Method Article
Isolation And Dendritic Cell-Uptake of Small Extracellular Vesicles from Echinococcus granulosus
In This Article
Summary
Here, we describe in vitro culture conditions, isolation, and increased generation of extracellular vesicles (EVs) from Echinococcus granulosus. The small EVs were characterized by dynamic light scattering and transmission electron microscopy. The uptake by bone marrow-derived dendritic cells and their phenotypic modulation were studied using confocal microscopy and flow cytometry.
Abstract
The secretion of extracellular vesicles by cestodes is crucial for enabling cellular communication not only among parasites but also with host tissues. In particular, small extracellular vesicles (sEVs) act as nano-carriers transferring natural antigens, which are critical in host immunomodulation and parasite survival. This article presents a step-by-step protocol to isolate sEVs from larval stage cultures of Echinococcus granulosus and analyzes their uptake by dendritic cells obtained from murine bone marrow, which acquire adhesion and antigen presentation capacity during their maturation after one week of in vitro culture. This article provides comprehensive information for generating, purifying, and quantifying sEVs using ultracentrifugation alongside parallel analyses of dynamic light scattering and transmission electron microscopy. Additionally, a detailed experimental protocol is outlined for isolating and cultivating mouse bone marrow cells and driving their differentiation into dendritic cells using Flt3L. These dendritic cells can present antigens to naïve T cells, thereby modulating the type of immune response in vivo. Thus, alternative protocols, including confocal microscopy and flow cytometry analysis, are proposed to check the acquired maturational phenotype of dendritic cells previously exposed to parasitic sEVs. Finally, it is worth noting that the described protocol can be applied as a whole or in individual parts to carry out parasite in vitro culture, isolate extracellular vesicles, generate bone marrow-derived dendritic cell cultures, and perform uptake assays with these cells.
Introduction
Echinococcus granulosus is a zoonotic parasitic helminth responsible for a long-term infection known as cystic echinococcosis1. In intermediate hosts, such as livestock and humans, the parasite infection primarily affects the liver and lungs, where the larval stage develops as fluid-filled cysts or metacestodes containing protoscoleces (a larva itself). Like all cestodes, this parasite lacks both digestive and excretory systems and has, therefore, evolved active endocytic and exocytic cellular processes to regulate the uptake and excretion of metabolites as well as the release of extracellular vesicles2,3. Extracellular vesicles (EVs) are lipid bilayer-enclosed particles secreted by apparently all cell types. In particular, small extracellular vesicles (sEVs), defined as EVs smaller than 200 nm regardless of their biogenesis origin4, can act as intercellular immune mediators. This function is especially significant in parasites, which rely on host immunomodulation to ensure their survival3. Immune manipulation is achieved through the uptake of sEVs by host dendritic cells, the only cells capable of activating naive T cells in vivo and initiating an adaptive immune response that will lead to chronic infection by these parasitic worms. Dendritic cells, professional antigen-presenting cells of the innate immune system, process and load antigenic peptides onto Major Histocompatibility Complex Class I and Class II (MHC I and MHC II) and exhibit them on their membranes for exclusive naïve T cell priming (CD8+ and CD4+ T cells, respectively)5. Following dendritic cells induce their maturation by induction of expression of the co-stimulatory markers CD80/CD86 and CD40 and MHC-II and migrate from peripheral tissues to secondary lymphoid organs upon recognizing foreign antigens, loading them for exclusive naïve T cell priming6. Thus, the overall goal of this protocol is to study the helminth parasite-host communication in a realistic manner, analyzing the packaging and delivery of parasitic components in the form of sEVs, which, upon reaching the host immune cells, influence the development of infection and the progression of the chronic parasitic disease.
Addressing the analysis of the helminth-host interface through the study of sEVs has several advantages. First, the tegument, the outer covering of flatworms, is a double membrane structure that constitutes a major crossing point between the parasite and its host, allowing sEVs to be readily generated or permeated from this structure7. Second, sEVs are highly loaded with protein antigens from all stages of the parasite life cycle, representing the natural way through which the host immune system samples antigens during worm infection8,9. Due to their biological production, ease of purification (without requiring tissue disruption or protein fractionation), and direct interaction with host cells, helminth sEVs enable the development of in vitro experiments to simulate the in vivo conditions of parasite-host interaction. Finally, sEVs represent the possibility of having parasitic structures that can be phagocytosed or internalized by host cells, overcoming the impossibility of doing so with whole parasites, particularly in cases of encysted worms.
Considering the advantages mentioned and the fact that helminthiases are prevalent and typically chronic diseases in which parasites presumably manipulate the host immune system as a survival strategy, the isolation of parasite-derived EVs and their study in interaction with dendritic cells provides a valuable framework to explore this immunomodulation10. In this sense, it has been described that the internalization of EVs from helminths, including nematodes and platyhelminths such as Schistosoma mansoni, Fasciola hepatica, Brugia malayi, and E. granulosus, induces the maturation and activation of dendritic cells9,11,12,13,14,15.
The isolation of helminth-derived EVs not only enables the study of immunological interactions, potentially leading to the development of protective vaccines or immunotherapeutic agents for allergic or autoimmune diseases, but also facilitates the exploration of other biological interactions and functions8,16,17. In this context, EVs, which play a role in the natural history of parasitic infections, could be utilized to investigate parasite development and interactions with specific host cells. Moreover, they could have potential applications as early or differential biomarkers for the diagnosis of parasitic diseases, monitoring therapeutic responses, and contributing to the control and management of parasitic infections17,18.
In addition, as previously demonstrated, the larval stage of E. granulosus is susceptible to changes in cytosolic calcium concentration, which, besides playing a role in parasite viability, also controls the exocytosis rate19,20. In this context, and knowing that intracellular calcium elevation enhances EV release, using an intracellular calcium enhancer as loperamide could be a crucial strategy to increase the number of EVs. This approach is particularly interesting for cellular systems that require large populations to generate an adequate quantity of EVs for cargo and functional analysis11,21,22. The current protocol (Figure 1) details the methods for obtaining pure cultures of E. granulosus larval stage and the conditions that enhance sEV production. It also describes the workflow for the isolation and characterization of these vesicles, as well as their uptake by murine dendritic cells, an essential step in the initial study of host immune system modulation.
Protocol
All procedures involving animals were evaluated and approved by the Animal Experimental Committee of the Faculty of Exact and Natural Sciences, Mar del Plata (permit numbers: RD544-2020; RD624-625-2021; RD80-2022). In this protocol, mice were euthanized, according to "Guide for the Care and Use of Laboratory Animals" published by the NIH and the guidelines of the National Health Service and Food Quality (SENASA).
1. Echinococcus larval stage cultivation
NOTE: All procedures were performed under aseptic conditions.
- E. granulosus protoscoleces obtention
- Aspirate with a 21 G needle and 10 mL syringe part of hydatid fluid from the lung or liver of infected cattle to reduce cyst turgor (Figure 2A).
NOTE: Infected lungs and livers come from cattle presented for routine slaughter at the abattoir. They must be kept at 4 °C and processed within 24 h of slaughter. - Open the cyst with scissors and remove the laminar and germinal layers from the cyst using forceps. Place them onto a sterile Petri dish, along with any remaining hydatid fluid (Figure 2B,C).
NOTE: At this point, it is recommended to observe the Petri dish under an inverted microscope to assess the quality of protoscoleces and brood capsules. Avoid pooling the biological material from cysts with more than 50% of protoscoleces collapsed, which are identified by their contracted soma, darker color, and disorganized rostellum with loss of hooks. - Wash the layers with sterile phosphate buffered saline (PBS) supplemented with antibiotics (100 μg/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL gentamicin 100 μg/mL) to remove the protoscoleces.
NOTE: All washes will be performed with PBS supplemented with antibiotics. - Transfer the protoscolex suspension to a sterile glass Khan tube using a Pasteur pipette.
- Wash the protoscoleces with supplemented PBS at 4 °C using a Pasteur pipette to remove dead parasites. Vigorously resuspend the suspension to break the brood capsules facilitating the protoscolex release. Allow the protoscoleces to settle for 1–2 min on the bottom of the tube; then discard the supernatant containing the dead protoscoleces.
NOTE: Due to a difference in density, living protoscoleces settle faster than dead parasites. - Repeat the washing process until all dead and floating protoscoleces have been removed.
NOTE: The dead parasites are removed when all settle at the same rate. - Determine the viability of protoscoleces using the methylene exclusion test.
- Resuspend the washed protoscoleces with a pipette and place a drop on a slide. Add a drop of 0.1 mg/mL methylene blue and cover with a coverslip. Wait for 2–3 min and observe under the microscope.
- Count the total number of living (unstained) and dead (blue-stained) protoscoleces, and calculate the percentage of viable protoscoleces (Figure 2D and inset).
NOTE: The viability of protoscoleces should be around 98% before establishing the cultures. The staining time should not exceed 10 min, as longer durations may result in the staining of living parasites.
- Aspirate with a 21 G needle and 10 mL syringe part of hydatid fluid from the lung or liver of infected cattle to reduce cyst turgor (Figure 2A).
- E. granulosus metacestodes obtention
- Produce an experimental secondary hydatid disease by intraperitoneally infecting female CF1 mice (body weight 25 ± 5 g) with 1500 protoscoleces (equivalent to 10 μL of the protocolex pellet) suspended in 0.5 mL of supplemented PBS (Figure 2E).
NOTE: It is recommended to maintain the protoscoleces in PBS supplemented with antibiotics at 4 °C for 24 h or in culture for 3–5 days prior to infection. - Metacestodes develop within 4-6 months post-infection (Figure 2F). During this period, house the animals under controlled laboratory conditions (temperature 20 ± 1 °C, 12 h light/dark cycle, and water and food provided ad libitum). Once the cysts have developed, anesthetize the mice using ketamine-xylazine (50 mg/kg/mouse-5 mg/kg/mouse) and euthanize them by cervical dislocation.
- Clean the ventral surface of the mouse with 70% alcohol and surgically open the peritoneal cavity to remove the developed metacestodes using scissors and forceps.
- Transfer the metacestodes masses to a sterile Petri dish.
NOTE: The metacestode masses consist of multiple internal cysts surrounded by connective tissue. - Release the cysts from the metacestode masses by carefully removing the connective tissue covering the metacestodes using forceps if required.
NOTE: This step ensures that the parasites are free from host tissue. - Wash the obtained metacestodes with supplemented PBS at 4 °C (Figure 2G).
- Produce an experimental secondary hydatid disease by intraperitoneally infecting female CF1 mice (body weight 25 ± 5 g) with 1500 protoscoleces (equivalent to 10 μL of the protocolex pellet) suspended in 0.5 mL of supplemented PBS (Figure 2E).
- E. granulosus protoscoleces and metacestodes cultivation
- Prepare the culture medium as follows: Add 100 μg/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL gentamicin, 4 mg/mL glucose, 50 mM Hepes buffer pH 7.5, and medium 199 to reach the desired final volume and mix gently by inversion.
- Transfer 5 mL of the prepared culture medium into each Leighton tube (Figure 2H–I).
- Add the parasites to the culture medium and incubate at 37 °C for 5 days without changing the medium. Incubate the Leighton tubes at a 15° angle to ensure even distribution of the biological material across the flat surface. This maximizes parasite exposure to the culture medium while preventing contact with the rubber stopper.
NOTE: Add 9,000–10,000 protoscoleces or 50 metacestodes (with diameters ranging between 5 mm and 15 mm distributed as 10 cysts per tube). - Optionally, add 20 μM loperamide (a sub-lethal concentration) dissolved in dimethyl sulfoxide for 16–24 h into the parasite culture medium to increase the cytosolic calcium level and enhance the EV release by the larval stage of E. granulosus.
NOTE: Given that an increase in intracellular calcium concentrations enhances EV production, treating the parasite with compounds that affect calcium homeostasis will raise the EV release.
2. Extracellular vesicles purification
- Collect the parasite culture medium from each Leighton tube and transfer it to a 15 mL conical tube.
NOTE: The culture medium can be stored for 24 h before first centrifugation with minimal impact on the concentration or size distribution of vesicles. Protoscoleces can be washed three times with PBS buffer, harvested, and stored at -20 °C in 1.5 mL tubes. - Centrifuge at 300 x g for 10 min at 4 °C and transfer the supernatant to a new 15 mL conical tube using a pipette.
NOTE: This step removes dead protoscoleces. After each centrifugation step, ensure that at least 0.5 cm of supernatant remains above the pellet to avoid contamination. - Centrifuge the supernatant at 2,000 x g for 10 min at 4 °C and transfer it to new 1.5 mL tubes using a pipette.
NOTE: This step removes larger cell debris. - Centrifuge at 10,000 x g for 30 min at 4 °C to remove smaller cell debris.
- Transfer the supernatant to a tube suitable for the ultracentrifuge rotor using a pipette. Mark one side of each tube with a marker before placing it in the ultracentrifuge rotor. Then, place the tube in the rotor with the marked side facing up.
NOTE: The mark serves as a reference point for locating the pellet after ultracentrifugation. Tubes must be three-quarters full and precisely balanced; therefore, if needed, add PBS. If the supernatant volume exceeds the capacity of a single tube, divide the samples into multiple tubes and combine them during resuspension. - Centrifuge at 100,000 x g for 1 h at 4 °C and pour off the supernatant in a quick action. Allow the tube to rest upside down for 1 min. The pellet may not be visible at this stage.
NOTE: The k-factor for the rotor used is 103. - Wash the pellet with at least 3 mL of PBS to remove contaminating proteins. Resuspend the pellet by pipetting up and down multiple times from the upper side of the tube to the bottom on all tube faces but principally on the marked side where the pellet is expected to be.
NOTE: If applicable, pool the resuspended pellet derived from the same supernatant into a single tube. - Centrifuge at 100,000 x g for 1 h at 4 °C and pour off the supernatant in a quick action. Allow the tube to rest upside down for 1 min.
NOTE: The k-factor for the rotor used is 103. - Resuspend the pellet in 30 µL of PBS following the process described in step 2.7.
NOTE: At this point, it is recommended to measure the total protein concentration in the resuspended pellet to estimate the amount of the secreted sEVs. Protein concentration can be determined by measuring absorbance at 280 nm using a microvolume spectrophotometer. - Transfer the sample to a 1.5 mL tube. Freeze the extracellular vesicles at -80 °C.
NOTE: To preserve vesicle integrity for downstream applications, freeze the resuspended pellet as quickly as possible and avoid repeated freeze-thaw cycles.
3. Characterization of the isolated vesicles
- Determination of EV size using dynamic light scattering (DLS)
NOTE: Dynamic Light scattering is a reliable and sensitive method for evaluating the size (based on the hydrodynamic radius, Rh) and shape of nanoparticles in complex fluids, regardless of their type. DLS and zeta potential measurements were performed using a He-Ne monochromatic laser beam at 633 nm. If the analysis is not possible on the sampling day, the samples may be frozen in a pre-filtered buffer before storage.- Defrost the samples and maintain them on ice until the measurements are taken.
NOTE: Freezing the samples could affect the particle distribution and integrity of sEVs. Therefore, avoid freezing and thawing cycles, as they can lead to a decrease in LS signal with reduced peak heights. - Filter the aqueous samples used for DLS through a 0.2 µm pore size filter.
NOTE: In LS experiments, it is essential to filter all solutions, buffers and aqueous samples to remove large particles and dust, which can interfere with measurements. - Dilute the samples 1:10 to 1:50 in pre-filtered PBS.
- Mix the samples by gentle inversion before each measurement to prevent sedimentation, as the LS intensity can decrease due to longer sample processing time.
- Add 1 mL of the sample into a clean cuvette, positioning the non-frosted side in the laser path on the left into the instrument. Close the lid and allow 2–3 min for equilibration before measuring the sample.
- Measure the size in terms of the hydrodynamic radius (Rh) before performing the zeta potential measurement. Record measurements at a single scattering angle (θ = 90° to 150°) at 25 °C ± 1 °C.
- Click on Control Panel to check the readings and start data acquisition.
NOTE: Based on the mean Rh values and the assessed size distributions for sEVs, a peak between 30 nm and 200 nm should be observed (Figure 3). Peaks below 15 nm in Rh are attributed to nucleic acids and proteins in suspension. Typically, size distributions are calculated from the mean hydrodynamic radius. However, the Z-average (mean particle size in the sample), the polydispersity index (PDI, which determines the size heterogeneity of a sample), and the angular dependency of scattered light intensity can also be reported.
- Defrost the samples and maintain them on ice until the measurements are taken.
- Determination of structure and particle size by transmission electron microscopy (TEM)
NOTE: Perform negative-stain transmission electron microscopy to assess the size, structure, and purity of sEVs, using a standard protocol that includes fixation, dehydration, resin embedding, and contrasting for whole-mount sEV preparations.- Defrost the concentrated sEV samples from step 2.10 and maintain them on ice.
- Fix sEVs in 1.5 mL tubes. Carefully apply 5–10 µL of 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7) to ~5 µL of the sEV sample pellet (required sEV concentration ≈ 1 x 108–1 x 109 mL-1) and incubate for 2 h at 4 °C.
NOTE: sEVs can be stored for up to 1 week at 4 °C in 0.1 M sodium cacodylate buffer before further processing. Therefore, if external technical services are required for TEM analysis, send the fixed sEV samples refrigerated in 1.5 mL tubes. - Deposit 5 µL of the resuspended pellets on the 300-mesh Formvar-carbon coated EM copper grids. Prepare two or three grids for each sample.
- Allow the sample to adsorb for 20 min in a dry environment and remove excess sample from the grid using filter paper.
- Place 100 µL drops of PBS onto a sheet of film. Using clean forceps, transfer the grids (with the adsorbed sample side down) into the PBS drops for washing.
- Dry the opposite side of the adsorbed sample, ensuring that the sample side of the grids does not dry during any of the following steps.
NOTE: For all subsequent steps, place drops of reagents onto a flat film and transfer the grids to the drops using forceps. - Transfer the grids to a 50 µL drop of 1% glutaraldehyde for 5 min.
- Wash the grids with a 100 µL drop of distilled water and let them stand for 3 min. Repeat nine times for a total of ten washes.
- Contrast the sample grids with a 50 µL drop of 1% w/v uranyl-acetate solution, pH 7, for 1 min.
- Contrast and embed the grids in a mixture of 100 µL of 4% uranyl acetate and 900 µL of 2% methyl cellulose.
- Air-dry the grids for 5–10 min while they remain on the loop, and observe them with an electron microscope at 80–100 kV with a resolution of 0.2 nm and magnification of 100,000x.
- Store the grids in dry storage boxes for long-term storage.
4. Bone marrow-derived dendritic cell generation
NOTE: This procedure should be performed using young mice, which are characterized by robust hematopoietic systems with active proliferation and differentiation capacities. In contrast, older mice exhibit declines in hematopoietic function, reduced stem cell reserves, altered niche interactions, and a more developed memory reservoir which is crucial for long-term immunity and response to pathogens or age-related changes such as immunosenescence.
- Euthanize a 5–8 week-old female CF-1 mouse according to institutional ethical guidelines, minimizing animal suffering.
- Spray the mouse with ethanol before placing it into a tissue culture hood.
NOTE: The following steps must be performed under sterile conditions. - Place the mouse on a dissecting board in the supine position. Using forceps and dissecting scissors, make a vertical "T" incision above the urethra and extend it horizontally to the top of the lower extremities, taking care not to break the peritoneum or puncture any organs, especially the gut.
- Separate the skin along both hind limbs to expose leg bones and tissues using forceps. With the hands, remove the skin of each leg pushing from the ankle towards the abdomen, pulling the skin to the opposite side. Both legs will then be free of skin.
NOTE: Discard the mouse body in the pathogen residue bag. - Using scissors and forceps, carefully remove the femur and tibia, avoiding breakage. Fasten the tip of each bone with forceps and cut tendons to remove muscle fascias around bones. Complete cleaning the muscle tissue with paper napkins.
- As each bone is removed, place it in a sterile 50 mL tube containing 2 mL of complete medium prepared with RPMI medium supplemented with 5% FBS, 100 U/ml penicillin, 100 U/mL streptomycin and 10 µg/mL gentamicin to remove debris. Following, aspirate and discard the culture medium and wash the bones twice with 70% ethanol for 5 min.
- Transfer the bones to a sterile Petri dish.
- Cut off the two bone epiphyses using sharp dissecting scissors to access bone marrow cells.
- Carefully flush bone marrow cells from each of the four bones into a sterile Petri dish using a 25 G needle attached to a 20 mL syringe containing complete medium. The bones will become more transparent as the bone marrow is extracted.
NOTE: A volume of 20 mL of the complete medium should be sufficient to elute the bone marrow cells from the two femurs and tibias. - Gently homogenize the medium with the eluted marrow by pipetting to remove bone connective tissue and cell lumps. Afterward, transfer the sample to a sterile 50 mL conical tube, passing the cells through a sterile 70 µm polypropylene cell strainer to remove connective tissue and bone debris.
- Centrifuge the cells at 450 x g for 7 min at 4 °C. Carefully remove and discard the supernatant, ensuring the pellet remains adhered to the tube wall.
- Incubate the cells for 1 min at room temperature (RT), then resuspend them in 500 µL of red blood cell (RBC) lysis buffer. Neutralize the lysis buffer by adding 3 mL of complete medium.
- Centrifuge the cells at 450 x g for 7 min at 4 °C. Discard the supernatant and resuspend the pellet in 5 mL of complete medium.
- Pass the cell suspension through a sterile 70 μm polypropylene cell strainer into a sterile 50 mL conical tube to remove cell aggregates from lysed erythrocytes.
- Count the cells using a hemocytometer.
- Add 300 ng/mL of recombinant murine Fms-related tyrosine kinase 3 ligand (Flt3L) to the culture medium.
- Plate the cells at a concentration of 1 x 106 cells/mL in a multiwell plate.
- Incubate the cells for 7 days at 37 °C in a humidified atmosphere with 5% CO2.
NOTE: Avoid shaking the cells while they differentiate and grow to prevent spontaneous maturation. - On day 3, remove 1 mL of medium from each well (avoiding disturbance of the cells) and replace it with 1 mL of pre-warmed fresh complete medium supplemented with 150 ng/mL recombinant murine Flt3L.
5. Interaction between bone marrow-derived dendritic cells and extracellular vesicles from E. granulosus
- Extracellular vesicle membrane staining
- Defrost the sEVs stored in step 2.10 and maintain them on ice.
- Resuspend 10 μL of purified sEVs in 10 μL of labeling vehicle (Diluent C).
- Add 20 μL of 2x PKH26 dye solution to achieve a final concentration of 2 μM. Mix gently using a pipette and incubate for 35 min at 37˚ C in darkness.
NOTE: The 2x PKH26 dye solution (4 μM) must be prepared immediately before staining by adding 0.5 μL of PKH26 ethanolic dye solution to 125 μL of Diluent C. - Mix gently every 3–5 min during the incubation to ensure homogeneous staining.
- Add 40 μL of BSA 1% and incubate for 10 min at RT to stop the staining process.
- Wash the sEVs with 1 mL of PBS and centrifuge at 100,000 x g for 1 h at 4 °C to remove excess dye.
NOTE: The k-factor for the rotor is 103. - Resuspend the pellet in 90 µL of PBS following the protocol described in step 2.7.
- Stimulation of murine bone marrow-derived dendritic cells with extracellular vesicles of E. granulosus
- Harvest bone marrow-derived dendritic cells (BMDCs) from the culture plate well and transfer them to 1.5 mL tubes.
NOTE: Handle carefully to avoid spontaneous cell maturation. - Centrifuge the medium at 450 x g for 5 min to pellet the cells.
NOTE: Reserve the supernatants from the centrifugation to replate the cells in subsequent flow cytometry analysis steps. - Resuspend BMDCs in 30 µL of unstained sEVs from step 2.10 for flow cytometry analysis or in 90 µL of PBS-containing stained-sEVs from step 5.1.7. for confocal microscopy. Incubate for 30 min at 37 °C in a humidified atmosphere with 5% CO2. Gently mix the sample every 10 min.
NOTE: To ensure effective contact between BMDCs and sEVs, the first 30 min of incubation should be performed in a minimal volume using 1.5 mL tubes. - For confocal microscopy, transfer the cells onto an Alcian blue-treated glass coverslip (12 mm diameter) placed in a 24-well plate. Incubate for an additional 30 min at 37 °C in a humidified chamber with 5 % CO2.
NOTE: The Alcian-blue treatment imparts a positive charge to the coverslip, facilitating the adherence of the negatively charged plasma membranes of BMDCs.- To prepare the coverslips, immerse them in a filtered 1% Alcian blue 8 GX dye and heat them in a microwave for 1–2 min without boiling. Incubate them in the hot solution for 10 min, swirling every 2 to 3 min.
- Then, wash the coverslips with deionized distilled water to remove excess Alcian blue, and dry them on paper towels. Finally, autoclave the coverslips and store them in sterile conditions until use.
- For flow cytometry analysis, transfer the BMDCs-sEVs back into the same well from which they were harvested (see step 5.2.1), add the reserved supernatants from step 5.2.2 and incubate for 18 h at 37 °C in a humidified atmosphere with 5% CO2.
NOTE: Leave approximately 500 µL of medium in the well to prevent desiccation of remaining cells after collection. - Use positive and negative controls to assess BMDC maturation. Stimulate the cells with 100 ng/mL of lipopolysaccharide (LPS) for 18 h as a positive control. For a negative control of endocytosis, incubate BMDCs with sEVs at 4 ˚C. Also, include an unstimulated dendritic cell control.
- Harvest bone marrow-derived dendritic cells (BMDCs) from the culture plate well and transfer them to 1.5 mL tubes.
- Confocal microscopy of extracellular vesicles from E. granulosus captured and internalized by bone marrow-derived dendritic cells.
- Once the incubation period described in step 5.2.4 has been completed, aspirate and discard the PBS from the coverslip.
- Fix BMDCs by adding 100 µL of 4% paraformaldehyde (PFA) over the coverslip and incubate for 10 min at RT.
NOTE: Ensure the volume maintains the surface tension on the coverslip. - Aspirate and discard the PFA, then wash the coverslip three times with PBS-BSA 2%.
- Add 100 µL of PBS containing anti-MHC class II-FITC antibody diluted 1/100 and incubate for 1 h at RT in darkness.
- Aspirate and discard the antibody solution, and wash the coverslip three times with PBS-BSA 2%.
- Add 100 µL of 50 ng/mL DAPI and incubate for 30 min at room temperature in a wet chamber to counterstain nuclei.
- Aspirate and discard the dye solution and wash the coverslip three times with PBS-BSA 2 %.
- Remove the coverslip using curved-fine point forceps and dry it on a paper towel to eliminate excess liquid.
- Mount the coverslip facing down onto a glass slide using a mounting medium composed of polyvinyl alcohol and glycerol. Let dry for 2 h at 37 °C or overnight at 4 °C in darkness.
- Remove any air bubbles between the coverslip and the glass slide by gently pressing down on the coverslip with forceps.
- Observe the mounted samples under a confocal microscope using a 60x oil immersion objective with an excitation/emission wavelength of 485/538 nm for FITC, 358/461 nm for DAPI, and 551/567 nm for PKH26.
- Phenotypic evaluation of extracellular vesicle-stimulated bone marrow-derived dendritic cells by flow cytometry
- After the incubation period described in step 5.2.5, harvest BMDCs by flushing the medium up and down several times with a pipette.
- Collect the medium-containing BMDCs and place it into 1.5 mL tubes.
- Centrifuge at 450 x g for 5 min at 4 °C to pellet the cells.
NOTE: Optionally, to analyze cytokine secretion, remove the supernatants with a pipette, transfer them into new 1.5 mL tubes, and store them at -20 °C for further enzyme-linked immunosorbent assay (ELISA) tests. - Resuspend the BMDCs in 100 µL of PBS containing fluorescein isothiocyanate (FITC), allophycocyanin (APC), or phycoerythrin-conjugated mAbs directed to CD11c, CD40, CD80, CD86, MHC class I and MHC class II and incubate for 15 min at 4 °C in the dark.
- Wash the BMDCs with PBS and centrifuge at 450 × g for 5 min at 4 °C.
- Resuspend BMDCs in 500 μL of 1% PFA to fix them and store them at 4 °C until acquisition in a flow cytometer.
Results
A flowchart summarizing the main steps for maintaining pure cultures of the E. granulosus larval stage, the isolation and characterization of sEVs, and their uptake by murine dendritic cells are shown in Figure 1. To achieve high sEV production from E. granulosus protoscoleces and metacestodes, an in vitro culture method previously developed in the laboratory was employed to maximize the survival and metabolic homeostasis of the studied parasite (Figure 2).

Figure 1: Overview of the experimental procedures for obtaining E. granulosus sEVs and BMDCs. Schematic representations describing the steps followed in this protocol from obtaining and cultivating the parasite material (STEP 1), isolating (STEP 2), and characterizing parasite EVs (STEP 3) to the generation of BMDCs (STEP 4) and their interaction with E. granulosus sEVs (STEP 5). Please click here to view a larger version of this figure.
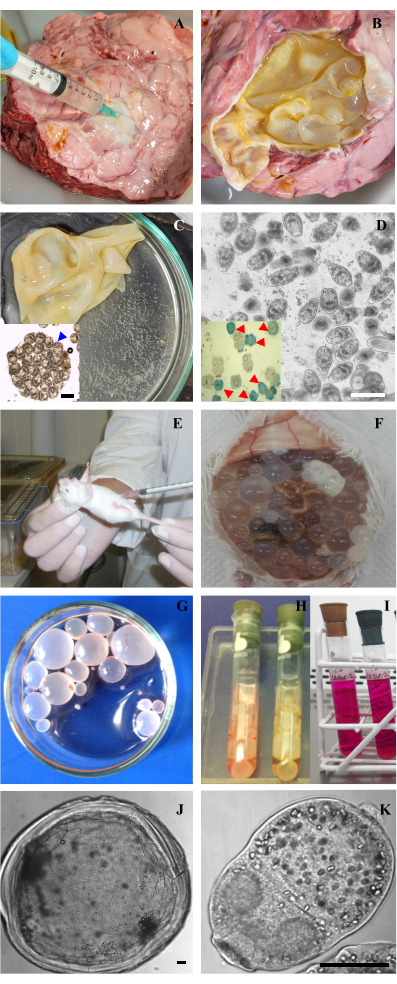
Figure 2: Acquiring samples and in vitro culture of protoscoleces and metacestodes of Echinococcus granulosus. (A) Puncture of pulmonary hydatid cyst of cattle to extract the hydatid fluid. (B) Opened Hydatid cyst with its membrane exposed. (C) Hydatid membrane and its hyaline fluid with brood capsules and free protoscoleces named “hydatid sand”. Inset: Brood capsule with protoscoleces inside with preserved morphology. (D) Optical microscopy of vital protoscoleces after removal of dead protoscoleces. Bar 200 μm. Inset: Methylene blue staining of protoscoleces showing alive protoscoleces (translucent) and dead protoscoleces (stained blue, indicated with red arrowheads). (E) Inoculation of protoscoleces into the peritoneal cavity of a CF-1 mouse. (F) Metacestodes within the abdominal cavity developed after 7 months of inoculation with protoscoleces. (G) Cysts isolated from a mouse and washed with PBS into a Petri dish. (H,I) In vitro maintenance of metacestodes and protoscoleces in Leighton tubes with M199 medium. (J,K) Optical microscopy of a metacestode and a protoscolex of E. granulosus. Bars indicate 50 μm. Please click here to view a larger version of this figure.
The characterization of EVs purified from the E. granulosus larval stage is shown in Figure 3. The EVs isolated following the current protocol were mainly sEVs with diameters ranging from 50 to 200 nm as determined by DLS (Figure 3A) and confirmed by MET (Figure 3B). TEM analysis also revealed that the sEVs exhibited the typical cup-shaped morphology of exosome-like vesicles (Figure 3B). In addition, TEM confirmed that sublethal concentrations of loperamide can act as an EV-release enhancer, as shown by an increased ratio of sEVs in loperamide-treated samples compared to controls. Likewise, the higher abundance of sEVs obtained from loperamide-treated parasites was further supported by protein concentration measurements (11 ± 1.5 μg/μL compared to 6 ± 1 μg/μL in controls, Figure 3C).

Figure 3: Characterization of extracellular vesicles purified from E. granulosus larval stage. (A) Dynamic Light Scattering (DLS) plot depicting the size distribution of isolated EVs from control (Co) and loperamide (Lp)-treated protoscoleces (PTS). (B) Transmission electron microscopy (TEM) photographs of negatively-stained EVs purified from control (a) or loperamide-treated PTS (d). Scale bars indicate 50 nm. Arrowheds indicate abundant exosome-like vesicles with the typical cup-shaped structure. (b-c) and (e-f) corresponds to amplifications of boxed areas from (a) and (d), respectively. (C) Protein concentration of ultracentrifugation pellet from seven independent assays. Data are presented as the mean ± SD. Asterisk indicates significant differences (Kruskal-Wallis with Dunn’s post-test, p < 0.05). Please click here to view a larger version of this figure.
Figure 4 illustrates the progression of BMDC generation through observations of variations in the quantity and morphology of bone marrow cells from the initial culture establishment to full differentiation. On day 0, hematopoietic cells were grown in a complete medium at a density of 1× 106 cells/mL supplemented with 300 ng/mL Flt3L, a cytokine and growth factor that activates hematopoietic progenitors to proliferate and differentiate into dendritic cell morphology. On days 1–2, the cells were still small and rounded in shape without significant morphological changes, although their quantity had increased. Between days 3–5, active cytoskeletal remodeling occurred, resulting in heterogeneous, elongated shapes with an increase in cytoplasmic extensions (dendrites). At this point, cultures contained adherent cells with pronounced cytoplasmic extensions, non-adherent cells undergoing morphological changes during dendritic cell differentiation, and spherical non-adherent cells that remained unresponsive to cytokine induction (Figure 4). After days 6–7, 80%–90% of the cultures mainly consisted of steady-state and fully differentiated BMDCs. These cells exhibited a stellate shape with extensive cytoplasmic processes optimized for antigen capture and presentation. On day 7, the BMDCs were harvested to analyze their phenotype and perform functional assays.
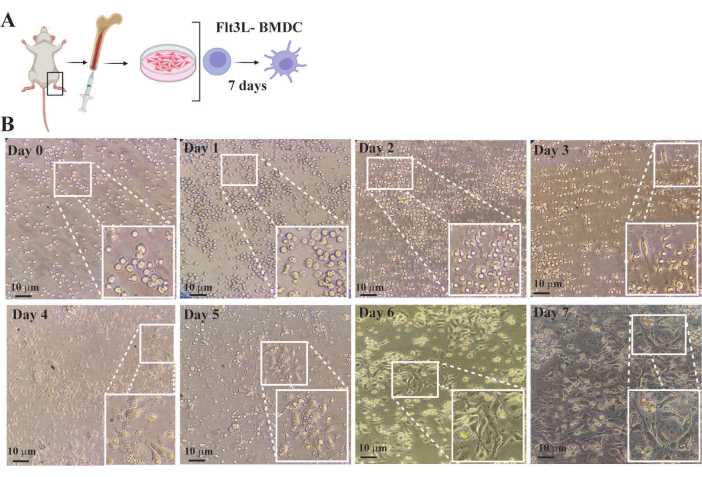
Figure 4: Monitoring of FTL3L bone marrow-derived dendritic cell differentiation using a conventional inverted optical microscope. (A) Schematic representation of hematopoietic precursors purification from mouse bone marrow and cell culture in complete medium supplemented with flt3L B- Images of bone marrow cell culture obtained at different time points. Insets are magnified regions of the boxed area from each image. (B) Day 0: Image of recently purified hematopoietic cells from bone marrow. It is observed that although the population is heterogeneous, the majority are cells with rounded morphology and small size. Days 1-5: culture images showing the increase in cell number and changes in morphology due to active proliferation induced by FLT3 signaling pathway activation. Cell morphology transitions from rounded to elongated with a progressive increase in cytoplasmic extensions (dendrites). These changes are most noticeable when the cells adhere to the wells of the culture plate. Days 6-7: images showing fully differentiated BMDCs at steady state characterized by irregular shape with a prominent nucleus and numerous fine branching projections, the cytoplasm is highly granular with vesicles involved in antigen presentation and immune response. Bar represents 10 µm. Please click here to view a larger version of this figure.
Since dendritic cells are essential mediators in the activation and orientation of the induced immune response, it is crucial to perform functional analyses of these cells to reveal the potential roles of sEVs in host-parasite interactions. The protocol presented here confirms that BMDCs capture parasite-sEVs after 1-hour incubation at 37 ˚C. Interestingly, the sEVs appear to be located in endosomal-lysosomal compartments where pre-formed MHCII molecules are stored as they are observed to colocalize (Figure 5).
Furthermore, dye-stimulated BMDCs without EVs were included as negative controls to validate labeling specificity. Only nonspecific extracellular fluorescence and 3%–4% of cells with diffuse dye signal were observed compared to over 40% of positively labeled cells exposed to stained sEVs.

Figure 5: Immunofluorescence confocal microscopy of BMDCs showing efficient uptake of labeled sEVs and recruitment of MHC class II to endosomes. (A) Schematic representation of the protocol described in item 5.3 showing the PKH26-labeled sEVs stimulation in BMDCs. (B) Confocal Image showing a negative control of PKH26 stimulated BMDCS without the presence of sEVs (red, PKH26) and (blue DAPI). No fluorescent signals from the dye inside the cell were observed without the sEVs. (C) Separate channels of confocal images showing BMDCs stimulated with stained-sEVs: differential interference contrast microscopy (DIC), DAPI (blue, nuclei), PKH26 (red, sEVs) and FITC (green, MHC class II). Scale bars represent 10 μm. Please click here to view a larger version of this figure.
Discussion
The protocol workflow for culturing parasites, isolating parasite-derived sEVs, differentiating dendritic cells from bone marrow, and analyzing sEV uptake by these cells is outlined in Figure 1. The aim was to describe in detail each protocol section that may be carried out as a whole or separately, highlighting the major considerations to guarantee the implementation of the methodology. The analysis of the population of EVs obtained from complete parasitic organisms has a concrete impact on the study of the parasite-host interrelationship, and precisely in this context, this experimental protocol was outlined.
The new insights into parasitic diseases have been hindered by the difficulty of in vitro culturing of some of these parasites. As parasite helminths fail to complete their life cycles under artificial conditions, they can only survive in a culture as full organisms. Therefore, the solution proposed by this protocol to study the worm-host interface is to carry out independently the cultivation of each parasitic form (adult worm and different larval forms) to enrich and isolate sEVs, and then confront them with the cells of the target organs or cells of the host immune system, allowing them to be internalized or phagocytosed, respectively. In addition, a critical step to establish a helminth culture to isolate EVs is to strictly select live parasites to start the in vitro culture and keep them at incubations as short as possible to limit parasite death and the generation of free residual membranes23. To make it in E. granulosus, recovered protoscoleces from hydatid cysts are subjected to intensive washing with cold PBS in small-diameter glass tubes. During vigorous dispersion with a pipette, the live protoscoleces sediment quickly. Thus, they are separated from the non-viable protoscoleces that remain suspended for a longer time in the liquid column of the tube, allowing them to be captured and eliminated from the mixture. In line with this purpose, during each washing step, vigorously mixing with a pipette also ensures the breaking of the brood capsules and the release of trapped protoscoleces from their interior, freeing them from the encircle membrane. Parasite cultures are established in glass tubes Leighton-type to avoid the sEVs adhesion and with a flat portion to enable a dispersed establishment of the parasite culture. In the case of metacestodes obtained from the peritoneal cavity of mice, it is essential to separate the adventitial layer that covers them and perform several washes with PBS supplemented with antibiotics to remove associated cells and intestinal microbiota, which may influence the purity of the sEVs derived from the helminth itself as also described by White et al.23. The tubes are plugged with sterile rubber stoppers, which contribute to generating an oxygen-deficient atmosphere required to emulate the fermentative metabolism that these parasites maintain in vivo. Finally, the M199 medium is very appropriate as a maintenance medium for the cestode larval stage for the long term24,25. The medium has the minimum components like salts, glucose, and essential amino acids, and, in addition, it contains non-essential amino acids, cholesterol, pyrimidines, water- and fat-soluble vitamins, and nucleic acid precursors including thiamine, riboflavin, and biotin. All these nutrients promote the maintenance of basic organic functions and the viability of these parasites. The M199 is particularly used for non-transformed cells, embryos, primary explants, and organ cultures, being optimal for cell mass cultivation26. Specifically, when used as a maintenance culture medium, M199 must be applied without fetal bovine serum supplementation since it promotes the obtention of vesicularized protoscoleces and their de-differentiation to microcysts27,28.
All conditions used for parasite cultures need to be documented to ensure the reproducibility of results that involve recovery and cell uptake of sEVs. In particular, one aspect that influences EV production is the addition of compounds that affect calcium homeostasis to the culture since they increase calcium-dependent exocytosis29,30. Here, the addition of loperamide, an intracellular calcium inductor in E. granulosus19,31, to parasite cultures is proposed to enhance sEV release. This method enhances the number of vesicles in cestodes (Figure 3) and could be a good strategy to improve production in cellular systems with low EV release. However, since this strategy, like other physical or chemical culture modifications, could alter EV properties and functionalities, careful attention must be considered when performing high-throughput analyses such as proteomics.
The cestode cultures allow achieving EV concentrations of orders of high magnitude, given that their external tegument surface is a syncytial cytoplasmic layer adapted to key processes of exocytosis and endocytosis32. The scale of the EV preparation will be subject to the experimental requirements. A total of 9,000 protoscoleces yield on average 30 μL of sEVs with a concentration of 1–2.5 ´ 108 sEV/mL, enough for carrying out DLS, TEM, and LC-MS-MS analysis in parallel. A limitation of the protocol is obtaining a high quantity of vesicles from metacestodes due to the presence of their laminar layer, which hinders the release of vesicles into the medium. Therefore, large amounts of parasites are required (step 1.3.3)11. An alternative approach is to also analyze sEVs from the hydatid fluid, as reported in other studies11,33,34,35,36.
In line with the broader trend observed in EV research, the majority of studies on helminth EVs to date have employed ultracentrifugation of excretory/secretory products as the primary method for EV isolation and separation37. In this protocol, ultracentrifugation was used to isolate sEVs, which is considered the gold standard method for EV recovery, even though it can entail the inclusion of contaminants jointly with the EVs15. A limitation of this method is the removal of soluble contaminants. Since sEVs are larger than soluble contaminants, an alternative to improve purification is to incorporate additional steps, such as using size exclusion chromatography (SEC)38,39. However, when the isolation of EVs is required for functional analyses, such as studying helminth parasite-host communication, strictly identifying the EV population is not as critical, as all of them carry antigenic cargo. Thus, depending on the needed purity for further experimentation, the isolation process can finish at ultracentrifugation, as performed here, or it could be combined with other methods such as filtration to eliminate large contaminants, sucrose or iodixanol density gradient centrifugation or size exclusion chromatography, which allow not only cleaning contaminants but also separating different EV populations37,38,40. Regardless, the method employed here is simple and represents the primary choice for harvesting and enriching sEVs from parasites39. It provides a high yield of sEVs at a relatively low cost since it only requires a laboratory equipped with an ultracentrifuge in which reusable tubes can be used.
The feature description of the isolated EVs is fundamental to determining the concentration, size, quality, and subtype of EVs. In addition, the analysis of the protein composition cooperates to determine which EV subpopulations are enriched in the sample, as well as estimates the presence of possible contaminants. The EV characterization can be performed by different methods that frequently require specialized equipment and facilities41. Here, the size of parasite sEVs was analyzed using DLS, which delivers reliable data in monodispersed suspensions but is less precise in suspensions with broad-distributed EVs42. Therefore, an alternative could be using other methods such as Nanoparticle Tracking Analysis (NTA), which accurately determines vesicle size and quantity, Tunable Resistive Pulse Sensing (TRPS), which offers a broader dynamic range than NTA, or the Nano-flow Cytometer (NanFCM) which possesses higher sensitivity than conventional flow cytometry43,44,45. Also, as part of the experimental routine and by expert recommendations, the TEM technique allows integrally to determine the purity, yield, and size of the obtained EVs, essentially in high EV harvests46. In addition, examination by TEM analysis is particularly recommended when, after ultracentrifugation, it is not possible to visualize the EV pellet to verify the composition of the recovered material.
These issues highlight the importance of utilizing robust techniques for the isolation and comprehensive characterization of helminth-derived EVs, which could facilitate the identification of specific markers to track their fate and, in turn, enable the evaluation of their functional roles.
Extracellular vesicles purified from E. granulosus represent natural antigen transport carriers with several characterized and unknown antigenic proteins9. These sEVs alone can stimulate antigen-presenting cells distributed throughout different tissues and secondary lymphoid organs of the host. Dendritic cells are unique professional antigen-presenting cells that can display antigens to naïve T cells, determining immune response type in vivo47,48.
This manuscript describes a protocol to detail a method for generating dendritic cells from mouse bone marrow cultures adapted from a previously published research49. Here, a well-established method for immune studies is proposed based on the in vitro culture of Flt3L-driven murine BMDCs, where the isolation of bone marrow is an essential step to achieve a successful in vitro culture50. Given that several isolation protocols of bone marrow cells produce heterogeneous cell populations, the BMDC cultures must be analyzed by flow cytometry during the first time of Flt3L differentiation to establish the cell phenotype for planned experiments51,52,53. Phenotypic characterization of BMDC under the Flt3L effect indicates that the majority are conventional dendritic cells (CD11b+, CD11c+, CD172a+), which more closely resemble dendritic cells found in vivo that load exogenous antigens. While a small percentage of BMDC corresponds to plasmacytoid dendritic cells (CD11c+ B220+ SinglecH+) and CD8+-like dendritic cells (CD11c+ CD24+ and CD172a-)11,53.
Some common troubling steps are the low yield of BMDCs in culture, which may result from bone breakage during the preparation, inefficient bone marrow extraction, cell clump formation, suboptimal culture conditions (e.g., medium composition, temperature), or insufficient cytokine stimulation. To address these issues, some alternatives are to cut behind the epiphyses, ensure complete flushing of bone marrow cells from both sides of femurs and tibias, exhaustly suspend the bone marrow before passing through the cell strainer, use freshly prepared cytokines at the recommended concentration and maintain the sterility, and pH stability of the culture medium.
It is imperative to ensure the dendritic cell baseline ‘resting’ state before inducing their maturation with antigen contact. Dendritic cells not only recognize a wide range of external antigens (PAMPs, pathogen-associated molecular pattern) and internal antigens (DAMPs, danger-associated molecular pattern) but also minimal changes in cell culture (pH, mild agitation, cell density) can induce their maturation. For these reasons, cell culture movements and vibrations must be minimized during handling in the centrifuge or incubator. Moreover, pipetting should be smooth without generating bubbles in the medium. In addition, the maintenance of primary cultures for more than 8 days may activate dendritic cells and promote cell death. Besides, a higher cell density than 2.5 x 106 produces greater MHCII expression54 and increases maturation before antigen contact. Also, all used reagents must be thoroughly free of endotoxins or LPS, a consequence of prior contamination with gram-negative bacteria, because the LPS induces stimulation or exhaustion of dendritic cells23,55. To maintain sterility during manipulation of the bone marrow cultures, besides incorporating the use of antibiotics in the media, it must be continually used 70% ethanol spray on the surface, gloved hands, and cabinet objects, preserving the contact between ethanol and bone marrow cells. Before exposing the instruments to bones or cells, ensure the ethanol has evaporated and air-dry them because it is poisonous to bone marrow cells.
Dendritic cells promote their maturation and cytokine production after recognizing and phagocytosing sEVs. E. granulosus sEVs induce the expression of IL-12, favoring the Th1 profile9. If dendritic cell phenotypic maturation is deficient, the time or the concentration of the stimulus achieved with the added sEVs must be optimized. Confocal microscopy is a powerful tool employed to visualize cargo internalization and colocalization with MHC molecules on dendritic cell endosomes. Moreover, after at least 8 h of the uptake of sEVs, flow cytometry can also be used to measure intracellular cytokines and surface molecules such as CD40, CD80, CD86, and MHCII in mature dendritic cells11,13.
Extensive research employing a wide range of techniques will provide a deeper comprehension of how parasite EV size, origin, and cargo can interfere with and modify host responses.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
The authors acknowledge Lic. Cecilia Gutiérrez Ayesta (Servicio de Microscopía Electrónica, CONICET, Bahía Blanca, Argentina) and Lic. Leonardo Sechi and Lic. Eliana Maza (INIFTA, Universidad Nacional de La Plata, Argentina) for the technical assistance with transmission electron microscopy and dynamic light scattering, respectively. We also thank Dra. Graciela Salerno, Dra. Corina Berón and Dr. Gonzalo Caló for the use of the ultracentrifuge at the INBIOTEC-CONICET-FIBA, Argentina. The authors gratefully acknowledge Lic. Kelly (SENASA, Mar del Plata, Argentina) and Lic. H. Núnez García (CONICET, Universidad Nacional de Mar del Plata, Argentina) for their collaboration in the welfare assessment of mice, and Med. Vet. J. Reyno, Med. Vet. S. Gonzalez, and Med. Vet L. Netti for their contribution to obtaining parasite material. This work, including the costs of experimentation, reagents, and equipment, was supported by the PICT 2020 No. 1651 financed by the ANPCyT.
Materials
| Name | Company | Catalog Number | Comments |
| 1.5 mL tubes | Henso | N14059 | |
| 24-well plate | JetBiofil | CAP011024 | Polystyrene, flat bottom, Sterile |
| 6-well plate | Henso Medical Co. Ltd. | N14221 | Flat-shape bottom, PS material, Sterile |
| 70 mm polypropylene cell strainer | Biologix Group Limited | 15-1070 | Sterile |
| Alcian blue 8 GX dye | Santa Cruz | sc-214517B | |
| Automatic CO2 incubator | Nuarire | UN-5100E/G DH | |
| Bovine Serum Albumin | Wiener lab | 1443151 | |
| CD11c Monoclonal antibody-PECy5 100 µg | eBioscience | 15-0114-82 | clone (N418) |
| CD40 Monoclonal antibody-FITC 100 µg | eBioscience | 11-0402-82 | clone (HM40-3) |
| CD80 Monoclonal antibody-APC 100 µg | eBioscience | 17-0801-82 | clone (16-10A-1) |
| CD86 Monoclonal antibody-FITC 100 µg | eBioscience | 11-0862-82 | clone (GL-1) |
| Centrifuge | Thermo Scientific | IEC CL31R Multispeed | |
| Confocal Microscope | Nikon | Nikon Confocal Microscope C1 | |
| Conical tubes 15 mL dia.17 x 120 mm | Citotest | 4610-1865 | |
| DAPI | Sigma | 107K4034 | |
| D-Glucose | Merk | 1.78343 | |
| Dimethyl Sulfoxide | Anedra | 6646 | |
| Fetal Bovine Serum 500 mL | Sigma-Aldrich | 12352207 | |
| Flow Cytometry System | BD Biosciences | BD FACSCanto™ II | |
| Folded Capillary Zeta Cell | Malvern Panalytical | DTS1070 | |
| Gentamicin sulfate salt | Sigma | G1264 | |
| Glutaraldehyde solution | Fluka | 49630 | |
| Hepes | Gibco | 11344041 | |
| Hypodermic needle 21 G x 1"25/8 | Weigao | Sterile | |
| Hypodermic needle 25 G x 5/8" | Weigao | Sterile | |
| Inverted microscope | Leica | DMIL LED Fluo | |
| Ketamine | Holliday | ||
| Lipopolysaccharide 5 mg | Invitrogen | tlrl-rslps | LPS from the Gram-negative bacteria E. coli K12 . TLR2/4 Agonists |
| Loperamide hydrochloride | Sigma-Aldrich | 5.08162 | |
| Medium 199 | Gibco | 11150059 | |
| Methylene Blue | Anedra | 6337 | |
| MHC class I (H2kb) Monoclonal antibody-PE 100 µg | eBioscience | 12-5958-82 | clone (AF6-88.5.5.3) |
| MHC class II (IA/IE) Monoclonal antibody-FITC 100 µg | eBioscience | 11-5321-82 | clone (M5/114.15.2) |
| Microscope | Olympus | CX31 | |
| Mouse recombinant murine Flt3L. | PrepoTech | 250-31L-10UG | |
| Nanodrop | Thermo Scientific | ND-One | |
| Paraformaldehyde | Agar Scientific | R1018 | |
| Penicillin G sodium salt | Sigma | P3032-10MU | |
| PKH26 | Sigma-Aldrich | MINI26 | |
| Potassium Phosphate Monobasic | Timper | For Phosphate Buffered Saline (PBS) | |
| RBC lysis buffer 100 mL | Roche | 11814389001 | |
| RPMI medium 1640 1x 500 mL | Sigma-Aldrich (Gibco) | 11875093 | |
| Sodium Cacodylate | Sigma-Aldrich | C0250 | |
| Sodium Chloride | Anedra | 7647-14-5 | For Phosphate Buffered Saline (PBS) |
| Sodium Phosphate Dibasic (Anhydrous) p. a. | Biopack | 1639.07 | For Phosphate Buffered Saline (PBS) |
| Streptomycin sulfate salt | Sigma | S9137 | |
| Syringe 10 mL | Bremen | Sterile | |
| Thickwall polycarbonate tubes | Beckman Coulter | 13 x 55 mm , nominal capacity 4 mL | |
| Transmission Electron Microscope | Jeol | JEOL JSM 100CX II | |
| Ultracentrifuge | Beckman | Optima LE-80k | 90 Ti rotor |
| Xylazine | Richmond | ||
| Zetasizer Nano | Malvern | Nano ZSizer-ZEN3600 | To perform Dynamic Light scattering and zeta potential measurements |
References
- Wen, H., et al. Echinococcosis: advances in the 21st century. Clin Microbiol Rev. 32 (2), e00075-e00118 (2019).
- Geary, T. G., Thompson, D. P. Development of antiparasitic drugs in the 21st century. Vet Parasitol. 115 (2), 167-184 (2003).
- Drurey, C., Maizels, R. M. Helminth extracellular vesicles: Interactions with the host immune system. Mol Immunol. 137, 124-133 (2021).
- Welsh, J. A., et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell Vesicles. 13 (2), e12404 (2024).
- Cresswell, P. Antigen processing and presentation. Immunol Rev. 207, 5-7 (2005).
- Buzas, E. I. The roles of extracellular vesicles in the immune system. Nat Rev Immunol. 23 (4), 236-250 (2023).
- Bennett, A. P. S., de la Torre-Escudero, E., Oliver, N. A. M., Huson, K. M., Robinson, M. W. The cellular and molecular origins of extracellular vesicles released by the helminth pathogen Fasciola hepatica. Int J Parasitol. 50 (9), 671-683 (2020).
- Drurey, C., Coakley, G., Maizels, R. M. Extracellular vesicles: new targets for vaccines against helminth parasites. Int J Parasitol. 50 (9), 623-633 (2020).
- Nicolao, M. C., et al. Characterization of protein cargo of Echinococcus granulosus extracellular vesicles in drug response and its influence on immune response. Parasit Vectors. 16 (1), 255 (2023).
- Sánchez-López, C. M., Trelis, M., Bernal, D., Marcilla, A. Overview of the interaction of helminth extracellular vesicles with the host and their potential functions and biological applications. Mol Immunol. 134, 228-235 (2021).
- Nicolao, M. C., Rodriguez, R. C., Cumino, A. C. Extracellular vesicles from Echinococcus granulosus larval stage: Isolation, characterization and uptake by dendritic cells. PLoS Negl Trop Dis. 13 (1), e0007032 (2019).
- Kuipers, M. E., et al. DC-SIGN mediated internalisation of glycosylated extracellular vesicles from Schistosoma mansoni increases activation of monocyte-derived dendritic cells. J Extracell Vesicles. 9 (1), 1753420 (2020).
- Murphy, A., et al. Fasciola hepatica extracellular vesicles isolated from excretory-secretory products using a gravity flow method modulate dendritic cell phenotype and activity. PLoS Negl Trop Dis. 14 (9), e0008626 (2020).
- Ricciardi, A., et al. Extracellular vesicles released from the filarial parasite Brugia malayi downregulate the host mTOR pathway. PLoS Negl Trop Dis. 15 (1), e0008884 (2021).
- Zhang, Q., Jeppesen, D. K., Higginbotham, J. N., Franklin, J. L., Coffey, R. J. Comprehensive isolation of extracellular vesicles and nanoparticles. Nat Protoc. 18 (5), 1462-1487 (2023).
- Brezgin, S., et al. Basic guide for approaching drug delivery with extracellular vesicles. Int J Mol Sci. 25 (19), 10401 (2024).
- Pinheiro, A. A., et al. Potential of extracellular vesicles in the pathogenesis, diagnosis, and therapy for parasitic diseases. J Extracell Vesicles. 13 (8), e12496 (2024).
- Barnadas-Carceller, B., Del Portillo, H. A., Fernandez-Becerra, C. Extracellular vesicles as biomarkers in parasitic disease diagnosis. Curr Top Membr. 94, 187-223 (2024).
- Nicolao, M. C., Denegri, G. M., Carcamo, J. G., Cumino, A. C. P-glycoprotein expression and pharmacological modulation in larval stages of Echinococcus granulosus. Parasitol Int. 63 (1), 1-8 (2014).
- Nicolao, M. C., Cumino, A. C. Biochemical and molecular characterization of the calcineurin in Echinococcus granulosus larval stages. Acta Trop. 146, 141-151 (2015).
- Savina, A., Furlan, M., Vidal, M., Colombo, M. I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 278 (22), 20083-20090 (2003).
- Ambattu, L. A., et al. High frequency acoustic cell stimulation promotes exosome generation regulated by a calcium-dependent mechanism. Commun Biol. 3 (1), 553 (2020).
- White, R., et al. Special considerations for studies of extracellular vesicles from parasitic helminths: A community-led roadmap to increase rigour and reproducibility. J Extracell Vesicles. 12 (1), e12298 (2023).
- Casado, N., Rodriguez-Caabeiro, F., Hernandez, S. In vitro survival of Echinococcus granulosus protoscolices in several media, at +4 degrees C and +37 degrees C. Z Parasitenkd. 72 (2), 273-278 (1986).
- Casado, N., Rodriguez-Caabeiro, F., Jiménez, A., Criado, A., de Armas, C. In vitro effects of levamisole and ivermectin against Echinococcus granulosus protoscoleces. Int J Parasitol. 19 (8), 945-947 (1989).
- Al-Lamki, R. S., Bradley, J. R., Pober, J. S. Human organ culture: updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front Med. 4, 148 (2017).
- Rodriguez-Caabeiro, F., Casado, N. Evidence of in vitro germinal layer development in Echinococcus granulosus cysts. Parasitol Res. 74 (6), 558-562 (1988).
- Loos, J. A., Cumino, A. C. In vitro anti-echinococcal and metabolic effects of metformin involve activation of AMP-activated protein kinase in larval stages of Echinococcus granulosus. PLoS One. 10 (5), e0126009 (2015).
- Erwin, N., Serafim, M. F., He, M. Enhancing the cellular production of extracellular vesicles for developing therapeutic applications. Phar. Res. 40 (4), 833-853 (2023).
- Sako, Y., et al. Identification of a novel small molecule that enhances the release of extracellular vesicles with immunostimulatory potency via induction of calcium influx. ACS Chem Biol. 18 (4), 982-993 (2023).
- He, L. P., Mears, D., Atwater, I., Rojas, E., Cleemann, L. Loperamide mobilizes intracellular Ca2+ stores in insulin-secreting HIT-T15 cells. Br J Pharmacol. 139 (2), 351-361 (2003).
- Dalton, J. P., Skelly, P., Halton, D. W. Role of the tegument and gut in nutrient uptake by parasitic platyhelminths. Can J Zool. 82 (2), 211-232 (2004).
- Dos Santos, G. B., et al. Excretory/secretory products in the Echinococcus granulosus metacestode: is the intermediate host complacent with infection caused by the larval form of the parasite. Int J Parasitol. 46 (13-14), 843-856 (2016).
- Siles-Lucas, M., et al. Isolation and characterization of exosomes derived from fertile sheep hydatid cysts. Vet Parasitol. 236, 22-22 (2017).
- Yang, J., et al. Identification of different extracellular vesicles in the hydatid fluid of Echinococcus granulosus and immunomodulatory effects of 110 K EVs on sheep PBMCs. Front Immunol. 12, 602717 (2021).
- Khosravi, M., et al. Characterisation of extracellular vesicles isolated from hydatid cyst fluid and evaluation of immunomodulatory effects on human monocytes. J Cell Mol Med. 27 (17), 2614-2625 (2023).
- Sotillo, J., et al. The protein and microRNA cargo of extracellular vesicles from parasitic helminths–current status and research priorities. Int J Parasitol. 50 (9), 635-645 (2020).
- Kuipers, M. E., et al. Optimized protocol for the isolation of extracellular vesicles from the parasitic worm Schistosoma mansoni with improved purity, concentration, and yield. J Immunol Res. 2022 (1), 5473763 (2022).
- Fernandez-Becerra, C., et al. Guidelines for the purification and characterization of extracellular vesicles of parasites. J Extracell Biol. 2 (10), e117 (2023).
- Chaiyadet, S., et al. Silencing of Opisthorchis viverrini tetraspanin gene expression results in reduced secretion of extracellular vesicles. Front Cell Infect Microbiol. 12, 827521 (2022).
- Théry, C., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 7 (1), 1535750 (2018).
- Szatanek, R., et al. The methods of choice for extracellular vesicles (EVs) characterization. Int J Mol Sci. 18 (6), 1153 (2017).
- Blundell, E. L. C. J., Mayne, L. J., Billinge, E. R., Platt, M. Emergence of tunable resistive pulse sensing as a biosensor. Anal Methods. 7 (17), 7055-7066 (2015).
- Vucetic, A., Filho, A., Dong, G., Olivier, M. Isolation of extracellular vesicles from Leishmania spp. Methods Mol Biol. , 555-574 (2020).
- Caputo, F., et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: Addressing some analytical challenges in the submicron size range. J Colloid Interface Sci. 588, 401-417 (2021).
- Crescitelli, R., Lässer, C., Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc. 16 (3), 1548-1580 (2021).
- Trombetta, E. S., Mellman, I. The cell biological basis of antigen presentation in vitro and in vivo. Ann Rev Immunol. 23 (1), 975-1028 (2005).
- Hammer, G. E., Ma, A. Molecular control of state dendritic cell maturation and immuno homeostasis. Ann Rev Immunol. 31 (1), 743-791 (2013).
- Weigel, B. J., et al. Comparative analysis of murine marrow–derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood. 100 (12), 4169-4176 (2002).
- March, N., et al. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 60, 3239-3246 (2000).
- Heath, W. R., et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 199 (1), 9-26 (2004).
- Naik, S., et al. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 174 (11), 6592-6597 (2005).
- Sauter, M., et al. Protocol to isolate and analyze mouse bone marrow derived dendritic cells (BMDC). STAR Protoc. 3 (3), 101664 (2022).
- Helft, J., et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+) MHCII(+) macrophages and dendritic cells. Immunity. 42 (6), 1197-1211 (2015).
- Borup, A., et al. Comparison of separation methods for immunomodulatory extracellular vesicles from helminths. J Extracell Biol. 1 (5), e41 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved