Tensile Strength of Resorbable Biomaterials
Przegląd
Source: Peiman Shahbeigi-Roodposhti and Sina Shahbazmohamadi, Biomedical Engineering Department, University of Connecticut, Storrs, Connecticut
For over 4000 years, sutures have been used as a medical intervention. The earliest records indicate linen was the biomaterial of choice. Catgut, which is still in use today, was reportedly used to treat gladiators around 150 AD. Today, there are numerous materials being used for sutures. Sutures are classified by their composition (natural or synthetic) and their absorption (non-resorbable or resorbable).
Resorbable (or absorbable) sutures degrade in the body through either enzymatic degradation or programmed degradation caused by the interaction of water with specific groups in the polymer chain. These sutures are often created from synthetic materials, such as polyglycolic acid, polydioxanone, and polycaprolactone, or natural biomaterials, such as silk. They are usually used for certain internal procedures, like general surgery. Resorbable sutures will hold the wound together for a time frame long enough for healing, but then they eventually disintegrate by the body. On the other hand, non-resorbable sutures do not degrade and must be extracted. They are usually derived from polypropylene, nylon, and stainless-steel. These sutures are usually implemented for orthopedic and cardiac surgery and require a medical professional to remove them at a later date.
Here, the tensile strength of two types of resorbable sutures will be tested after exposing them to neutral, acidic, and alkaline solutions, which correspond to the different pH environments found within the human body. The test will consist of two parts. First, control samples will be prepared and analyzed via tensile testing. Then, samples will be tested after the continuous exposure to solutions of varying pH over the course of several weeks.
Zasady
Material degradation describes the loss of performance and change in material properties, such as tensile strength, color, and shape, after exposure to one or more environmental factors. These factors include heat, light, mechanical forces or chemical exposure, such as of acids, alkalis or salts. One way to control degradation is by surface engineering. This is accomplished by shielding a surface with a protective layer or by modifying the material itself, for example, through crosslinking.
Here, samples of commercially available specimens are tested in a testing machine with a force transducer. The samples are placed securely into the clamps of the testing machine (UTM), the UTM is zeroed, and a displacement speed of 6 mm/min is initiated until failure. After failure, the peak force is recorded. The experimental design is shown in the figure below.
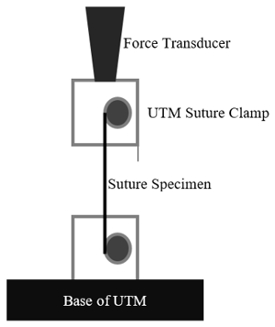
Figure 1: Experimental Design.
Two resorbable sutures will be used in this experiment: Polyglyconate sutures and polydioxanone sutures. Synthetic polyglyconate sutures are prepared from a reaction requiring glycolide and trimethylene carbonate. Upon forming poly(glycolide-co-trimethylene carbonate), they are polymerized. These polyglycolides have a linear structure of (C8H10O7)n,, which is shown below in Figure 2. On the other hand, PDS II Violet Monofilament sutures are synthesized from polydioxanone polymers, with a linear structure of (C4H6O3)n. Polydioxanone is shown below in Figure 3.
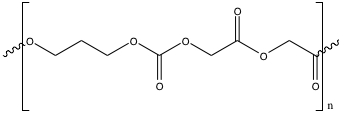
Figure 2: Poly(glycolide-co-trimethylene carbonate) used to polymerize polyglyconate sutures.
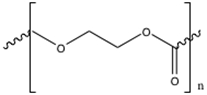
Figure 3: Polydioxanone used to polymerize sutures.
Both sutures follow hydrolysis reactions through the degradation process. For polyglycolide, a two-step hydrolysis occurs. First, the polymer is first converted into its monomer (glycolic acid) by cleavage of its internal ester bonds. Then, a hydrolytic attack on the crystalline portions of the polymer occurs. As the crystalline regions dissolve, the polymer collapses. Likewise, polydioxanone sutures are safely degraded through the ester radical. The degraded compounds from both suture types are non-toxic and safely secreted through urination or exhalation.
Over time, the solution becomes more acidic, due to the increased presence of hydroxyl ions. Carboxylic acid radicals are generated from the ends of the degraded monomers, which lowers the pH of the surrounding solution. Recent studies have shown that polyglycolide and polydioxanone structures degrade faster in vivo than in vitro, brought on by cellular enzymatic activity2. Effects due to the presence of biological enzymes are not observed during this in vitro procedure.
Procedura
1. Sample preparation
- Create six labels containing the information below, and attach the labels onto screw-top test tubes.
- Date: month and day
- Sample type: polyglyconate or polydioxanone
- Solution type: acidic (A), alkaline (B), or neutral (N) solution with pH ranging between 2-14.
- Open the suture packaging and remove the suture. Cut off the needle and discard it into the sharps container.
- Cut the suture into 3 pieces that are approximately 10-12 in long.
- Note the color and physical characteristics of the suture.
- Use a caliper to measure the diameter of each suture.
- Weigh each suture and place one sample into each test tube.
- Fill the test tubes labeled "N" with enough deionized water to cover the suture, and then cap the test tubes.
- Use a pipette to fill the test tubes labeled "A" with enough 0.001 M HCl solution to cover the suture. Remember to cap the test tubes.
- Use a pipette to fill the test tubes labeled "B" with enough 0.001 M NaOH solution to cover the suture. Remember to cap the test tubes.
- Place all six test tubes in the metal rack in an oven at 37 ºC.
2. Control Sample Tensile Testing
- Obtain a fresh suture, the control sample, and place it in the fixture of the UTM and secure into place.
- Prior to initiating tension on the specimen, zero the UTM by pressing the F1 (zero force) and F2 (zero ext) keys. Record the displacement speed setting on the data sheet.
- Make sure peak hold is displayed on the UTM display panel.
- Start the UTM by pressing the up-arrow key. The force and displacement will start to change on the UTM.
- Load the specimen until failure. Then stop the UTM.
- Record the peak force from the UTM display.
3. Strength Loss Profile
- Remove one of each sample (A, B, & N) from the oven every week for five weeks.
- Measure the pH of the solution in the test tube with pH paper.
- Rinse the suture with deionized water, and note any physical or color changes to the material in each sample.
- If necessary, pat the sample dry with a paper towel.
- Weigh each sample and record the new weight
- Place the specimen in the grips of the UTM and lock it into place.
- Prior to initiating tension on the specimen, zero the UTM by pressing the F1 (zero force) and F2 (zero ext) keys.
- Make sure peak hold is displayed on the UTM display panel, and verify that the displacement speed on the UTM is the same as when you tested the control sample.
- Load the specimen until failure. Then stop the UTM.
- Record the peak force at failure from the UTM display.
Wyniki
Over the course of five weeks, all treated specimens were tested and analyzed. From the overall trials, the average tensile strengths were calculated using Equation 1:
 (1)
(1)
The standard deviations of all the forces at failure with respect to suture type and solution environment were also calculated. Finally, the percent tensile strength retained was determined using average tensile strength. Below are the graphs showing representative results.
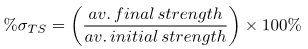 (2)
(2)
The average strength-loss profile for the polyglyconate sutures across all pH ranges were around 81%, 76%, 66%, and 54% for the first four weeks, respectively. During the first four weeks of the experiment, this profile is nearly identical to the manufacturer claims for these sutures. It is also evident that the original polyglyconate profile degrades at a slightly faster rate than the experimental in vitro sutures. This is attributed to the fact that the manufacturer performed in vivo tests, where factors such as enzymatic degradation were present. The presence of biological enzymes can greatly increase the rate of degradation and reabsorption of biomaterials. In vivo testing subjects the specimen to different stresses and biochemical interactions that in vitro procedures lack. In vivo testing is generally preferred over in vitro testing because it allows for the overall effects of an experiment on a living subject to be observed.

Figure 4: Acidic Solution: Suture Tensile Strength.

Figure 5: Neutral Solution, Suture Tensile Strength.

Figure 6: Alkaline Solution, Suture Tensile Strength.
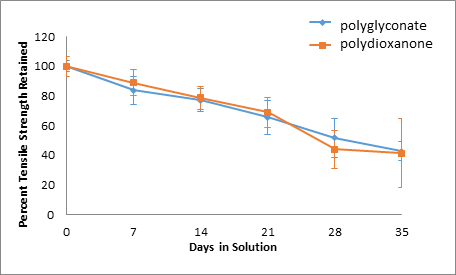
Figure 7: Acid Solution, Percent Tensile Strength Retained.
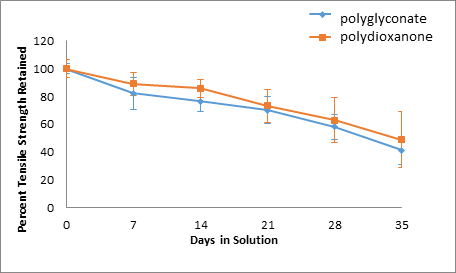
Figure 8: Neutral Solution, Percent Tensile Strength Retained.
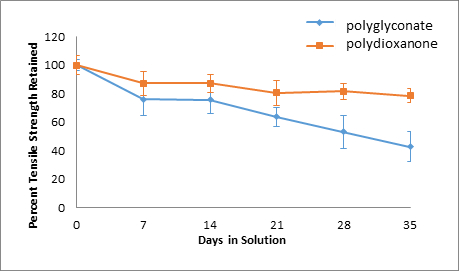
Figure 9: Basic Solution, Percent Tensile Strength Retained.
| Control | 7 days | 14 days | ||||||
| Average pH | Average pH | Average pH | ||||||
| N/A | Acid | Neutral | Base | Acid | Neutral | Base | ||
| 5 | 6 | 8 | 4 | 6 | 9 | |||
| Force (N) | Force (N) | Force (N) | ||||||
| 93.63 | 83.67 | 85.67 | 78.40 | 74.63 | 83.53 | 78.40 | ||
| 102.07 | 98.53 | 93.50 | 82.77 | 71.73 | 77.30 | 80.83 | ||
| 101.43 | 78.13 | 81.03 | 86.77 | 75.08 | 81.73 | 80.33 | ||
| 97.80 | 79.50 | 75.73 | 82.40 | 76.50 | 74.67 | 81.17 | ||
| 86.43 | 79.93 | 81.63 | 75.33 | 67.00 | 87.10 | 94.80 | ||
| 94.23 | 96.80 | 98.07 | 89.27 | 91.43 | 87.47 | |||
| 21 days | 28 days | 35 days | ||||||
| Average pH | Average pH | Average pH | ||||||
| Acid | Neutral | Base | Acid | Neutral | Base | Acid | Neutral | Base |
| 4 | 6 | 9 | 4 | 6 | 8 | 4 | 6 | 8 |
| Force (N) | Force (N) | Force (N) | ||||||
| 56.53 | 58.70 | 85.97 | 51.53 | 58.57 | 73.22 | 36.37 | 38.77 | 74.67 |
| 60.73 | 65.33 | 75.80 | 49.70 | 51.43 | 72.20 | 24.20 | 34.83 | 67.70 |
| 58.27 | 63.53 | 69.23 | 56.87 | 72.20 | 83.20 | 36.30 | 42.37 | 73.27 |
| 64.93 | 66.83 | 81.60 | 40.63 | 28.40 | 72.90 | 21.60 | 36.83 | 74.63 |
| 68.57 | 63.90 | 81.90 | 29.70 | 58.70 | 80.93 | 42.00 | 40.97 | 75.67 |
| 75.20 | 76.17 | 61.63 | 20.83 | 69.47 | 83.33 | 31.37 | 45.33 | 81.77 |
| 85.63 | 94.17 | 85.00 | 36.37 | 78.13 | 76.73 | 87.53 | 90.77 | 81.83 |
| 60.33 | 75.83 | 80.47 | 52.33 | 66.67 | 85.83 | |||
Table 1: Overall 5-Week Polydioxanone Suture Data, Forces at Failure
| Control | 7 days | 14 days | ||||||
| Average pH | Average pH | Average pH | ||||||
| N/A | Acid | Neutral | Base | Acid | Neutral | Base | ||
| 4 | 6 | 9 | 4 | 6 | 9 | |||
| Force (N) | Force (N) | Force (N) | ||||||
| 170.80 | 131.37 | 147.03 | 146.23 | 122.07 | 117.87 | 135.17 | ||
| 170.93 | 147.70 | 142.60 | 152.63 | 129.30 | 132.13 | 129.87 | ||
| 167.70 | 134.00 | 153.80 | 120.13 | 107.93 | 113.13 | 101.57 | ||
| 162.37 | 112.90 | 102.87 | 111.07 | 139.63 | 120.47 | 111.20 | ||
| 156.70 | 153.20 | 124.63 | 103.80 | 123.80 | 131.47 | 129.57 | ||
| 152.87 | 145.90 | 123.33 | 143.57 | 146.13 | 144.57 | |||
| 21 days | 28 days | 35 days | ||||||
| Average pH | Average pH | Average pH | ||||||
| Acid | Neutral | Base | Acid | Neutral | Base | Acid | Neutral | Base |
| 4 | 6 | 8 | 4 | 6 | 8 | 4 | 5 | 7 |
| Force (N) | Force (N) | Force (N) | ||||||
| 110.63 | 109.13 | 115.27 | 93.67 | 93.40 | 74.57 | 50.43 | 54.03 | 44.80 |
| 115.10 | 113.13 | 87.90 | 75.40 | 100.50 | 77.93 | 82.47 | 78.67 | 78.70 |
| 120.50 | 128.93 | 116.37 | 111.43 | 108.00 | 109.73 | 80.47 | 42.83 | 80.20 |
| 114.03 | 116.43 | 101.03 | 84.23 | 87.17 | 80.10 | 69.40 | 81.13 | 77.10 |
| 118.83 | 110.93 | 107.43 | 51.47 | 66.90 | 81.60 | 68.70 | 81.50 | 46.97 |
| 78.33 | 87.90 | 115.57 | 59.87 | 93.77 | 61.07 | 76.87 | 82.73 | 82.53 |
| 131.20 | 141.07 | 107.83 | 105.60 | 111.73 | 112.21 | 68.00 | 57.27 | 86.23 |
| 80.47 | 122.70 | 91.67 | 103.67 | 110.10 | 105.67 | |||
Table 2: Overall 5-Week Polyglyconate Suture Data, Forces at Failure
Over time, the tensile strengths of all suture specimens decreased. In addition, for polydioxanone sutures, an acidic environment was the most damaging as only 41.46% of the original tensile strength was retained, whereas 78.58% and 48.95% of the original tensile strength was retained for polydioxanone sutures in alkaline and neutral solutions, respectively. On the other hand, the strength retention percentages over time for polyglyconate sutures across different pH solutions were all similar. The greatest decrease in tensile strength for the polyglyconate sutures was observed in a neutral environment, where only 41.22% of the original strength was retained. In acidic and alkaline environments, 42.79% and 42.81% of original tensile strength was retained for the polyglyconate sutures, respectively.
If the sutures were incubated at a higher temperature, they would have degraded faster due to the increased inherent energy found within the system. This would allow for more spontaneous depolymerization into monomers to occur. In other words, as the temperature increases, the tensile strength is negatively affected. In addition, if the sutures were held at constant stress, chances of decay would also increase. This would be due to creep deformation; stretching the sutures creates weaker locales that are prime for absorption. If the sutures were to be tied into knots, a similar scenario would occur.
Wniosek i Podsumowanie
In this experiment, the tensile strength of sutures in different pH environments were evaluated. Over five weeks, the tensile strengths of two different types of sutures were explored after exposure to acidic, alkaline, and neutral solutions. The results overwhelmingly indicate that bioabsorbable sutures will degrade over time in any pH environment.
Although the polyglyconate sutures degrade at a faster rate, the remain stronger compared to the polydioxanone sutures. The experimental results also show that in prolonged time frames, polydioxanone sutures retain more of their strength than the polyglyconate sutures, as the faster degradation rate of polyglyocnate sutures becomes more evident. Nonetheless, since the experiment was performed in vitro, no substantial conclusions can be drawn for the effectiveness of either the polyglyconate or polydioxanone sutures in a more active biochemical model. Enzymatic degradation is a critical aspect that must be considered. Regardless, both sutures are viable candidates for surgical procedures. This study confirms the importance of this type of research.
Resorbable sutures provide temporary wound support, allowing the wound to heal well enough to withstand normal forces. Generally, resorbable sutures are used for internal procedures, so additional surgical procedures would be unnecessary for suture removal. Upon disintegration, little to no traces of the suture remain. Resorbable sutures are also used in patients that cannot return for a suture removal procedure. On the other hand, non-resorbable sutures are commonly used in epidermal would closure, where the sutures can be easily removed after a certain amount of time. Additionally, non-resorbable sutures are often used in stressful internal environments as well, when resorbable sutures are incapable of providing enough wound support. Internal structures such as the heart, which consistently withstands various pressures and movements, require non-resorbable sutures. Other applications of non-resorbable sutures include orthopedic surgeries and sternal closure in cardiac surgeries. Since resorbable sutures are used in internal and more critical parts of the body, it is important to test their strength and analyze the product quality.
Materials List
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Suture | |||
| Ruler | |||
| Scissors | |||
| Calipers | |||
| Tweezers | |||
| Scale | |||
| Tinius Olsen Tester | |||
| Oven | |||
| Sample Container | |||
| Beakers | |||
| Pipette | |||
| Pipette fillers | |||
| Pipette tube | |||
| Glassware | |||
| Chemicals | |||
| De-ionized water | |||
| Hydrochloric Acid (HCL) | |||
| Sodium Hydroxide (NaOH) |
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Tensile Strength of Resorbable Biomaterials
Biomedical Engineering
7.7K Wyświetleń

Imaging Biological Samples with Optical and Confocal Microscopy
Biomedical Engineering
36.3K Wyświetleń

SEM Imaging of Biological Samples
Biomedical Engineering
24.1K Wyświetleń

Biodistribution of Nano-drug Carriers: Applications of SEM
Biomedical Engineering
9.6K Wyświetleń

High-frequency Ultrasound Imaging of the Abdominal Aorta
Biomedical Engineering
14.8K Wyświetleń

Quantitative Strain Mapping of an Abdominal Aortic Aneurysm
Biomedical Engineering
4.6K Wyświetleń

Photoacoustic Tomography to Image Blood and Lipids in the Infrarenal Aorta
Biomedical Engineering
5.9K Wyświetleń

Cardiac Magnetic Resonance Imaging
Biomedical Engineering
15.0K Wyświetleń

Computational Fluid Dynamics Simulations of Blood Flow in a Cerebral Aneurysm
Biomedical Engineering
12.0K Wyświetleń

Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Biomedical Engineering
8.4K Wyświetleń

Noninvasive Blood Pressure Measurement Techniques
Biomedical Engineering
12.1K Wyświetleń

Acquisition and Analysis of an ECG (electrocardiography) Signal
Biomedical Engineering
107.1K Wyświetleń

Micro-CT Imaging of a Mouse Spinal Cord
Biomedical Engineering
8.3K Wyświetleń

Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Biomedical Engineering
8.3K Wyświetleń

Combined SPECT and CT Imaging to Visualize Cardiac Functionality
Biomedical Engineering
11.2K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone