Method Article
Surgical Technique for Lumbar Spinal Catheter Insertion in Pigs Enabling Continuous Access to the Thecal Sac in a Terminal Setup
In This Article
Summary
We present a technique for inserting a lumbar spinal catheter at the L4-L5 level in a 3-month-old Danish Landrace pig as part of a terminal research protocol, enabling continuous infusion or CSF sampling from the thecal sac.
Abstract
Pigs are increasingly used as a large animal model for pharmacologic CNS research due to the anatomical and physiological similarities between the porcine and human central nervous systems (CNS). However, accessing the cerebrospinal fluid (CSF) in larger pig breeds by conventional lumbar puncture techniques can be challenging due to an oblique orientation of the spinal spinous processes and a limited interlaminar space. Accordingly, an open surgical procedure for inserting a lumbar spinal catheter for continuous CSF sampling at the L4/L5 level in pigs is thoroughly described in this work. After positioning the pig and identifying the anatomical landmarks, a dorsal midline surgical incision is made to expose the spinous processes. By advancing the introducer needle, the spinal catheter is inserted inside the thecal sac of the spinal canal while leaving the bone structures of the spine intact. This method allows continuous infusion into or sampling from the porcine thecal sac with minimal bleeding or CSF leakage. The procedure is simple, time-efficient, and reproducible across different experimental setups, offering significant potential for various pre-clinical studies, including pharmacokinetic research, surgical training, and spinal cord injury models.
Introduction
Animal models are essential when ethical or practical limitations prevent the use of human subjects to investigate diseases or test surgical methods. While rodents are commonly used due to their low cost, their translational relevance is limited by significant differences from humans1. Pigs, however, offer several advantages compared to rodents, including anatomical and physiological similarities to humans - especially in the context of CNS research1,2. Canine models have historically served as experimental models for CNS research, but ethical considerations have constrained the use of dogs in recent years3. Furthermore, the comparable size of porcine organs to human enhances their use in surgical research and procedural training4. The porcine CNS and spine closely mirror that of humans, with similarities in brain and spinal cord architecture and functionality1,5,6. Importantly, the dimensions of the vertebral column and spinal canal in pigs make them suitable for various pre-clinical studies7,8, including surgical procedural training9,10, drug penetration11,12,13, and spinal cord injuries14.
Access to the CSF in porcine models is crucial in many experimental setups. While lumbar puncture provides a method for singular CSF sampling or intrathecal drug administration, repeated lumbar punctures are impractical. They pose a potential risk of intraspinal hematomas, nerve damage, and CSF contamination with blood. In human patients, spinal microcatheters are commonly used for continuous lumbar CSF drainage in aneurismal subarachnoid hemorrhages and should, due to size similarities, be equally suited for continuous CSF sampling in pigs. However, species-specific anatomical differences in pigs present unique challenges for CSF access. For example, the presence of overlapping laminae, ossified ligaments, and abundant epidural adipose tissue makes conventional percutaneous lumbar puncture techniques less reliable15. In Göttingen minipigs, a minimally invasive percutaneous method has been employed, which enables serial CSF sampling16. This method relies upon manual identification of the lumbar intervertebral spaces, and the catheterization itself is performed without visualization of the introducer. However, this technique is less suitable for larger pigs, as anatomical variations in vertebral size, spinous processes, and the amount of epidural adipose tissue make percutaneous catheterization more difficult15. Therefore, more invasive methods involving exposure of the spine may be required in larger porcine models to ensure reliable catheter placement.
The purpose of this manuscript is to describe the surgical procedure for inserting a spinal catheter into the porcine thecal sac at the L4/L5 level. The procedure involves positioning the subject, planning the surgical incision based on anatomical landmarks, and accessing the posterior bone structures of the spine prior to catheterization.
Protocol
Subjects were housed in compliance with local regulations under the approval of the Danish Animal Experiments Inspectorate (license no. 2020-15-0201-00401). Subject information: Domestic swine, female, approximately 40 kg, 3 months of age.
1. Subject housing and preoperative fasting
- House subjects in groups at 12 h light/dark cycles in approved housing pens for at least 14 days prior to the procedure to ensure proper acclimatization and reduce stress17.
- Ensure that subjects have been on a food withdrawal regime for 12 h prior to the planned anesthesia to reduce the risk of regurgitation. If the subjects' diet includes alfalfa or other types of hay, this must be excluded from the diet 2-3 days prior to the procedure, as this can delay gastric emptying time further18.
2. Anesthesia and monitoring
- Anesthetize the subject with an intramuscular injection of 2 mL/10 kg bodyweight of a mixture of Ketamine 6.25 mg/mL, Zolazepam 6.25 mg/mL, Tiletamine 6.25 mg/mL, butorphanol 1.25 mg/mL, and xylazine 6.25 mg/mL (Zoletil).
- Place the subject in a supine position on top of a heating blanket to support thermoregulation.
- Intubate the subject with a size 6.5 tube19, and ventilate it mechanically with non-humidified air, a tidal volume of 8-10 mL/kg, and a respiratory rate of 16-22 breaths/min according to the expiratory end-tidal CO2 concentrations < 6.0 kPa.
NOTE: CO2 readings confirm the correct intratracheal location of the tube. - Maintain anesthesia by inhalation of 3%-4% vaporized sevoflurane18.
- Apply ophthalmic ointments carefully bilaterally to avoid dryness during anesthesia.
- Ensure a sufficient degree of anesthesia by checking for muscle relaxation and absence of palpebral movement every 10th min18.
- Insert a bladder catheter with a thermometer into the subject's bladder through the urethra19 to monitor temperature and collect urine in a suitable catheter bag.
- Insert a peripheral venous catheter in a suitable superficial ear vein by percutaneous puncture and use it for continuous saline (NaCl, 0.9%) infusion, drug infusion, and euthanasia at the end of the study.
- Insert a femoral artery catheter (6 Fr sheet) in the right femoral artery through a percutaneous puncture. Use this access for continuous invasive blood pressure monitoring.
- Monitor the subject's vital signs every 5 min throughout the procedure.
NOTE: Vital signs include pulse, continuous invasive arterial blood pressure, intravesical temperature, and end-tidal CO2 concentration.
3. Animal positioning
- Place the subject in a prone position centrally on the operating table. Ensure the spine of the subject is straight to avoid any scoliosis.
- Place a sandbag beneath the lumbar aspect of the spine to increase the angulation between the laminae.
- Shave the hair from the surgical site with a trimmer.
- Apply iodine solution to the surgical site in centrifugal patterns. Repeat this process until the entire surgical site is covered.
- Tilt the subject slightly to an upright position.
4. Preparation of surgical equipment
- Prepare the surgical equipment listed in the Table of Materials.
5. Identifying key anatomical landmarks
- Identify the iliac crest at each side of the subject's lumbar spine and follow the contours medially until the sacrum is identified (Figure 1).
- Identify the intervertebral space in the midline between the cranial aspect of the sacrum and the spinous process of L6.
- Identify the spinous processes of L6, L5, and L4 (Figure 1, Figure 2).
6. Exposing the spinous processes
- Make a midline incision along the spinous processes L4-L6 using scalpel no. 24, cutting through skin and subcutis.
- Use a monopolar to cauterize small bleeding from superficial veins and arterioles.
- Wipe off the blood with a surgical swamp and check for active bleeding; use the monopolar accordingly.
NOTE: It is important to stop even minor bleeding to avoid hematomas. - Insert the surgical retractor and expand the opening.
- Identify the supraspinous ligament dorsal to the spinous processes.
- Expand the incision gradually with the monopolar along the lateral aspect of the spinous processes until approximately 1 cm of the spinous processes is visible (Figure 3).
NOTE: If the person performing the procedure is right-handed, one should consider following the subject's right lateral aspect of the spinous process to ease the insertion of the introducer later. - Identify the interspinous ligament between L4/L5 (Figure 3).
- Check for active bleeding and apply the monopolar for cauterizing accordingly.
7. Access to the thecal sac
- Identify the L4/L5 intervertebral space between the lamina of the spinous processes by manual palpation.
- Place the introducer with its bevel and lumen oriented in a cranial direction angled towards the L4/L5 intralaminar space (Figure 2, Figure 4, Figure 5).
- Ensure the introducer is kept at a 30° horizontal and 45° cranial inclination (Figure 5).
NOTE: Aim towards the intralaminar space between L4/L5. - Gradually advance the introducer until gentle resistance is felt; this represents the ligamentum flavum.
NOTE: The sensation of blunt resistance indicates that the introducer is stopped by the spinous process. If this occurs, retract the introducer 1 cm and advance again at a slightly different inclination. - Apply a firm yet very careful pressure and advance the introducer millimeter by milliliter through the flavum ligament until a sudden loss of resistance is felt.
NOTE: If the subject exhibits motor reflexes in the lumbar musculature or hind legs, it is due to direct contact with the nerve roots and not insufficient anesthesia. - Follow each advancement of the introducer by removing the trocar to check for visible CSF flow.
- Confirm the correct placement of the introducer within the spinal canal by visual confirmation of CSF flow from the introducer after it has penetrated the ligamentum flavum and, subsequently, the dura mater.
NOTE: Spontaneous flow of CSF may be slow. Confirmation can be expedited by filling the introducer with sterile saline and observing for pulsation. - Reinsert the trocar into the introducer to avoid excessive loss of CSF while preparing the catheter.
8. Insertion of catheter into the thecal sac
- Insert the guidewire into the catheter.
- Remove the trocar from the introducer.
- Insert the catheter, containing the guidewire, into the introducer until gentle resistance is felt.
- Measure 5 cm distally from the introducer and set a mark with the surgical marker.
- Apply gentle yet firm pressure as the catheter is advanced into the thecal sac until the previously measured mark reaches the introducer.
NOTE: Due to medullary reflexes, the subject might twitch/move despite being sufficiently anesthetized. - Retract the introducer carefully while keeping the catheter in its position.
NOTE: Apply a firm grip on the catheter as soon as it is visible above the skin to avoid misplacement as the introducer is removed. - Remove the guidewire while keeping a firm grip on the catheter at skin level (Figure 6).
- Attach a 2 mL single-use plastic syringe to the catheter.
- Confirm location within the spinal canal by aspiration CSF from the catheter.
- In the case of lacking CSF in the syringe, gently retract the catheter a few millimeters to restore patency.
- Secure the spinal catheter to the surgical retractor and the skin by tape to avoid misplacement.
9. Administration of lipopolysaccharide
- Administer a 400 μg of E. coli lipopolysaccharide (LPS) (OH:143) into the central venous sheet.
- Start a timer.
10. CSF sampling
- Obtain CSF samples hourly for the following 24 h to measure total leucocyte count, CSF-albumin, and CSF IgG. Draw a maximum of 0.5 mL of CSF in each sample.
11. Euthanasia
- Administer a bolus of pentobarbital (50 mg/kg) through the peripheral venous catheter.
- Observe the pulse, blood pressure, and end-tidal CO2 concentration curves at the respirator for flatline as a confirmation of the cardiac arrest.
Results
The prone positioning of the pig optimizes surgical access to the lumbar vertebrae. The use of supportive sandbags increases the angulation between adjacent lumbar spinous processes, thereby improving access to the spinal canal.
The present study aimed to investigate the inflammatory response within the CSF compartment following intraventricular inoculation with E. coli lipopolysaccharide. A total of 10 pigs underwent the procedure, and subsequent CSF analyses revealed an increase in mean total CSF leukocyte from 30 x 106/L (range 17-39) at 0 h to 19,720 x 106/L (11,353-30,546) at 12 h and an increase in CSF albumin 0.05 g/L (0.03-0.07) at 0 h to 0.15 g/L (0.14-0.17) at 12 h - thereby confirming cerebral inflammation and that all spinal catheters were placed correctly using the presented technique (Figure 7). In the present study, key anatomical landmarks -- specifically the iliac crests, the sacrum, and the spinous processes of L4, L5 and L6 -- were used to accurately locate the vertebral midline prior to the incision (Figure 1). Following the incision through the skin and subcutaneous tissues, the supraspinous ligament and the spinous processes of L4, L5, and L6 were exposed, confirming the anatomical midline (Figure 2). Continued dissection and exposure of the laminae of the spinous processes allowed the identification of the intervertebral spaces between L4/L5, L5/L6, and L6/S1 by manual palpation. These spaces were particularly suitable as anatomical landmarks due to their proximity to the underlying spinal canal (Figure 3).
The gradual insertion of the introducer at the 30° horizontal and 45° cranial inclination led to the successful entry into the thecal sac, confirmed by the visible spontaneous flow of CSF. Ensuring the bevel of the introducer faced cranially allowed for smooth catheter insertion after catheterization. Once the guidewire was removed, proper placement of the catheter was validated by the aspiration of CSF. The overall success of the procedure was determined by the following criteria: (a) no major bleeding occurred during the incision, (b) the introducer remained free of blood upon entry into the spinal canal, (c) there was no CSF leakage in the surgical field, and (d) CSF could be aspirated repeatedly from the catheter.
Minor hemorrhages should be expected during the initial stages of incision due to the well-vascularized nature of the tissue. Smaller arteries were frequently encountered, highlighting the need for cauterization. The surgical retractor was also used to apply pressure and achieve hemostasis. During deeper incisions, small intervertebral veins were occasionally encountered, which could be difficult to control due to their location. As it is difficult to apply pressure or cauterize in this area, careful surgical technique is crucial to avoid such venous bleeding. Since the catheter was intended for long-term placement, it was essential to prevent bleeding to avoid hematoma formation. In the event of such venous bleeding, hemostasis can be achieved through cottonoids applied with localized direct pressure.
The integrity of the spinal canal's epidural space was assessed by inspecting the tissue surrounding the catheter for CSF leakage. Any leakage would appear as a water-like fluid accumulation in the tissue. In this case, the dura mater surrounding the catheter was confirmed to be intact, as no CSF leakage was observed during or after the procedure (Figure 6). Lastly, consistent CSF aspiration through the catheter further validated the correct placement within the lumbar cistern of the thecal sac.
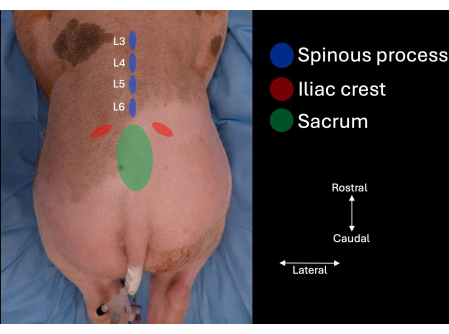
Figure 1: Picture of the pig's lumbar spine with anatomical landmarks identified by manual palpation. The sacrum, the iliac crests, and the spinous processes of L4, L5, and L6. Please click here to view a larger version of this figure.
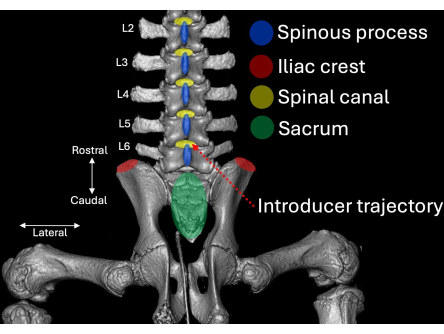
Figure 2: Three-dimensional computed tomography scan of the lumbar aspect of the porcine spine visualized from a posterior perspective. Anatomical landmarks are highlighted in blue (spinous processes), red (iliac crests), and green (sacrum) with their respective relation to the spinal canal (yellow). The insertion route of the introducer is highlighted in dotted red. Please click here to view a larger version of this figure.
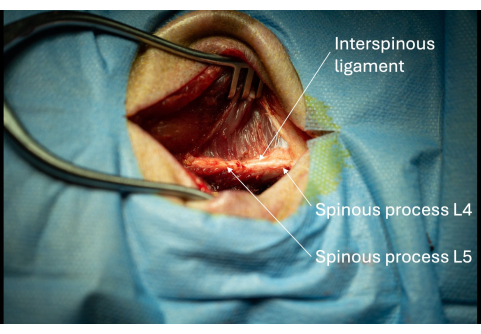
Figure 3: Picture of the exposed spinous processes of L5 and L6 with adjacent interspinous ligament seen from a lateral perspective. The presence of these spinous processes confirms the correct anatomical location of the surgical opening. Please click here to view a larger version of this figure.
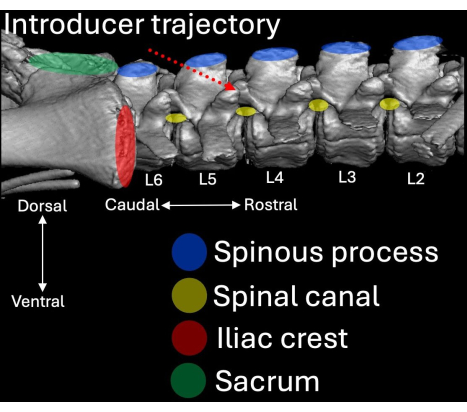
Figure 4: Three-dimensional computed tomography scan of the lumbar aspect of the porcine spine visualized from a lateral perspective. Anatomical landmarks are highlighted in blue (spinous processes), red (iliac crests), and green (sacrum) with their respective relation to the spinal canal (yellow). The insertion route of the introducer is highlighted in dotted red. Please click here to view a larger version of this figure.
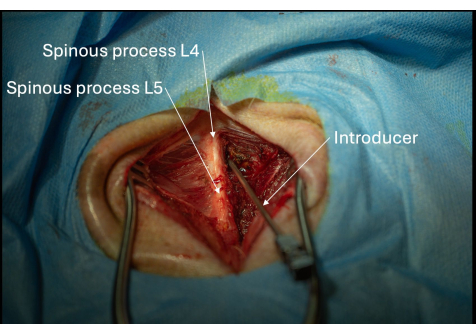
Figure 5: Picture of the correct positioning of the introducer angled towards the L4/L5 interlaminar space seen from a posterolateral perspective. The introducer must be advanced gradually millimeter by millimeter until the ligamentum flavum has been penetrated. Please click here to view a larger version of this figure.
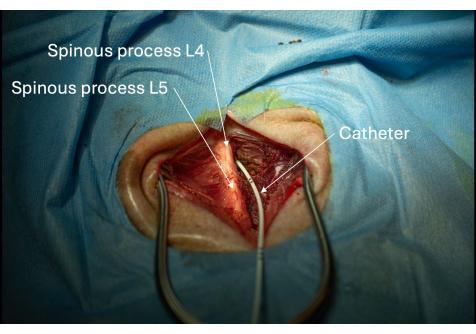
Figure 6: Picture of the catheter inserted into the spinal canal through the L4/L5 interlaminar space. The placement of the catheter within the spinal canal is confirmed if CSF can be aspirated repeatedly. Please click here to view a larger version of this figure.

Figure 7: Time-concentration graphs of total CSF leukocyte counts (orange) and CSF albumin (blue) following intraventricular LPS inoculation measured at 0 h, 12 h and 24 h. Please click here to view a larger version of this figure.
Discussion
The demonstrated procedure for lumbar catheter insertion for continuous CSF sampling in pigs involves several crucial steps. Firstly, the correct vertebral level must be exposed to ensure optimal conditions for successful catheterization. The porcine spinal cord extends further caudally compared to humans, reaching the S2-S3 level15, in contrast to the human conus medullaris, which terminates at the L1-L2 level20. Consequently, accessing the lumbar cistern for CSF sampling is more challenging in pigs, particularly at higher lumbar levels where the spinal cord occupies more of the intrathecal space. In our experience, the L4/L5 or the L5/L6 levels are suitable sections for catheterization as the spinal cord occupies a slightly smaller fraction of the spinal canal at these segments21.
The surgical approach presented here contrasts with less invasive methods, such as the minimally invasive CSF collection model developed by Bergadano et al., which utilized percutaneous lumbar puncture in Göttingen minipigs16. In their technique, the intervertebral spaces of the lumbar spine are identified through manual palpation and targeted for introducer insertion at a 45° horizontal inclination. Confirmation of correct introducer placement within the epidural space relies on the perception of loss of resistance during catheterization. While minimally invasive techniques offer the advantage of reduced tissue damage and quicker recovery times, they also present potential limitations in larger pig breeds like the Danish Landrace. For instance, Pleticha et al. identified several anatomical features, such as overlapping laminae, ossified ligaments, and the abundance of epidural fat in large pigs, which make lumbar puncture more unreliable without imaging guidance15. Consequently, multiple puncture attempts can be necessary, which increases the risk of bleeding, CSF leakage, and blood contamination of CSF samples.
In comparison, Pleticha et al. successfully employed a CT-guided lateral lumbar puncture technique to overcome these anatomical challenges, achieving precise intrathecal access in larger pigs15. The argument for utilizing imaging guidance is that manual palpation cannot identify anatomical landmarks with the necessary reproducibility. Consequently, image guidance (such as ultrasound or fluoroscopy) are indeed needed for exact anatomical identification15,22. While this lateral method presented by Pleticha et al. offers significant advantages in facilities equipped with imaging capabilities, it may not be feasible in all laboratory settings due to the need for CT equipment. The surgical method described here, though more invasive, provides a practical alternative that can be easily implemented in laboratories without access to CT imaging. By exposing the spinous processes and intervertebral spaces, this approach allows for direct visual confirmation of the catheters' entry into the lumbar cistern, reducing the need for repeated attempts and minimizing complications.
Another important consideration is the tissue damage and postoperative complications associated with any open surgical approach. While the method described here provides excellent visualization of the spine and reliable catheter placement, it also causes more tissue disruption compared to minimally invasive techniques. This may limit its application in survival studies, where preserving muscle integrity and reducing the risk of infection is crucial. However, with careful surgical technique and the use of monopolar cautery to control bleeding, the risk of postoperative complications can be minimized. Although the presented method was used in a non-survival study of cerebral inflammation following intraventricular inoculation with E. coli lipopolysaccharide in 10 pigs, we believe that the method can be adapted to survival studies, as the fascia and skin can be sutured around the placed catheter. In a potential survival study, it is crucial to monitor the subject for signs of neurological deficits or infections in the postoperative phase.
Overall, the surgical method presented here offers several advantages over less invasive techniques. By exposing a larger portion of the spine, this approach allows for precise placement of the introducer and catheter on the first attempt, thereby reducing the risk of CSF contamination with blood and ensuring consistent CSF sampling. In our experience, this method enables reliable collection of CSF at a rate of approximately 0.5 mL/h over a 24-h period. If CSF flow diminishes, slight retraction of the catheter by a few millimeters often restores patency. Additionally, direct observation of the surgical field allows for the detection of potential complications, such as CSF leakage. This method also provides flexibility for future studies that may require the implantation of multiple catheters or additional instruments.
In conclusion, we consider the presented method to be simple and reproducible, with significant applications in various future porcine models relevant to the porcine spinal cord, particularly in models requiring extensive CSF sampling or instrumentalization of the porcine spinal canal.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to express our sincere gratitude for the experience shared by the personnel at the Biomedical Laboratory, Aalborg University Hospital, Denmark.
Materials
| Name | Company | Catalog Number | Comments |
| Adjustable operating table | N/A | N/A | |
| Bair Hugger heater | 3M | B5005241003 | |
| Bair Hugger heating blanket | 3M | B5005241003 | |
| Endotracheal tube size 6.5 | DVMed | DVM-107860 | Cuffed endotracheal tube |
| Euthasol Vet | Dechra Veterinary Products A/S | 380019 | phentobarbital for euthanazia, 400mg/mL |
| Foley Catheter 12F | Becton, Dickinson and Company | D175812E | Catheter with in-built thermosensor |
| Intravenous peripheral catheter | Avantor | BDAM381344 | Size G18 |
| Intravenous sheath | Coris Avanti | Avanti Cordis Femoral Sheath 6F | |
| Monopolar, ForceTriad System | Medtronic | ||
| Plastic Syringe, 2 mL | Becton, Dickinson and Company | 300928 | |
| Primus respirator | Dräger | Respirator with in-built vaporiser for supplementary Sevofluran anesthisa | |
| Self-retaining retractor | World Precission Instruments | 501722 | Weitlander retractor, self-retaining, 14 cm blunt |
| Silicone Lumbar Catheter incl. Introducer | Integra | NL8508330 | |
| Sterile Saline | Fresnius Kabi | 805541 | 1000 mL |
| Sterile surgical swaps | |||
| Surgical scalpel no 24 | Swann Morton | 5.03396E+12 | Swann Morton Sterile Disposable Scalpel No. 24 |
| Zoletil Vet | Virbac | Medical mixture for induction of anesthesia |
References
- Meurens, F., Summerfield, A., Nauwynck, H., Saif, L., Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 20 (1), 50-57 (2012).
- Bassols, A., et al. The pig as an animal model for human pathologies: A proteomics perspective. Proteomics Clin Appl. 8 (9-10), 715-731 (2014).
- Yaksh, T. L., Rudy, T. A. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 17 (6), 1031-1036 (1976).
- Lunney, J. K., et al. Importance of the pig as a human biomedical model. Sci Transl Med. 13 (621), eabd5758 (2021).
- Lind, N. M., et al. The use of pigs in neuroscience: Modeling brain disorders. Neurosci Biobehav Rev. 31 (5), 728-751 (2007).
- Hoffe, B., Holahan, M. R. The use of pigs as a translational model for studying neurodegenerative diseases. Front Physiol. 10, 838 (2019).
- Toossi, A., et al. Comparative neuroanatomy of the lumbosacral spinal cord of the rat, cat, pig, monkey, and human. Sci Rep. 11 (1), 1955 (1955).
- Busscher, I., Ploegmakers, J. J. W., Verkerke, G. J., Veldhuizen, A. G. Comparative anatomical dimensions of the complete human and porcine spine. Eur Spine J. 19 (7), 1104-1114 (2010).
- Säteri, T., et al. Ex vivo porcine models are valid for testing and training microsurgical lumbar decompression techniques. World Neurosurg. 155, e64-e74 (2021).
- Yamanouchi, K., et al. Validation of a surgical drill with a haptic interface in spine surgery. Sci Rep. 13 (1), 598 (2023).
- Hanberg, P., Bue, M., Birke Sørensen, H., Søballe, K., Tøttrup, M. Pharmacokinetics of single-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone determined by microdialysis. Spine J. 16 (3), 432-438 (2016).
- Hvistendahl, M. A., et al. Cefuroxime concentrations in the anterior and posterior column of the lumbar spine - an experimental porcine study. Spine J. 22 (9), 1434-1441 (2022).
- Mariager, T., et al. Continuous evaluation of single-dose moxifloxacin concentrations in brain extracellular fluid, cerebrospinal fluid, and plasma: a novel porcine model. J Antimicrob Chemother. 79 (6), 1313-1319 (2024).
- Thygesen, M. M., et al. A 72-h sedated porcine model of traumatic spinal cord injury. Brain Spine. 4, 102813 (2024).
- Pleticha, J., et al. Pig lumbar spine anatomy and imaging-guided lateral lumbar puncture: A new large animal model for intrathecal drug delivery. J Neurosci Methods. 216 (1), 10-15 (2013).
- Bergadano, A., et al. A minimally-invasive serial cerebrospinal fluid sampling model in conscious Göttingen minipigs. J Biol Methods. 6 (1), e107 (2019).
- Maxwell, A. R., Castell, N. J., Brockhurst, J. K., Hutchinson, E. K., Izzi, J. M. Determination of an acclimation period for swine in biomedical research. J Am Assoc Lab Anim Sci. 63 (6), 651-654 (2024).
- Costea, R., Ene, I., Pavel, R. Pig Sedation and anesthesia for medical research. Animals. 13 (24), 3807 (2023).
- Ettrup, K. S., et al. Basic surgical techniques in the göttingen minipig: Intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. J Vis Exp. 52, e2652 (2011).
- Grogan, J. P., Daniels, D. L., Williams, A. L., Rauschning, W., Haughton, V. M. The normal conus medullaris: CT criteria for recognition. Radiology. 151 (3), 661-664 (1984).
- Bessen, M. A., et al. Characterising spinal cerebrospinal fluid flow in the pig with phase-contrast magnetic resonance imaging. Fluids Barriers CNS. 20 (1), 5 (2023).
- Weber-Levine, C., et al. Porcine model of spinal cord injury: A systematic review. Neurotrauma Rep. 3 (1), 352-368 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved