Method Article
Isolation of Live Myeloid and Epithelial Cell Populations from the Mouse Lung
In This Article
Summary
This protocol details the isolation of live immune and non-immune populations from the mouse lung at a steady state and following influenza infection. It also provides gating strategies for identifying epithelial and myeloid cell subsets.
Abstract
The lung is continuously exposed to pathogens and other noxious environmental stimuli, rendering it vulnerable to damage, dysfunction, and the development of disease. Studies utilizing mouse models of respiratory infection, allergy, fibrosis, and cancer have been critical to reveal mechanisms of disease progression and identify therapeutic targets. However, most studies focused on the mouse lung prioritize the isolation of either immune cells or epithelial cells, rather than both populations concurrently. Here, we describe a method for preparing a comprehensive single-cell suspension of both immune and non-immune populations suitable for flow cytometry and fluorescence-activated cell sorting. These populations include epithelial cells, endothelial cells, fibroblasts, and a variety of myeloid cell subsets. This protocol entails bronchoalveolar lavage and subsequent inflation of the lungs with dispase. Lungs are then digested in a liberase mixture. This method of processing liberates a variety of diverse cell types and results in a single-cell suspension that does not require manual dissociation against a filter, promoting cell survival and yielding high numbers of live cells for downstream analyses. In this protocol, we also define gating schemes for epithelial and myeloid cell subsets in both naïve and influenza-infected lungs. Simultaneous isolation of live immune and non-immune cells is key for interrogating intercellular crosstalk and gaining a deeper understanding of lung biology in health and disease.
Introduction
The lung is composed of the airways, alveoli, and interstitium. Immune and non-immune cells reside within these compartments to contribute to both homeostatic lung function (gas exchange) and host defense against environmental insults, such as viral infection. The large and small airways, or the bronchi and bronchioles, are lined by epithelial cells. The predominant epithelial cells in these regions are club and ciliated cells which are responsible for secreting protective molecules and facilitating mucociliary clearance1. The alveoli are the most distal structures in the lung, lined by two epithelial cell types, alveolar type I cells (ATIs) and alveolar type II cells (ATIIs). ATIs are responsible for gas exchange, and ATIIs secrete and recycle surfactants to ensure appropriate surface tension2,3. ATIIs are self-renewing and can also differentiate into ATIs, a role especially relevant following lung damage4. Additionally, ATIIs provide a supportive niche for the major immune cell type populating the alveolar niche, alveolar macrophages (AMs)5,6. Beyond the epithelium, fibroblasts, endothelial cells, and interstitial macrophages (IMs) (which can be both nerve- and vessel-associated) comprise the interstitium7,8,9,10. In response to infection and injury, numerous lung cells die, and immune cells, including monocytes and neutrophils, infiltrate into the tissue11,12. Lung-infiltrating monocytes differentiate into macrophages and can contribute to the macrophage compartment long-term13.
Current methods to prepare single-cell suspensions from the mouse lung are generally collagenase-based and require physical dissociation of tissue14. This can result in low numbers of viable non-immune cell populations. Some protocols to isolate epithelial cells are dispase-based and yield higher proportions of live epithelial cells; however, these protocols generally do not investigate immune cell yields and viability15,16,17. Flow cytometry is a common method used to distinguish cell populations within a digested tissue. At baseline, flow cytometry gating for AMs, IMs, monocytes, and neutrophils is clearly delineated. However, during inflammation, the gating process becomes variable and challenging to interpret due to the continuum in surface marker expression of infiltrating monocytes differentiating into macrophages. Therefore, the protocol presented herein also outlines gating strategies to identify myeloid cell populations of interest following infection.
Robust dissociation of lung epithelial and myeloid cells is essential to discern their homeostatic and inflammatory functions. A method to isolate these cell compartments in parallel will enable the downstream analyses of key cell types that both maintain health and drive disease. A schematic overview of this protocol's workflow can be found in Figure 1.
Protocol
This protocol complies with the guidelines of the Institutional Animal Care and Use Committee at Harvard Medical School (Grant numbers: R35GM150816 and P30DK043351). Female C57BL/6J mice aged 8-12 weeks were used for the experiments. This protocol is also suitable for male mice. The details of the reagents and equipment used in this study are provided in the Table of Materials.
1. Preparation of materials
- Thaw necessary enzymes on ice, including dispase, liberase, and DNAse.
- Prepare other necessary reagents. Fill a 10 mL syringe with 5 mL of 2 mM EDTA in DPBS and fit it with a 27 G needle. Fill the 1 mL syringe with DPBS and attach the catheter.
2. Harvesting the lungs
- Euthanize the mouse by intraperitoneal injection of 400-500 mg/kg and 25-100 mg/kg ketamine/xylazine mixture (following institutionally approved protocols). Proceed with dissection once the mouse is unresponsive to the foot pad pinch (~5 min).
NOTE: A ketamine/xylazine mixture is used for euthanasia instead of carbon dioxide to prevent suffocation-induced hemorrhage and immune cell infiltration that can confound results. - Spray the mouse with 70% ethanol. Dissect the mouse using fine surgical scissors and #7 forceps. Make an incision into the abdomen using scissors and cut laterally through the peritoneum.
- Make a small cut into the diaphragm to release the vacuum. Cut the diaphragm and lower rib cage out of the body cavity to expose the lungs and heart.
- Perfuse the lungs with 5 mL of 2 mM EDTA in DPBS in a 10mL syringe fitted with a 27 G needle. Perfusion is performed by entering the base of the heart with the needle, directed from the right into the left ventricle, and slowly dispensing liquid from the syringe18.
NOTE: Perfusion should be performed slowly to prevent ruptured vasculature. - Open the ribcage at sternum by cutting away sides of ribcage, then cut vertically through the sternum to expose the trachea19. Cut away as much muscle and connective tissue as possible using fine scissors and #7 forceps without puncturing the trachea or lungs.
- Wrap the trachea in suture (size 2-0) as if to tie it off but without tightening. Nick trachea laterally and insert the catheter. Secure the suture tightly around the catheter by pulling the suture tight. Do not insert the catheter more than 1 cm into the lung.
- Inflate lungs with 1 mL of DPBS. Pull out the DPBS by slowly pulling back on the syringe. Re-inflate the lungs with the same DPBS and once again pull back on the syringe to harvest bronchoalveolar lavage fluid (BALF).
- Disconnect the syringe from the catheter, leaving the catheter inserted. Dispense BALF into a microcentrifuge tube.
- Fill the same 1 mL syringe with 1 mL of dispase and reattach to the catheter. Dispense syringe to inflate lungs with dispase. While removing the catheter, tighten the suture around the trachea to prevent leakage of dispase.
- Cut through the trachea laterally. Remove the lungs from the body cavity by cutting through the connective tissue along the back of the ribcage while using forceps to pull the lungs and trachea upwards.
- Remove the heart from the lungs. Place the lungs in 5 mL of DPBS on ice.
- At this step, take the separated BALF and spin down at 400 x g for 5 min at 4 °C. The supernatant may be aliquoted and stored as required. There should be a visible cell pellet that can be resuspended in 100 µL of RPMI.
NOTE: One can wait up to 2 hr to proceed with digestion, which allows multiple mice to be harvested at a time.
3. Digesting the lungs
- Prepare the digest mix. Add 83 µg/mL liberase (50 µL stock solution), and 100 µg/mL DNAse (15 µL stock solution) in 3 mL of RPMI per lung.
- Dissect the five lobes of the lungs and discard any connective tissue19,20. Chop the lung with scissors for 1 min on a glass slide.
- Transfer the chopped lung into a 50 mL centrifuge tube using dissection scissors. Add cell pellet recovered from BALF and resuspended in RPMI into the centrifuge tube. Add 3 mL of digest mix to the tube containing the sample using a 5 mL serological pipette.
- Pipet lung digest mixture up and down 2-3 times. Place at 37 °C in an orbital shaker at 140 rpm with digestion tubes placed at a 45-degree angle for 40 min.
4. Preparing the single-cell suspension
- Remove the 50 mL centrifuge tubes from the orbital shaker and place them on ice. Pipette lung digest mixture up and down 5-6 times. Filter through a 70 μm cell strainer into a new 50 mL tube. Wash any remaining cells through the filter and neutralize enzymes with 10 mL 5% FBS in RPMI.
- Spin for 5 min 4 °C at 600 x g. Aspirate the supernatant using a vacuum aspirator or pipette. Resuspend in 1 mL of ACK lysis buffer and incubate for 3 min at room temperature to lyse red blood cells (RBCs). Add 9 mL of PBS and pipette up and down.
- Spin for 5 min at 4 °C at 600 x g. Aspirate supernatant using a vacuum aspirator or pipette. Resuspend in 1 mL of FACS buffer (1% FBS in PBS) and filter through 64 μm mesh into a microcentrifuge tube using a p1000 pipette.
5. Flow cytometry and downstream analyses
- If performing flow cytometry or fluorescence-activated cell sorting in a 96-well plate, stain approximately 1-2 million cells per well, or an 8% fraction of the lung suspension.
- Spin the cells down. All spins in a 96-well plate should be performed for 2.5 min (4 °C) at 600 x g. Flick off the supernatant and wash cells once by resuspending in 200 μL of DPBS. Spin down and flick.
- If cells are to be stained for flow cytometry analysis, proceed with the following staining procedure.
- Resuspend cells in live/dead Zombie stain (1:150) and Fc block (1:250) in 25 μL of DPBS for 10 min at room temperature in the dark.
- Prepare the antibody staining mix at a 2x concentration in the FACS buffer.
NOTE: A staining mix should include all of the markers in the myeloid panel (Table 1) or epithelial panel (Table 2) at their indicated concentrations. - Add 25 μL of staining mix to 25 μL of lung suspension in a plate and incubate on ice for 30 min in the dark. The total staining volume should now be 50 μL, and primary antibodies should be at their final 1x staining concentration.
- Add 150 µL of FACS buffer, spin down for 2.5 min at 4 °C at 600 x g, and flick off the supernatant. Wash twice in 200 μL of FACS buffer.
- If intracellular staining is required, fix the cells with an intracellular fixation kit. If intracellular staining is not required, cells may be fixed for 20 min in 4% PFA in DPBS.
- Wash off the fixative with 200 μL of FACS buffer three times. Samples may be stored resuspended in 200 μL of FACS buffer at 4 °C in the dark for 2-3 days. Before running samples, counting beads may be added to quantify cell numbers.
- If cells are to be sorted, proceed with the following staining procedure.
- Resuspend cells in 1x staining antibody mix with Fc block (1:250). Incubate for 30 min on ice in the dark.
- Add 150 μL of FACS buffer, spin down for 2.5 min at 4 °C at 600 x g, and flick off the supernatant. Wash three times with 200 μL of FACS buffer and resuspend in 200 μL of FACS buffer before sorting.
NOTE: It is recommended to add a viability dye, such as DAPI, to cells before sorting.
Results
A successful digest will result in approximately 20-25 million cells with 90%-95% viability. If approximately 25,000 counting beads are added to an 8% fraction of the lung, beads should compromise 1%-3% of collected events. After gating on singlets, approximately 90%-95% of cells should be Zombie Aqua negative (indicating viability) (Figure 2A, Figure 3A).
Of CD45+ cells, CD64+F4/80+ cells are defined as macrophages (Figure 2B). At baseline, 80%-90% of all macrophages in the lung are defined as AMs (Siglec-F+CD11c+), and the rest can be classified as IMs (Siglec-F-CD11c-) (Figure 2C). However, during infection and inflammation, this definition shifts, and the IM gating will contain monocyte-derived macrophages (moMacs) in addition to IMs (Siglec-F-CD11clo/hi). Furthermore, the ratio shifts to approximately 4% AMs and 90% IMs/moMacs at 9 days post-infection (dpi) (Figure 2C).
Cells that are CD64-F4/80+ can be further gated by Siglec-F and Ly6C. Siglec-F+ cells are eosinophils (Figure 2D). Ly6C+Siglec-F-CD11b+ cells can be identified as monocytes (Figure 2D). Cells that are negative for both CD64 and F4/80 (Figure 2B) can be further gated as CD11b+Ly6G+ to identify neutrophils (Figure 2E).
To gate epithelial cells, first CD45+ cells are to be gated out (Figure 3). Then, endothelial cells (CD31+) and fibroblasts (Pdgfra+) can be identified (Figure 3B). Subsequently, CD31-Pdgfra- cells that are EpCAM+ are identified as epithelial cells (Figure 3C). Epithelial cells may be further subsetted into ATIIs (MHC-II+CD104-), airway cells (club and ciliated cells) (CD104+), and other epithelial cells (MHC-II-CD104-) (Figure 3D). The proportion of ATII cells decreases during infection.
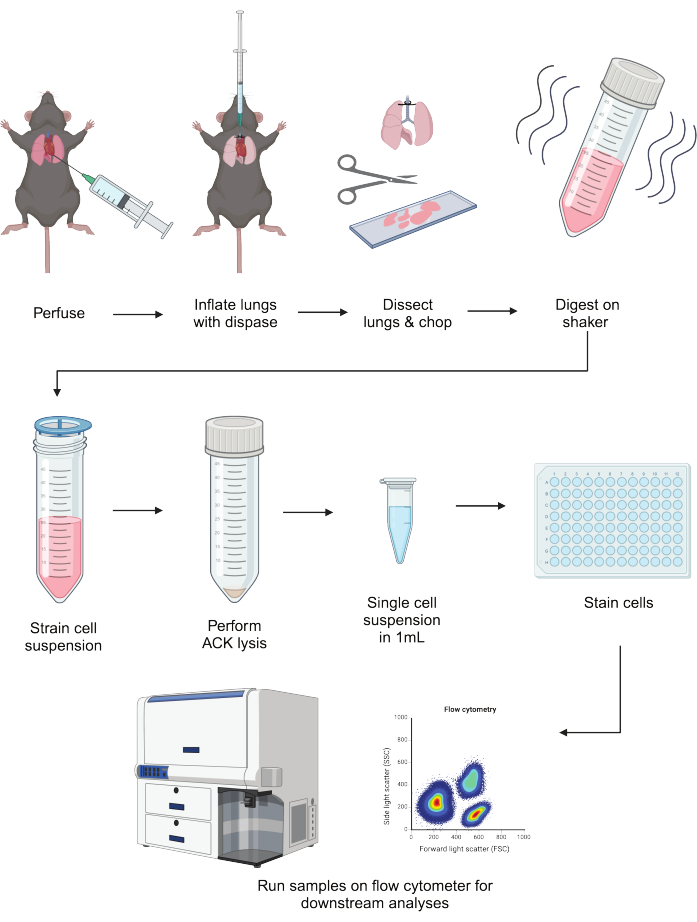
Figure 1: Schematic representation of the protocol. Mouse lungs are perfused, inflated with dispase, and dissected. After chopping, they are digested in a liberase/DNAse mixture with shaking for 40 min. Digests are run through a cell strainer, and RBCs are lysed. After resuspension in FACS buffer, cells can be plated and stained before downstream applications, including flow cytometry. This figure was created using BioRender. Please click here to view a larger version of this figure.

Figure 2: Myeloid cell gating strategy. Representative gating of flow cytometry analyses of mouse lungs that are uninfected, 9 days, or 14 days post-infection (dpi) with Influenza A/Puerto Rico/8/34 (H1N1). (A) Gating of live CD45+ cells. (B) CD45+ cells gated CD64 by F4/80. (C) CD64+F4/80+ cells (macrophages) gated into AMs and IMs/moMacs using Siglec-F by CD11c. (D) CD64-F4/80+ cells gated into eosinophils and monocytes. (E) CD64-F4/80- cells gated to identify neutrophils. Please click here to view a larger version of this figure.
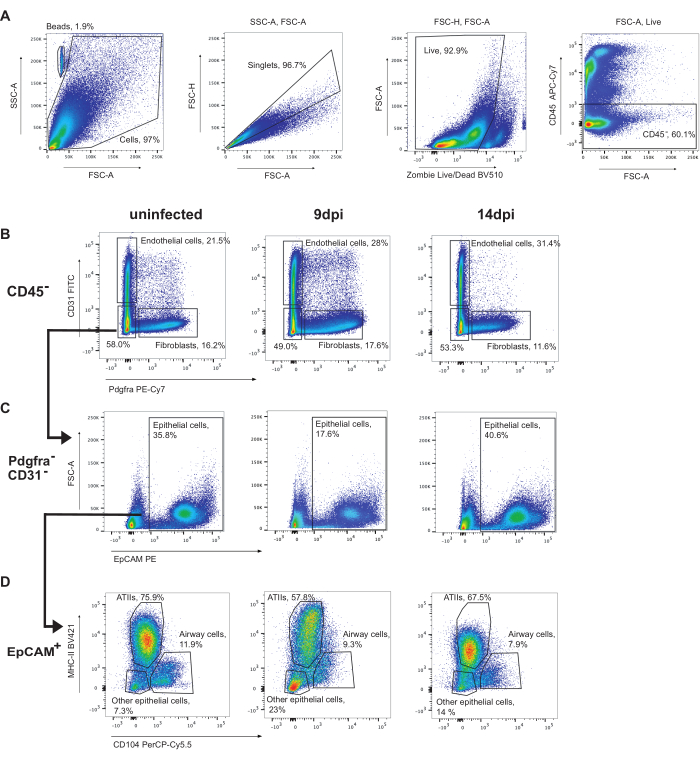
Figure 3: Epithelial cell gating strategy. Representative gating of flow cytometry analyses of uninfected mouse lungs, 9 dpi, or 14 dpi with Influenza A/Puerto Rico/8/34 (H1N1). (A) Gating of live CD45- cells. (B) CD45-cells gated on CD31 (endothelial cells) by Pdgfra (fibroblasts). (C) CD31-Pdgfra-EpCAM+ cells are epithelial cells. (D) Gating epithelial cells MHC-II by CD104 identifies ATIIs (MHC-II+, CD104-), airway epithelial cells (including ciliated and club cells) (CD104+), and other epithelial cells (MHC-II-CD104-). Please click here to view a larger version of this figure.
| Antibody | Fluorophore | Staining Concentration | Clone |
| anti-mouse CD11c | AF-488 | 1:300 | N418 |
| anti-mouse CD170 (Siglec-F) | PE | 1:200 | 1RNM44N |
| anti-mouse CD45 | BV711 | 1:200 | 30-F11 |
| anti-mouse CD64 (FcγRI) | APC | 1:200 | X54-5/7.1 |
| anti-mouse F4/80 | PE/Cy7 | 1:100 | BM8 |
| anti-mouse Ly-6C | BV605 | 1:500 | HK1.4 |
| anti-mouse Ly-6G | PerCP/Cyanine5.5 | 1:300 | 1A8 |
| anti-mouse/human CD11b | BV421 | 1:800 | M1/70 |
| Live/Dead stain | BV510 | 1:150 | n.a. |
Table 1: Antibody index for myeloid panel. Antibodies used for the myeloid gating strategy.
| Antibody | Fluorophore | Staining Concentration | Clone |
| anti-mouse CD104 | PerCP/Cyanine5.5 | 1:200 | 346-11A |
| anti-mouse CD140a (Pdgfra) | PE/Cyanine7 | 1:200 | APA5 |
| anti-mouse CD31 | FITC | 1:200 | 390 |
| anti-mouse CD326 (Ep-CAM) | PE | 1:200 | G8.8 |
| anti-mouse CD45 | APC/Cyanine7 | 1:200 | 30-F11 |
| anti-mouse I-A/I-E (MHC-II) | BV421 | 1:400 | M5/114.15.2 |
| Live/Dead stain | BV510 | 1:150 | n.a. |
Table 2: Antibody index for epithelial panel. Antibodies used for the epithelial gating strategy.
Discussion
This protocol outlines a mouse lung digest that isolates approximately 20-25 million cells per mouse with 90%-95% viability. It additionally allows for the collection of BALF for further analysis. The resultant cell suspension is compatible with multiple laboratory techniques, including flow cytometry and fluorescence-activated cell sorting to isolate cells for sequencing or cell culture. Briefly, after perfusion, BALF is collected, and lungs are inflated with dispase. Lungs are then chopped and digested in a liberase/DNase solution. After lysis and filtering, one may proceed with downstream applications, including flow cytometry analysis of myeloid and epithelial subsets during influenza virus infection.
If BALF cannot be retrieved and/or lungs do not properly remain inflated, it may be due to incorrect catheter placement. If the catheter is placed too far into the trachea/bronchi, it can break through the tissue into the peritoneum. This will result in a lack of inflation and an inability to retrieve BALF. If the lungs have inflated but liquid cannot be pulled out of one or more lobes of the lung, this may be due to the catheter being directed into one bronchus. Pulling the catheter slightly further out should remedy this issue. Sufficient perfusion is required to eliminate contamination from circulating immune cells21. In severely damaged lungs, hemorrhages and decreased barrier integrity can make the alveoli more susceptible to bursting from the pressure of perfusion22. Therefore, it is critical to perfuse mice with damaged lungs very slowly.
Below are some guidelines for best practices in flow cytometry staining. Regarding myeloid gating, if a Siglec-F+CD11c- population is present in the macrophage gate (CD45+CD64+F4/80+), it is likely contamination from eosinophils. We have found eosinophils to be autofluorescent in both PE and BV605 channels - therefore, it is critical to avoid using a CD64 antibody conjugated with these fluorophores. Furthermore, although this digestion method can isolate live lymphocytes, many lymphocyte-specific surface markers, such as CD4 and CD8, are cleaved in the process. As a result, this protocol is not suitable for flow cytometry analysis of T cell populations. In such cases, a collagenase-based approach may be a more effective alternative23,24.
There are many experimental questions that require the analysis of both immune and non-immune fractions from a single mouse25,26. For example, when studying interactions between different cell types, it is critical to collect and analyze populations from within a single mouse to minimize potential artifacts that can arise due to biological variability between individual animals. In addition, comparing immune and non-immune cell populations from the same microenvironment allows for a more complete understanding of tissue dynamics and their roles in both maintaining homeostasis and driving disease processes.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (R35GM150816 and P30DK043351), Charles H. Hood Foundation, and Harvard Stem Cell Institute. We thank Alexander Mann and all other members of the Franklin laboratory for their help and advice in designing and refining the flow cytometry gating schemes and analyses. We also thank the Immunology Flow Cytometry Core at Harvard Medical School. Flow cytometry analysis was performed using FlowJo. Figure schematics were created using BioRender.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL syringe with Slip Tip | VWR | BD309659 | |
| 1.7 mL microcentrifuge tube | DOT Scientific | RN1700-GMT | |
| 10 mL pipettes (disposable) | Fisher Scientific | 12-567-603 | |
| 10 mL Syringe with BD Luer-Lok Tip | VWR | 75846-756 | |
| 123 count eBeads Counting Beads | Thermo Scientific | 01-1234-42 | |
| 12-channel pipette (30-300ul) | USA Scientific | 7112-3300 | |
| 16% paraformaldehyde | VWR | 100503-917 | |
| 23 G needle with regular bevel | VWR | 305194 | |
| 27 G needle with regular bevel | VWR | BD305109 | |
| 5 mL pipettes (disposable) | Thermo Fisher Scientific | 170373 | |
| 50 mL centrifuge tubes | Olympus | 28-108 | |
| 96-well round bottom plate | Corning | 3797 | |
| ACK lysing buffer | Gibco | A100492-01 | |
| Alexa Fluor 488 anti-mouse CD11c | BioLegend | 117311 | |
| Anti-F4/80 Rat Monoclonal Antibody (PE (Phycoerythrin)/Cy7) | BioLegend | 123114 | |
| APC anti-mouse CD64 (FcγRI) | BioLegend | 139306 | |
| APC/Cyanine7 anti-mouse CD45 | BioLegend | 103115 | |
| BD Insyte Autoguard Shielded IV Catheters | VWR | 381423 | |
| Brilliant Violet 421 anti-mouse I-A/I-E (MHC-II) | BioLegend | 107632 | |
| Brilliant Violet 421 anti-mouse/human CD11b | BioLegend | 101235 | |
| Brilliant Violet 605 anti-mouse Ly-6C | BioLegend | 128036 | |
| Brilliant Violet 711 anti-mouse CD45 | BioLegend | 103147 | |
| C57BL/6J mice | Jackson Laboratories | ||
| Cd140a (PDGFRA) Monoclonal Antibody (APA5), PE-Cyanine7, eBioscience | Life Technologies | 25-1401-82 | |
| CD170 (Siglec F) Monoclonal Antibody (1RNM44N), PE | Life Technologies | 12170280 | |
| Cell strainers | Corning | 352350 | |
| Centrifuge | Eppenodorf | Centrifuge 5910R | |
| Deoxyribonuclease I from bovine pancreas (DNase) | Millipore Sigma | DN25-100MG | Reconstituted at 20 mg/mL in DPBS as stock solution stored at -20 °C |
| Dispase | VWR | 76176-668 | Thawed once and stored as 1mL aliquots at -20 °C |
| Dissection forceps (Dumont #7) | Fine Science Tools | 11297-00 | |
| Dissection scissors | Fine Science Tools | 14060-09 | |
| DPBS | Thermo Fisher Scientific | 14190250 | |
| eBioscience fixation kit | Life Technologies | 00-5523-00 | |
| EDTA | Life Technologies | AM9260G | |
| Ethanol | VWR | TX89125170HU | |
| FBS | GeminiBio | 100-106 | Thawed once and heat-inactivated before long-term storage as aliquots at -20 °C |
| FITC anti-mouse CD31 Antibody | BioLegend | 102406 | |
| Gibco RPMI 1640 Medium | Fisher Scientific | 11-875-093 | |
| Glass slides | Fisher Scientific | 12-552-3 | |
| graduated reservoir | USA Scientific | 1930-2235 | |
| Ice bucket | Corning | 432128 | |
| Ketamine hydrocholoride injection (100 mg/mL) | Dechra | Ketamine and xyalazine euthanization mixture can be kept at 30 mg/mL ketamine hydrochloride and 4.5mg/mL xylazine in sterile DPBS for up to one month. | |
| Liberase | Millipore Sigma | 5401119001 | Reconstituted at 5 mg/mL in DPBS as stock solution stored at -20 °C |
| Lids for 96-well plates | Fisher Scientific | 07-201-731 | |
| Orbital Incubator Shaker | Barnstead Lab-Line | SHKE4000 | |
| p1000 pipette | Eppenodorf | 3123000063 | |
| p1000 tips | USA Scientific | 1122-1830 | |
| p200 pipette | Eppenodorf | 3123000055 | |
| p200 tips | USA Scientific | 1110-1700 | |
| PE anti-mouse CD326 (Ep-CAM) | BioLegend | 118206 | |
| PerCP/Cyanine5.5 anti-mouse CD104 Antibody | BioLegend | 123614 | |
| PerCP/Cyanine5.5 anti-mouse Ly-6G | BioLegend | 127616 | |
| Pipet-Aid | Drummond | 4-000-101 | |
| Purified anti-mouse CD16/32 | BioLegend | 101302 | Referred to as "Fc block" in text |
| Spray bottle | VWR | 23609-182 | |
| Suture (Size 2-0) | VWR | 100190-026 | |
| Underpads | VWR | 56617-014 | |
| Xysed (xylazine 100mg/mL) | Pivetal | See ketamine hydrocholoride notes above. | |
| Zombie Aqua Fixable Viability Kit | BioLegend | 423102 |
References
- Wanner, A., Salathé, M., O'Riordan, T. G. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 154 (6), 1868-1902 (1996).
- Mason, R. J., Williams, M. C. Type II alveolar cell. Defender of the alveolus. Am Rev Respir Dis. 115 (1), 81-91 (1977).
- Fehrenbach, H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2 (1), 33-46 (2001).
- Basil, M. C., Alysandratos, K. -. D., Kotton, D. N., Morrisey, E. E. Lung repair and regeneration: Advanced models and insights into human disease. Cell Stem Cell. 31 (4), 439-454 (2024).
- Hussell, T., Bell, T. J. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 14 (2), 81-93 (2014).
- Gschwend, J., et al. Alveolar macrophages rely on GM-CSF from alveolar epithelial type 2 cells before and after birth. J Exp Med. 218 (1), e20210745 (2021).
- Gillich, A., et al. Capillary cell-type specialization in the alveolus. Nature. 586 (7831), 785-789 (2020).
- Schyns, J., et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat Commun. 10 (1), 3964 (2019).
- Chakarov, S., et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science. 363 (6432), eaat3773 (2019).
- Tsukui, T., et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 11 (1), 1920 (1920).
- Aegerter, H., et al. Influenza-induced monocyte-derived alveolar macrophages confer prolonged antibacterial protection. Nat Immunol. 21 (2), 145-157 (2020).
- Johansson, C., Kirsebom, F. C. M. Neutrophils in respiratory viral infections. Mucosal Immunol. 14 (4), 815-827 (2021).
- Li, F., et al. Monocyte-derived alveolar macrophages autonomously determine severe outcome of respiratory viral infection. Sci Immunol. 7 (71), eabj5761 (2022).
- Moll, H. P., et al. Orthotopic transplantation of syngeneic lung adenocarcinoma cells to study PD-L1 expression. J Vis Exp. (143), e58101 (2019).
- Warshamana, G. S., Corti, M., Brody, A. R. TNF-α, PDGF, TGF-β1 expression by primary mouse bronchiolar-alveolar epithelial and mesenchymal cells: TNF-α induces TGF-β1. Exp Mol Pathol. 71 (1), 13-33 (2001).
- Corti, M., Brody, A. R., Harrison, J. H. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 14 (3), 309-315 (1996).
- Quantius, J., et al. Influenza virus infects epithelial stem/progenitor cells of the distal lung: Impact on Fgfr2b-driven epithelial repair. PLoS Pathog. 12 (5), e1005544 (2016).
- Wu, J., et al. Transcardiac perfusion of the mouse for brain tissue dissection and fixation. BIO Protoc. 11 (8), e3988 (2021).
- Morton, J., Snider, T. A. Guidelines for collection and processing of lungs from aged mice for histological studies. Pathobiol Aging Age Relat Dis. 7 (1), 1313676 (2017).
- Bijgaart, R. J. E., van den Kong, N., Maynard, C., Plaks, V. Ex vivo live imaging of lung metastasis and their microenvironment. J Vis Exp. (108), e53741 (2016).
- Shi, W., et al. Isolation and purification of immune cells from the liver. Int Immunopharmacol. 85, 106632 (2020).
- Short, K. R., et al. Influenza virus damages the alveolar barrier by disrupting epithelial cell tight junctions. Eur Respir J. 47 (3), 954-966 (2016).
- Laidlaw, B. J., et al. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 41 (4), 633-645 (2014).
- D'Agostino, M. R., et al. Protocol for isolation and characterization of lung tissue-resident memory T cells and airway-trained innate immunity after intranasal vaccination in mice. STAR Protoc. 3 (2), 101652 (2022).
- Waal, A. M., de Hiemstra, P. S., Ottenhoff, T. H., Joosten, S. A., vander Does, A. M. Lung epithelial cells interact with immune cells and bacteria to shape the microenvironment in tuberculosis. Thorax. 77 (4), 408-416 (2022).
- Bhattacharya, J., Westphalen, K. Macrophage-epithelial interactions in pulmonary alveoli. Semin Immunopathol. 38 (4), 461-469 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved