysis to systematically identify the ions present in a solution. It is important to understand acid/base properties, ionic equilibria, redox reactions, and pH properties to identify ions successfully.
While there is a qualitative test for virtually every elemental and polyatomic ion, the identification process typically begins with knowing a "class" of ions being analyzed; cations or anions, elemental or polyatomic, groups or periods, transition or main group. In this experiment, both types of ions, cations and anions, are identified. The cations include polyatomic ions as well.
Принципы
Identifying cations and anions is based on known chemical reactions between the unknown ion and given reactant. Sometimes, it may be the lack of a reaction that positively identifies the ion as well. All ionic compounds are composed of a cation and an anion, and when a reaction occurs between two different ionic compounds, the cation of one compound is electrostatically attracted to the anion of another, forming a new ionic compound. (NOTE: Some unique ionic compounds have one or more cations or ions. An example would be KNaC4H4O6 or (NH4)2Fe(SO4)2. The overall charge of the ionic compound must still sum to zero.) This type of reaction is known as a metathesis, or double displacement, reaction and is shown below:
wAB(aq) + xCD(aq)→yAD(s) + zCB(aq)
molecular reaction
where A and C are cation reactants, B and D are anion reactants, and the compounds are in molar proportions w and x, respectively. The same follows for products AD(s) and CB(aq) with molar ratios of y and z. When a reaction takes place in aqueous solution, the molecular reaction can be written as a combination of free ions and insoluble products known as an ionic reaction:
A+(aq) + B-(aq) + C+(aq) + D-(aq) →AD(s) + B-(aq) + C+(aq)
ionic reaction
An ionic reaction shows both the ions involved in the reaction as well as those that do not participate, known as spectator ions. The formation of the insoluble product AD(s) identifies the reacting ions or could be used to determine a solubility rule for those ions. In all cases, a net ionic reaction underlies all observations, which is a simplified form of the ionic reaction and shows only the ions involved in the reaction.
A+(aq) + D-(aq)→ AD(s)
net ionic reaction
Observing a chemical reaction producing an insoluble product, or precipitate, is a marker for the participants of a net ionic reaction.
Reactions may be unique to a certain cation or anion, or common to all ions within a group or class of reagents. For example, all transition metal ions react with the sulfide ion, S2-, to form insoluble precipitates. Many alkaline earth metals form white precipitates in the presence of carbonate or phosphate ions. More selective identification analyses can be performed with mixed solutions through a combination of solubility rules and chemical reactivity. For example, a solution containing zinc, silver, nickel, and iron could be separated according to the flowchart in Figure 1. Chloride is first added to the solution, precipitating out silver chloride, AgCl. The remaining metals are all precipitated in hydroxide, with excess hydroxide re-dissolving the zinc. The zinc is confirmed in the presence of potassium hexacyanoferrate, forming a green precipitate. The remaining iron and nickel precipitates are collected and excess ammonia is added to dissolve the nickel and the solid iron complex is collected. The iron is re-dissolved in the presence of acid and confirmed with thiocyanate ion. Nickel is positively identified by adding dimethylglyoxime, forming a solid reddish precipitate.
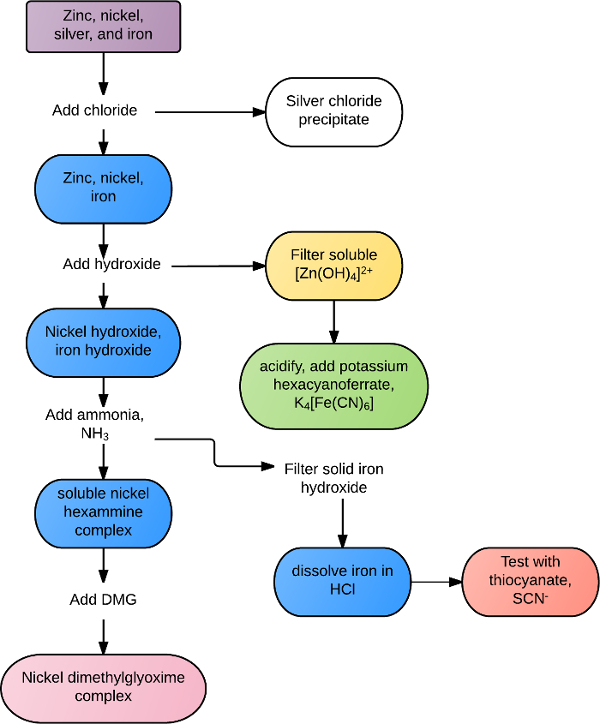
Figure 1. Example flowchart of solution separation.
Процедура
1. General Methods
- Preparing for Qualitative Analysis
- Reactions are generally done in small test tubes with volume of 5 mL or less.
- Solutions need to be fully soluble and should be relatively dilute, typically ~0.1 M.
- Reagents should be slowly added drop-wise and observed carefully.
- Several common "test solutions" are required to establish solubility rules or identify an unknown ion. These contain ions known to react specifically with certain chemical species (cations or anions).
- Common solutions include CaNO3, BaCl2, (NH4)2MoO4, HCl, AgNO3, and NaOH, and other solutions as needed.
- Mixing
- Mix solutions by tapping or swirling the test tube in a vertical direction. Use a cork or stopper to prevent splashing the solution.
- Remove the cork or stopper, then gently heat the solutions with a water bath or cool flame to induce a reaction. Point the test tube away from any individuals in the lab.
- Observation and Recovery
- Separate the supernatant (non-reacting solution) and precipitate using centrifugation. If more precipitate forms when additional test ion is added, the reaction is incomplete. Continue adding test ion until no more precipitate forms.
- Wash the precipitate using centrifugation and pouring or decanting off the supernatant. Add more water and repeat the process for a total of three washings.
- Wash large quantities of precipitate by vacuum filtration and recover the dried precipitate from the filter paper.
- Note the formation of a precipitate as well as the properties of the precipitate such as color, thickness (gelatinous, cloudy, fine), and crystal formation.
- Safety and Waste
- Always wear safety eyewear while performing qualitative analysis experiments. Gloves may also be necessary based on the reagents used and products formed.
- Proper waste disposal methods must be followed closely. Harmful products can be formed when multiple reactants are combined in one container.
2. Anion Analysis
- Identifying phosphate, carbonate, chloride, and sulfide ions; PO43-, CO32-, Cl-, S2-
- Phosphate
- Add a solution containing phosphate, PO43-, to another solution containing calcium ions, Ca2+. The formation of a white precipitate indicates the formation of calcium phosphate, Ca3(PO4)2.
- Since many cations form insoluble products with calcium, a more specific reaction is possible. Add H+ (acid) to Ca3(PO4)2 to dissolve the solid and form HPO42-. Then combine the HPO42- with ammonium molybdate, (NH4)2MoO4. A positive test yields the yellow precipitate ammonium phosphomolybdate, NH4)3PO4(MoO3)12(s). The net ionic reactions are as follows:
3 Ca2+(aq) + 2 PO43-(aq) → Ca3PO4(s)
Ca3PO4(s) + 2 H+(aq) → 3 Ca2+ + 2 HPO42-(aq)
HPO42-(aq) + 12 (NH4)2MoO4(aq) + 23 H+(aq) → (NH4)3PO4 (MoO3)12(s) + 21 NH4+(aq) + 12 H2O(l)
- Carbonate
- Carbonate salts are generally insoluble except in the presence of Group 1 and ammonium cations. Add a few drops of calcium chloride, CaCl2, to the carbonate-containing solution. In solutions with high carbonate concentrations, a white precipitate forms and indicates the possible formation of calcium phosphate, CaCO3. The reaction has many interferences, including other anions like phosphate.
Ca2+(aq) + CO32-(aq) → CaCO3(s) - Add H+ (acid) to a solution containing carbonate, CO32-. The formation of bubbles indicates presence of CO2, signifying CO32- as a reactant. Carbonate ion behaves as a base in the presence of strong acid to form carbon dioxide gas and water.
CO32-(aq) + H+(aq) → CO2(g) + H2O(l)
- Carbonate salts are generally insoluble except in the presence of Group 1 and ammonium cations. Add a few drops of calcium chloride, CaCl2, to the carbonate-containing solution. In solutions with high carbonate concentrations, a white precipitate forms and indicates the possible formation of calcium phosphate, CaCO3. The reaction has many interferences, including other anions like phosphate.
- Chloride
- Add silver nitrate to a chloride-containing solution. The formation of a white precipitate indicates the formation of AgCl(s):
Ag+(aq) + Cl-(aq) → AgCl(s)
- Add silver nitrate to a chloride-containing solution. The formation of a white precipitate indicates the formation of AgCl(s):
- Sulfide
- Add a copper chloride solution to a solution containing sulfide. The formation of a black precipitate indicates the formation of copper sulfide, CuS. In general, solutions containing sulfide ions, S2-, react with metal ions to yield an insoluble metal sulfide.
S2- + Cu2+ → CuS(s).
The value of the solubility product, Ksp = 6.3 x 10-36, indicates the high degree of insolubility of the product.
- Add a copper chloride solution to a solution containing sulfide. The formation of a black precipitate indicates the formation of copper sulfide, CuS. In general, solutions containing sulfide ions, S2-, react with metal ions to yield an insoluble metal sulfide.
- Phosphate
3. Cation Analysis
- All alkali metals (group 1) and some alkaline earth metals (group 2) are soluble except under specific conditions.
- Nearly all Group 3–13 metals are considered insoluble in the presence of sulfide, carbonate, phosphate, and hydroxide. The color and type of precipitate will vary.
- Place a chromium solution in a hydroxide solution. A green precipitate will be observed. The general reaction of a +2 metal with a hydroxide is shown below:
M2+ + OH- → M(OH)2(s) - It is not possible to differentiate most metal ions based on solubility alone with some notable exceptions:
- The addition of silver, Ag+, mercury, Hg22+, or lead, Pb2+ to chloride, bromide, or iodide results in precipitate formation.
- The addition of strontium, Sr2+, barium, Ba2+, mercury, Hg22+, or lead, Pb2+ results in a precipitate in the presence of sulfate.
- Ba2+ forms a yellow solid in the presence of CrO42-, BaCrO4(s). This is pigment used in oil-based paint commonly known as "barium yellow".
- Place a chromium solution in a hydroxide solution. A green precipitate will be observed. The general reaction of a +2 metal with a hydroxide is shown below:
- Limited insolubility of metal ions allows for other qualitative tests to positively identify each metal. While some form precipitates, others undergo unique color changes in the presence of chelating ions or molecules. Cation identifications include nickel, iron, aluminum, and zinc; Ni2+, Fe3+, Al3+, Zn2+.
- Add nickel (II) in the presence of dimethylglyoxime (H2dmg) to form the rose-red precipitate Ni(H2dmg):
Ni2+(aq) + 2 H2dmg(aq) → Ni(Hdmg)2(s) + 2 H+(aq) - Add Iron (III) to thiocyanate ion, SCN- to form the blood-red [FeNCS]2+] complex:
Fe3+(aq) + SCN-(aq) → [FeNCS]2+(aq) - Aluminum ion
- Combine aluminum (III) with pyrocatechol violet in a pH 6 ammonium acetate buffer solution to form a blue solution.
- Aluminum (III) is also precipitated in the presence of weak base to form the gelatinous-white Al(OH)3(s) compound. Addition of more base causes the compound to form the clear and colorless [Al(OH)4]-(aq) soluble complex.
- Zinc ion
- Add zinc (II) to a small amount of base to form a white precipitate. Then add more base to re-dissolve the precipitate and form the soluble [Zn(OH)4]2- complex.
- Add zinc (II) to potassium hexacyanoferrate, K4[Fe(CN)6] to form the light green precipitate K2Zn3[Fe(CN)6]2(s):
3 Zn2+(aq) + 2 K4[Fe(CN)6](aq) → K2Zn3[Fe(CN)6]2(s) + 6 K+(aq)
- Add nickel (II) in the presence of dimethylglyoxime (H2dmg) to form the rose-red precipitate Ni(H2dmg):
Заявка и Краткое содержание
The reactions shown here can be used to identify the presence of a class of cations or anions or be used very specifically for a certain ion. Because two reagents are used in the analyses, either reagent can be typically detected using the other. For example, instead of analyzing for the presence of chloride using silver ion, silver ion can be identified using chloride. A combination of common rules of precipitation followed by specific colorimetric or precipitation tests can be used to positively identify nearly every ion, atomic or polyatomic, available. At the same time, most of those same rules can be established by reacting anions and cations together systematically to generate a set of rules for cation and anion solubility.
Qualitative analysis and rules related to solubility are common experiments in the general chemistry laboratory. This is due, in part, to the ease, speed, and inexpensive nature of the tests. It is for these reasons that qualitative tests are also used in field-based analyses and confirmatory lab tests. For example, a geology firm may wish to know if significant quantities of nickel exist in stream runoff from a mine. A simple test by adding the water to dimethylgloxime is selective for nickel ion. Similarly, water-quality authorities can use barium (or some other group 2 metals) to detect carbonate in water, thus detecting the level of water hardness. Advanced instrumentation is used, however, where quantitative results are required or multiple ions need to be identified at very low levels. This includes various forms of mass spectroscopy as well as ion chromatography and light spectroscopy.
Ссылки
- Eaton, A. Standard Methods for the Examination of Water & Wastewater. Centennial ed. Washington, DC: American Public Health Association (2005).
Перейти к...
Видео из этой коллекции:

Now Playing
Determining the Solubility Rules of Ionic Compounds
General Chemistry
141.3K Просмотры

Common Lab Glassware and Uses
General Chemistry
655.5K Просмотры

Solutions and Concentrations
General Chemistry
273.8K Просмотры

Determining the Density of a Solid and Liquid
General Chemistry
555.7K Просмотры

Determining the Mass Percent Composition in an Aqueous Solution
General Chemistry
383.3K Просмотры

Determining the Empirical Formula
General Chemistry
180.8K Просмотры

Using a pH Meter
General Chemistry
345.2K Просмотры

Introduction to Titration
General Chemistry
424.0K Просмотры

Ideal Gas Law
General Chemistry
78.3K Просмотры

Spectrophotometric Determination of an Equilibrium Constant
General Chemistry
158.3K Просмотры

Le Châtelier's Principle
General Chemistry
264.9K Просмотры

Freezing-Point Depression to Determine an Unknown Compound
General Chemistry
160.6K Просмотры

Determining Rate Laws and the Order of Reaction
General Chemistry
195.9K Просмотры

Using Differential Scanning Calorimetry to Measure Changes in Enthalpy
General Chemistry
44.4K Просмотры

Coordination Chemistry Complexes
General Chemistry
91.3K Просмотры
Авторские права © 2025 MyJoVE Corporation. Все права защищены